Abstract
Aims:
There exists a discrepancy regarding the relationship between obstructive sleep apnoea (OSA) and circadian variation during the onset of acute myocardial infarction (MI). We hypothesized that OSA patients show a characteristic circadian variation and that the severity of OSA significantly affects this variation.
Methods and results:
The present study included 288 patients with first acute MI who underwent percutaneous coronary intervention within 12 h of symptom onset. The diagnosis of OSA required an apnoea–hypopnoea index (AHI) of ≥5 events/h. A total of 216 patients fulfilled the OSA criteria. The incidence of MI onset between 06:00 and 11:59 hours was significantly higher in OSA patients than in control patients (38 vs. 25%, p=0.039). Circadian variation in the morning peak of MI onset was attenuated in mild OSA (as defined by AHI, 5.0–14.9 events/h; 33 vs. 25%, p=0.240). Moderate-to-severe OSA (as defined by AHI ≥15.0 events/h) clearly increased the incidence of MI onset between 06:00 and 11:59 hours (43 vs. 25%, p=0.014). Multiple logistic regression adjusting for AHI (≥15.0 events/h), age, body mass index, hypertension, and current smoking showed that moderate-to-severe OSA significantly contributed to MI onset between 06:00 and 11:59 hours (odds ratio 2.00, p=0.010).
Conclusions:
OSA showed a morning peak with regard to MI onset, and moderate-to-severe OSA significantly enhanced this circadian variation.
Keywords: Acute myocardial infarction, circadian variation, obstructive sleep apnoea
Introduction
The time of onset of acute myocardial infarction (MI) shows an apparent circadian pattern with a peak incidence between 06:00 and 12:00 hours.1,2 Diabetes and prior use of β-blockers attenuate this occurrence.3,4 Cumulative evidence indicates that untreated obstructive sleep apnoea (OSA) increases the risk of developing cardiovascular disease, including acute MI.5,6 Increased sympathetic nerve activity throughout the day, altered variation in blood pressure, and repetitive hypoxia and blood pressure elevation during sleep are cardiovascular actions related to OSA,7 which may constitute a different circadian variation of MI onset compared with patients without OSA. However, there exists a discrepancy between the time of onset of acute MI and sleep apnoea. Kuniyoshi et al.8 demonstrated that patients with OSA have an increased risk of MI between 00:00 and 06:00 hours compared with non-OSA patients. In contrast, two studies demonstrated that patients with sleep apnoea experienced a higher incidence of MI onset in the morning than that observed in control patients.9,10
Our study is the largest to have examined the relationship between OSA and MI onset. We hypothesized that OSA patients show a distinct circadian variation of MI onset compared with control patients and that circadian variation may be significantly affected by OSA severity.
Methods
Between June 2006 and September 2011, 314 patients admitted to Nagasaki Citizens Hospital with first acute MI and who underwent primary percutaneous coronary intervention (PCI) within 12 h of onset were initially considered to be included in the present study. After informed consent was obtained, all patients with acute MI were scheduled to undergo polysomnography before being discharged. Acute MI was defined as ischaemic symptoms lasting for >30 min with ST-segment elevation or depression (≥1mm), an increase in creatine kinase to twice the normal upper value, and an elevation in cardiac troponin T levels (≥0.1 ng/ml). Inclusion criteria were as follows: (1) time of onset clearly determined based on patient’s symptoms and (2) polysomnography showing normal sleep or OSA. Twenty-six patients with central sleep apnoea were excluded from the study, and the study group consisted of the remaining 288 patients. This study complied with the Helsinki Declaration and was approved by all relevant committees at our institution.
Polysomnography
All study patients underwent polysomnography between 14 and 21 days. Electroencephalograms, electro-oculograms, and chin electromyograms were recorded to evaluate the stage of sleep. Sleep states and arousal were scored on the basis of standard criteria. Naso–oral air flow and thoraco–abdominal movements were used to determine the type of apnoea. Arterial oxyhaemoglobin saturation (SaO2) was recorded using a pulse oximeter and electrocardiographic recordings were taken from a single lead. Apnoea was defined as complete cessation of air flow lasting for ≥10 s. Hypopnoea was defined as a ≥50% reduction in air flow lasting for ≥10 s associated with a 3% decrease in oxygen saturation or arousal. The apnoea–hypopnoea index (AHI) was defined as the average number of apnoea and hypopnoea events per hour. Obstructive apnoea was defined as the absence of air flow in spite of respiratory movement or exertion. Central sleep apnoea was defined as the absence of both air flow and respiratory movement. OSA was defined as AHI ≥5 events/h, of which > 50% were obstructive. The severity of OSA was assessed based on AHI. Mild OSA was defined as AHI 5.0–14.9 events/h, and moderate-to-severe OSA as AHI ≥15.0 events/h.
Analysis of circadian variation of MI onset
The day was divided into four 6-h intervals: 0:00 to 05:59, 06:00 to 11:59, 12:00 to 17:59, and 18:00 to 23:59 hours. The difference in circadian variation of MI onset was examined between OSA and control patients. The impact of OSA severity on circadian variation was also evaluated.
Statistics
Continuous variables were expressed as the median values and interquartile ranges because all continuous variables, except for systolic blood pressure on admission, did not show normal distribution using the Shapiro–Wilk test. Comparisons of continuous variables were made between OSA and control patients using the Wilcoxon rank-sum test. Dichotomous variables were expressed as counts with percentages and were compared using the χ2 test or Fisher’s Exact test when cells had counts <5. The presence of circadian variation across four 6-h intervals was tested using the χ2 goodness-of-fit test for uniform distribution in all study, control, and OSA patients. Difference in frequency of MI onset during each 6-h interval was tested between OSA and control patients using the χ2 test. The impact of severity of OSA on circadian variation of onset was also evaluated. The relationship of risk factors including age, OSA severity, body mass index (BMI), current smoking, diabetes, hypercholesterolaemia, and hypertension with MI onset during the second quarter of the day was examined using univariate logistic regression analysis. Subsequently, multiple logistic regression analysis including all variables with a p-value <0.10 in univariate analysis was used to determine the predictive factors of MI onset during the second quarter of the day. The following variables were entered into the model: AHI ≥15.0, age, BMI, hypertension, and current smoking. The validation of the model was examined using the Hosmer–Lemeshow goodness-of-fit test. Statistical significance was defined as two-sided p<0.05. Data analyses were performed using SPSS statistical software version 19.0 (SPSS, Chicago, IL, USA).
Results
A total of 216 patients (75%) fulfilled the criteria for OSA and 72 (controls) had an AHI of <5 events/h. Patient characteristics for the two groups are presented in Table 1. BMI was significantly higher in OSA than in the control patients. The incidence of other risk factors including hypertension, hypercholesterolaemia, diabetes, and current smoking was not different between the two groups. Infarct size estimated by peak creatine kinase value and final Thrombolysis in Myocardial Infarction (TIMI) flow grade were not different for the two groups, despite a longer time from symptom onset to hospital visit in controls.
Table 1.
Patient characteristics.
| Control (n=72) | OSA (n=216) | p-value | |
|---|---|---|---|
| Age (years) | 66 (55–76) | 66 (57–76) | 0.429 |
| Male | 52 (72) | 168 (78) | 0.336 |
| BMI (kg/m2) | 22.5 (20.9–23.7) | 23.7 (21.8–26.1) | <0.001 |
| Hypertension | 41 (57) | 131 (61) | 0.579 |
| Hypercholesterolaemia | 37 (51) | 130 (60) | 0.190 |
| Diabetes | 19 (26) | 73 (34) | 0.243 |
| Current smoking | 27 (38) | 96 (44) | 0.337 |
| Time to admission (h) | 3.0 (1.7–5.8) | 2.2 (1.3–4.0) | 0.006 |
| Pre-infarct angina <24 h | 31 (43) | 73 (34) | 0.157 |
| Anterior infarct | 31 (43) | 106 (49) | 0.376 |
| SBP (mmHg) | 130 (109–152) | 138 (116–158) | 0.822 |
| LVEDP (mmHg) | 10 (7–16) | 12 (8–16) | 0.343 |
| Peak CK (IU/l) | 1704 (750–3429) | 1898 (836–3502) | 0.328 |
| Multivessel disease | 30 (42) | 101 (47) | 0.452 |
| Stent use | 63 (88) | 196 (91) | 0.429 |
| Final TIMI grade 3 flow | 72 (100) | 210 (97) | 0.175 |
Values are median (interquartile range) or n (%).
BMI, body mass index; CK, creatine kinase; LVEDP, left ventricular end-diastolic pressure; SBP, systolic blood pressure.
Sleep-related symptoms and polysomnographic findings are presented in Table 2. Witnessed apnoea was noted more frequently in OSA patients compared with controls. Patients with OSA showed significant hypoxia during sleep as estimated by 3% oxygen desaturation index, mean arterial oxygen saturation (SaO2), lowest SaO2, and cumulative time of SaO2 <90%. The arousal index was also higher in OSA patients.
Table 2.
Sleep-related symptoms and polysomnographic findings.
| Control (n=72) | OSA (n=216) | p-value | |
|---|---|---|---|
| Snoring | 29 (40) | 107 (50) | 0.173 |
| Insomnia | 17 (24) | 70 (32) | 0.159 |
| Day-time sleepiness | 9 (13) | 45 (21) | 0.117 |
| Witnessed apnoea | 8 (11) | 64 (30) | 0.002 |
| Total sleep time (min) | 356 (316–404) | 360 (303–400) | 0.795 |
| AHI (events/h) | 1.95 (1.13–3.67) | 16.3 (8.9–26.7) | <0.001 |
| 3% ODI (events/h) | 2.85 (1.03–4.70) | 14.0 (8.18–22.5) | <0.001 |
| Mean SaO2 (%) | 96 (95–97) | 95 (94–96) | 0.006 |
| Lowest SaO2 (%) | 91 (89–93) | 86 (83–89) | <0.001 |
| Cumulative time of SaO2< 90% | 0 (0–0.4) | 2 (0.5–9) | <0.001 |
| Arousal index (events/h) | 4.60 (1.90–10.6) | 17.7 (11.2–26.1) | <0.001 |
Values are median (interquartile range) or n (%).
AHI, apnoea-hypopnoea index; ODI, oxygen desaturation index; SaO2, arterial oxyhaemoglobin saturation.
Circadian variation of MI onset
The circadian variation of MI onset is presented in Figure 1. The χ2 goodness-of-fit test indicated uneven distributions with a morning peak of onset in all study patients and OSA patients (p=0.001 and p<0.001, respectively). The incidence of MI onset between 06:00 and 11:59 hours was significantly higher in OSA patients than in controls (38 vs. 25%, p=0.039).
Figure 1.
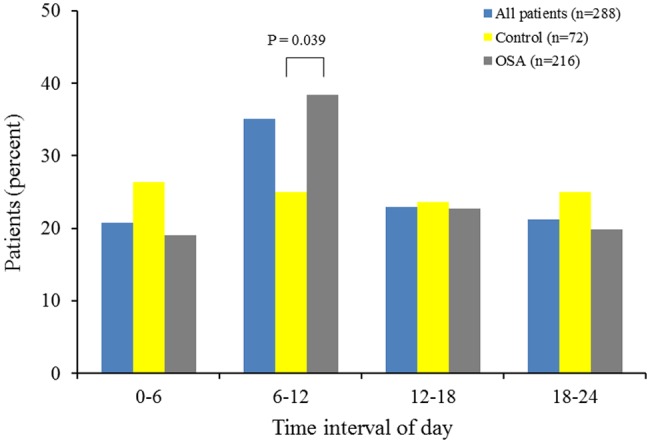
Circadian variations in the frequency of MI onset based on 6-h intervals for all study, control, and OSA patients.
The impact of OSA severity on circadian variation is presented in Figures 2 and 3. The circadian variation in the morning peak of MI onset was attenuated in mild OSA (33 vs. 25%, p=0.240). In contrast, moderate-to-severe OSA clearly increased the incidence of MI onset between 06:00 and 11:59 hours (43 vs. 25%, p=0.014).
Figure 2.
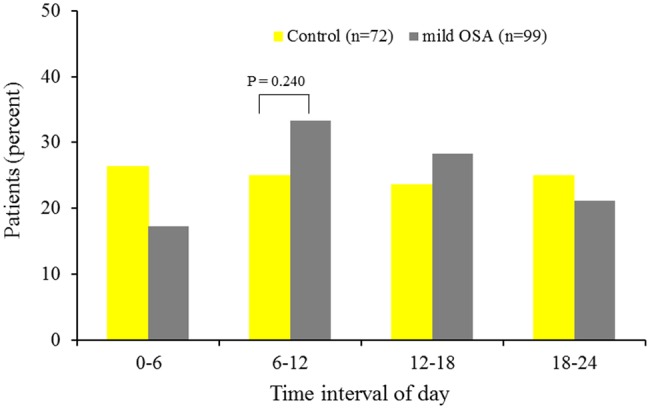
Circadian variations in the frequency of MI onset based on 6-h intervals for control and mild OSA patients.
Figure 3.
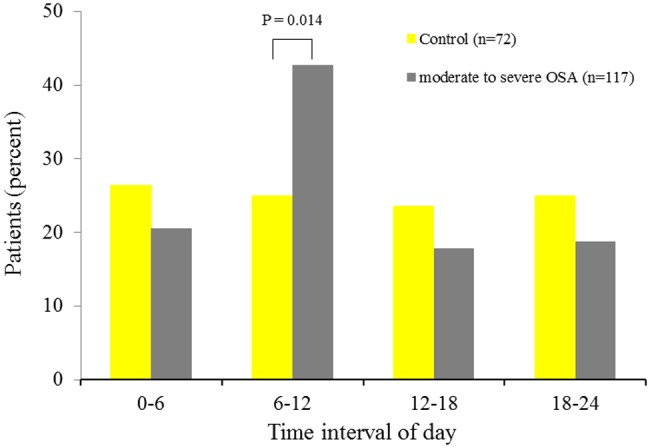
Circadian variations in the frequency of MI onset based on 6-h intervals for control and moderate-to-severe OSA patients.
Predictors for morning MI onset
The significant coronary risk factors for MI onset between 06:00 and 11:59 hours are given in Table 3. Univariate analysis showed that AHI ≥15.0 events/h; (moderate-to-severe OSA), age, hypertension, and current smoking were related to morning onset of MI, but mild OSA was not related to morning onset. Multivariate analysis adjusted for AHI ≥15.0 events/h, age, BMI, hypertension, and current smoking showed that AHI ≥15.0 events/h was positively correlated with morning onset (odds ratio 2.00, p=0.010). Hypertension was negatively correlated with this circadian variation. Validation of this model was confirmed by the Hosmer–Lemeshow goodness-of-fit test (p=0.828).
Table 3.
Predictors for morning onset in acute MI.
| Unadjusted OR | p-value | Adjusted OR | p-value | |
|---|---|---|---|---|
| AHI ≥15.0 | 1.83 (1.12–2.99) | 0.016 | 2.00 (1.12–3.41) | 0.010 |
| AHI 5.0–14.9 | 0.99 (0.53–1.49) | 0.655 | ||
| Age | 1.03 (1.01–1.05) | 0.013 | 1.02 (0.996–1.05) | 0.095 |
| BMI | 0.93 (0.995–1.15) | 0.068 | 0.95 (0.89–1.03) | 0.221 |
| Hypertension | 0.59 (0.36–0.97) | 0.037 | 0.47 (0.27–0.80) | 0.005 |
| Current smoking | 0.60 (0.36–0.98) | 0.043 | 0.79 (0.42–1.36) | 0.353 |
| Diabetes | 0.98 (0.58–1.65) | 0.994 | ||
| Dyslipidaemia | 0.85 (0.52–1.39) | 0.521 |
Values in parentheses are 95% confidence interval.
AHI, apnoea-hypopnoea index; BMI, body mass index; MI, myocardial infarction; OR, odds ratio.
Discussion
The main finding of our study is that OSA significantly increased the incidence of acute MI between 06:00 and 11:59 hours. In particular, moderate-to-severe OSA, rather than mild OSA, was positively correlated with this characteristic circadian variation. OSA may be a main risk factor for the morning peak of MI onset because control patients showed an even distribution of frequency of MI onset across the four 6-h intervals.
Coronary thrombosis following unstable plaque disruption is the principal mechanism for the occurrence of acute MI.11–13 A high incidence of OSA (43–65.7%) defined as AHI ≥15 events/h in patients with acute MI has been reported.14,15 A long-term follow-up study for an average of 10.1 years indicated that patients with severe OSA had a higher incidence of fatal or non-fatal cardiovascular events compared with untreated mild to moderate OSA patients, simple snorers, and healthy participants.6 The above findings suggest that OSA may be closely involved in plaque rupture. Increased oxidative stress with lowered antioxidant capacity,16–18 decreased endothelial nitric oxide production,19 activated inflammation,20,21 and increased apoptosis of endothelial cells,22 all of which are known as unfavourable cardiovascular effects of OSA, may result in endothelial dysfunction and unstable plaque formation. Repetitive increases in sympathetic nerve activity and blood pressure elevation associated with apnoea–hypopnoea could trigger plaque rupture during sleep. However, the incidence of MI between 0:00 and 05:59 hours was not different between OSA and control patients in our study. Previous studies have indicated that sympathetic nerve activation and catecholamine release,23–26 which increase cardiac oxygen demand through an increase in heart rate, blood pressure, and vascular resistance, can trigger acute MI because increased cardiac workload may increase the shear stress of blood flow against an unstable plaque, resulting in plaque rupture. Higher sympathetic nerve activity persisted throughout the day in patients with OSA compared with non-OSA subjects.27 Further increase in sympathetic nerve activity evoked by increased physical activity and emotional stress after awakening may contribute to the increased likelihood of plaque rupture between 06:00 and 11:59 hours. Progressive blood pressure elevation from the onset of sleep to early morning or persistent blood pressure elevation throughout the day noted in hypertensive OSA patients28 may also be related to increase in incidence of plaque rupture in the morning. Platelet activation, plasma fibrinogen level, and plasma viscosity were higher in patients with OSA than in control subjects.29–31 Thus, coronary thrombosis may occur more rapidly in patients with OSA in the event of plaque rupture. It is conceivable that moderate-to-severe OSA patients show a more characteristic circadian variation of MI onset compared with mild OSA patients as a result of various enhanced cardiovascular effects from OSA. Continuous positive airway pressure (CPAP) treatment significantly decreased cardiovascular or cardiac death in OSA patients without cardiac disease and those undergoing PCI, by reducing disease severity.6,32 It is an interesting question as to whether CPAP treatment attenuates the morning peak of onset in OSA patients.
Two studies, which included both central and OSA patients, found that those with sleep apnoea had an increased risk of MI between 06:00 and 12:00 hours,9,10 and these findings were corroborated by our study. In a study reported by Aboyans et al.,10 no patient with sleep apnoea had MI between 0:00 and 5:59 hours. Only Kuniyoshi et al.8 examined the differences in circadian variation of MI onset between OSA and control patients. They found that OSA patients were at an increased risk of MI between 00:00 and 06:00 hours compared with non-OSA patients, in contradiction to our findings. Patients in that study were prospectively collected and the incidence of OSA was defined as AHI ≥5 events/h, which is comparable to our study methods. The possible reasons for this discrepancy may be posited. First, our study patient group (n=288) was approximately 3-times higher than that observed in their study (n=92); a smaller sample size may underestimate or overestimate the incidence of OSA in individual 6-h intervals. Another feature of our study is that all our patients showed angiographic confirmation of critical stenosis or complete coronary artery occlusion and underwent primary PCI. Second, BMI in our OSA patients was lower than that observed in their study (23.7 vs. 31 kg/m2, respectively). It is known that most Asian patients with OSA are non-obese33 and it is possible that obese OSA patients may show different circadian variations of MI onset from non-obese OSA patients. Racial differences may therefore have contributed to discrepancy in findings between the two studies.
Study limitations
All our study patients were Japanese. The relationship between racial difference and circadian variation of MI onset in OSA patients seems to be an important concern because ethnic groups were different between our study and the study by Kuniyoshi et al.8 The lesser number of control subjects (n=72) than OSA patients in our study could be a limiting factor for reaching a definite conclusion regarding this issue.
The impact of OSA severity on circadian variation of MI onset is another important issue. Because the number of patients with severe OSA was small, we combined moderate OSA patients defined as AHI 15.0–29.9 events/h (n=73) and severe OSA patients defined as AHI ≥30.0 events/h (n=44) into one group. Although the moderate-to-severe OSA patients demonstrated a more characteristic circadian variation compared with the mild OSA patients, it is uncertain whether severe OSA further increases the incidence of the morning peak of onset when compared with moderate OSA. Therefore, a much larger-scale study would be needed to elucidate this important issue.
Conclusion
OSA further increases the peak of MI onset between 06:00 and 11:59 hours. Moderate-to-severe OSA is a significant risk factor for morning MI onset.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest: The authors declare that there are no conflicts of interest.
References
- 1. Morning peak in the incidence of myocardial infarction: experience in the ISIS-2 trial. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group Eur Heart J 1992; 13: 594–8 [PubMed] [Google Scholar]
- 2. Muller JE, Stone PH, Turi ZG, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 1985; 313: 1315–22 [DOI] [PubMed] [Google Scholar]
- 3. Rana JS, Mukamal KJ, Morgan JP, et al. Circadian variation in the onset of myocardial infarction: effect of duration of diabetes. Diabetes 2003; 52: 1464–8 [DOI] [PubMed] [Google Scholar]
- 4. Willich SN, Linderer T, Wegscheider K, et al. Increased morning incidence of myocardial infarction in the ISAM Study: absence with prior beta-adrenergic blockade. ISAM Study Group. Circulation 1989; 80: 853–8 [DOI] [PubMed] [Google Scholar]
- 5. Shah NA, Yaggi HK, Concato J, et al. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath 2010; 14: 131–6 [DOI] [PubMed] [Google Scholar]
- 6. Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365: 1046–53 [DOI] [PubMed] [Google Scholar]
- 7. Luthje L, Andreas S. Obstructive sleep apnea and coronary artery disease. Sleep Med Rev 2008; 12: 19–31 [DOI] [PubMed] [Google Scholar]
- 8. Kuniyoshi FH, Garcia-Touchard A, Gami AS, et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol 2008; 52: 343–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Junker-Neff A, Eberle R TVA, et al. Besteht ein Zusammenhang zwischen dem Schlafapnoesyndrom und der zirkadianen Haufung von Myokardinfarkten in den Morgenstunden? [Is there an association between the sleep apnea syndrome and the circadian peak of myocardial infarction in the morning hours?]. Dtsch Med Wochenschr 2005; 130: 2818–22 [DOI] [PubMed] [Google Scholar]
- 10. Aboyans V, Cassat C, Lacroix P, et al. Is the morning peak of acute myocardial infarction’s onset due to sleep-related breathing disorders? A prospective study. Cardiology 2000; 94: 188–92 [DOI] [PubMed] [Google Scholar]
- 11. Arroyo LH, Lee RT. Mechanisms of plaque rupture: mechanical and biologic interactions. Cardiovasc Res 1999; 41: 369–75 [DOI] [PubMed] [Google Scholar]
- 12. Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation 1995; 92: 657–71 [DOI] [PubMed] [Google Scholar]
- 13. Davies MJ, Thomas A. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. N Engl J Med 1984; 310: 1137–40 [DOI] [PubMed] [Google Scholar]
- 14. Lee CH, Khoo SM, Tai BC, et al. Obstructive sleep apnea in patients admitted for acute myocardial infarction. Prevalence, predictors, and effect on microvascular perfusion. Chest 2009; 135: 1488–95 [DOI] [PubMed] [Google Scholar]
- 15. Nakashima H, Katayama T, Takagi C, et al. Obstructive sleep apnoea inhibits the recovery of left ventricular function in patients with acute myocardial infarction. Eur Heart J 2006; 27: 2317–22 [DOI] [PubMed] [Google Scholar]
- 16. Barcelo A, Barbe F, de la Pena M, et al. Antioxidant status in patients with sleep apnoea and impact of continuous positive airway pressure treatment. Eur Respir J 2006; 27: 756–60 [DOI] [PubMed] [Google Scholar]
- 17. Yamauchi M, Nakano H, Maekawa J, et al. Oxidative stress in obstructive sleep apnea. Chest 2005; 127: 1674–9 [DOI] [PubMed] [Google Scholar]
- 18. Christou K, Markoulis N, Moulas AN, et al. Reactive oxygen metabolites (ROMs) as an index of oxidative stress in obstructive sleep apnea patients. Sleep Breath 2003; 7: 105–10 [DOI] [PubMed] [Google Scholar]
- 19. Jelic S, Padeletti M, Kawut SM, et al. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation 2008; 117: 2270–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 2003; 107: 1129–34 [DOI] [PubMed] [Google Scholar]
- 21. Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation 2002; 105: 2462–4 [DOI] [PubMed] [Google Scholar]
- 22. El Solh AA, Akinnusi ME, Baddoura FH, et al. Endothelial cell apoptosis in obstructive sleep apnea: a link to endothelial dysfunction. Am J Respir Crit Care Med 2007; 175: 1186–91 [DOI] [PubMed] [Google Scholar]
- 23. Strike PC, Perkins-Porras L, Whitehead DL, et al. Triggering of acute coronary syndromes by physical exertion and anger: clinical and sociodemographic characteristics. Heart 2006; 92: 1035–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kloner RA. Natural and unnatural triggers of myocardial infarction. Prog Cardiovasc Dis 2006; 48: 285–300 [DOI] [PubMed] [Google Scholar]
- 25. Kloner RA. Can we trigger an acute coronary syndrome? Heart 2006; 92: 1009–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muller JE, Tofler GH, Verrier RL. Sympathetic activity as the cause of the morning increase in cardiac events. A likely culprit, but the evidence remains circumstantial. Circulation 1995; 91: 2508–9 [DOI] [PubMed] [Google Scholar]
- 27. Somers VK, Dyken ME, Clary MP, et al. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96: 1897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Noda A, Okada T, Hayashi H, et al. 24-hour ambulatory blood pressure variability in obstructive sleep apnea syndrome. Chest 1993; 103: 1343–7 [DOI] [PubMed] [Google Scholar]
- 29. Steiner S, Jax T, Evers S, et al. Altered blood rheology in obstructive sleep apnea as a mediator of cardiovascular risk. Cardiology 2005; 104: 92–6 [DOI] [PubMed] [Google Scholar]
- 30. Hui DS, Ko FW, Fok JP, et al. The effects of nasal continuous positive airway pressure on platelet activation in obstructive sleep apnea syndrome. Chest 2004; 125: 1768–75 [DOI] [PubMed] [Google Scholar]
- 31. Nobili L, Schiavi G, Bozano E, et al. Morning increase of whole blood viscosity in obstructive sleep apnea syndrome. Clin Hemorheol Microcirc 2000; 22: 21–7 [PubMed] [Google Scholar]
- 32. Cassar A, Morgenthaler TI, Lennon RJ, et al. Treatment of obstructive sleep apnea is associated with decreased cardiac death after percutaneous coronary intervention. J Am Coll Cardiol 2007; 50: 1310–14 [DOI] [PubMed] [Google Scholar]
- 33. Li KK, Kushida C, Powell NB, et al. Obstructive sleep apnea syndrome: a comparison between Far-East Asian and white men. Laryngoscope 2000; 110: 1689–93 [DOI] [PubMed] [Google Scholar]


