Abstract
Aims:
It is widely thought that ST-elevation myocardial infarction (STEMI) is more likely to occur without warning (i.e. an unanticipated event in a previously healthy person) than non-ST-elevation myocardial infarction (NSTEMI), but no large study has evaluated this using prospectively collected data. The aim of this study was to compare the evolution of atherosclerotic disease and cardiovascular risk between people going on to experience STEMI and NSTEMI.
Methods:
We identified patients experiencing STEMI and NSTEMI in the national registry of myocardial infarction for England and Wales (Myocardial Ischaemia National Audit Project), for whom linked primary care records were available in the General Practice Research Database (as part of the CALIBER collaboration). We compared the prevalence and timing of atherosclerotic disease and major cardiovascular risk factors including smoking, hypertension, diabetes, and dyslipidaemia, between patients later experiencing STEMI to those experiencing NSTEMI.
Results:
A total of 8174 myocardial infarction patients were included (3780 STEMI, 4394 NSTEMI). Myocardial infarction without heralding by previously diagnosed atherosclerotic disease occurred in 71% STEMI (95% CI 69–72%) and 50% NSTEMI patients (95% CI 48–51%). The proportions of myocardial infarctions with no prior atherosclerotic disease, major risk factors, or chest pain was 14% (95% CI 13–16%) in STEMI and 9% (95% CI 9–10%) in NSTEMI. The rate of heralding coronary diagnoses was particularly high in the 12 months before infarct; 4.1-times higher (95% CI 3.3–5.0) in STEMI and 3.6-times higher (95% CI 3.1–4.2) in NSTEMI compared to the rate in earlier years.
Conclusions:
Acute myocardial infarction occurring without prior diagnosed coronary, cerebrovascular, or peripheral arterial disease was common, especially for STEMI. However, there was a high prevalence of risk factors or symptoms in patients without previously diagnosed disease. Better understanding of the antecedents in the year before myocardial infarction is required.
Keywords: Cardiovascular diseases, epidemiology, myocardial infarction, risk factors
Introduction
The extent to which first acute myocardial infarction (AMI) with or without ST-elevation is heralded by previous symptomatic atherosclerotic disease, major risk factors, or symptoms has important implications for understanding the aetiology of each phenotype, as well as the provision of optimal services. Studies which retrospectively evaluate medical history suggest that prior atherosclerotic disease is common in people with AMI,1–5 and patterns differ according to ST-elevation myocardial infarction (STEMI) and non-ST-elevation myocardial infarction (NSTEMI). Patients with NSTEMI tend to have higher levels of angina,6,7 heart failure symptoms,8 coronary artery bypass graft (CABG) and percutaneous coronary intervention (PCI),6,9,10 and peripheral vascular disease7 compared to patients with STEMI (Supplementary Table 1, available online).
However many of these studies take a single, retrospective snapshot of medical history and have important limitations. They may underestimate the burden of prior disease (i.e. falsely inflating the estimate of unheralded AMI) and may poorly reflect the timing of initial and subsequent manifestations of disease. To our knowledge, no large-scale study to date has evaluated the extent and nature of STEMI and NSTEMI heralding using prospectively collected information on the onset of atherosclerotic disease (in coronary, cerebral, and peripheral circulations) and other risk factors.
Therefore, this paper aims to compare the evolution of atherosclerotic disease and cardiovascular risk between people going on to experience STEMI and NSTEMI. Using prospectively collected longitudinal primary care data linked to detailed hospital data on acute coronary syndromes, we describe the initial manifestation, distribution and timing of different atherosclerotic presentations before first STEMI and NSTEMI, and the proportion of AMIs that occur without any previously diagnosed atherosclerotic disease, cardiovascular risk factors, or chest pain.
Methods
Study design
As part of the CALIBER research programme (Cardiovascular disease research using Linked Bespoke studies and Electronic Records, www.caliberresearch.org),11 the records of patients presenting with STEMI and NSTEMI in the Myocardial Ischaemia National Audit Project (MINAP) were linked to longitudinal electronic health records from primary care from the General Practice Research Database (GPRD).
MINAP
MINAP is the national registry of patients admitted to hospitals in England and Wales with acute coronary syndrome (ACS).12 The MINAP dataset records timing of symptom onset and admission, clinical features and investigations (including ECG results and cardiac biomarkers), past medical history, hospital treatment, and discharge diagnosis.12
GPRD
The GPRD is a primary care database containing anonymized patient records from general practices for approximately 8% of the UK population (5.2 million patients).13 General practitioners (GPs) play a key role in the UK healthcare system as they are responsible for primary health care and specialist referrals. Patients are affiliated to a practice, which centralizes the medical information from the GP (diagnoses, symptoms, prescriptions, treatments, and health behaviours), specialist referrals, and hospitalizations, so that GP data provide a comprehensive longitudinal health record. Around 40% of the general practices in GPRD permit linkage of individual patient records with other data sources.14 Data from these practices, all in England, are used in the current study.
Linkage
Linkage of MINAP with GPRD permits researchers to establish a longitudinal patient journey before and after ACS, while providing greater clinical detail on ACS events than is reliably available within GPRD. All linked patients had general practice data and were a representative 4% sample of AMI cases from MINAP. The pseudoanonymized dataset was created using a Trusted Third Party to perform the linkage, based on patient NHS number, date of birth, gender, and postcode.15
Definition of acute myocardial infarction
STEMI or NSTEMI was defined by details recorded in MINAP, following the joint American Heart Association/European Society of Cardiology definition.16 In order to confine the analysis to first AMI, we excluded patients with a history of AMI noted in their MINAP record, or with evidence of AMI in their GPRD record prior to the first AMI recorded in MINAP. We included patients fulfilling the following criteria: at least 18 years of age at AMI, first AMI occurring between 1 January 2003 and 31 December 2008; registered with the GPRD practice at the time of AMI, with at least one year of observation before AMI and at least one consultation during pre-AMI follow up to allow prevalent diagnoses to be recorded once a patient joins a practice, as patients can register with a practice and not attend for many months or years (Supplementary Figure 1).
Identifying atherosclerotic cardiovascular disease and risk factors in the linked data
MINAP and GPRD data were used to identify AMI, other atherosclerotic disease and cardiovascular risk factors among study patients. Any disease, risk factor, or chest pain recorded at any time prior to AMI was defined as ‘heralding’ the AMI. Atherosclerotic disease included cardiac disease (stable angina, unstable angina, cardiac arrest, heart failure, coronary heart disease (CHD) not otherwise specified, receipt of PCI and CABG), ischaemic cerebrovascular disease, including stroke, non-stroke cerebrovascular disease, and transient ischaemic attack, and peripheral arterial disease (PAD), including abdominal aortic aneurysm.
Risk factors investigated were smoking (categorized as non, ex, current, or unknown at the time of AMI), hypertension (either diagnosed hypertension or three consecutive raised (>140/90 mmHg) measurements), dyslipidaemia (abnormal lipid measurements or management of high lipids), and diabetes (diagnosed diabetes or insulin prescription) and were defined by codes in the primary care or MINAP hospital record. We also determined whether patients had been prescribed blood pressure-lowering, lipid-lowering, or antiplatelet medications in the 6 months before AMI. Missing data from MINAP variables and absence of any diagnostic codes in the GPRD were taken to indicate absence of the risk factor or morbidity.
Determining onset and duration of diagnosed atherosclerotic disease before AMI
For patients whose AMI was heralded by a diagnosis of atherosclerotic disease, we took the earliest record of any atherosclerotic disease before AMI in the GPRD to be the date of onset (data on timing of prior disease are not recorded in MINAP). Where this code was for a prevalent diagnosis (e.g. ‘history of stroke’) or the morbidity was recorded only in MINAP, the date of onset was recorded as missing. The earliest date of each subtype of atherosclerotic disease (coronary, cerebrovascular, peripheral arterial) was ascertained using the same method. This allowed calculation of the duration of diagnosed disease before AMI and the rate of diagnosis before STEMI and NSTEMI.
Consultation or admission for chest pain in the linked data
In patients without diagnosed atherosclerotic disease, we assessed the frequency of primary care consultations for chest pain.
Statistical analysis
The proportions with diagnosed atherosclerotic disease and risk factors were calculated for STEMI and NSTEMI patients. Since the age and sex profiles of STEMI and NSTEMI patients differed, we included each atherosclerotic disease/risk factor in turn in an age- and sex-adjusted logistic regression model to determine whether the odds of prior disease/risk factor differed between STEMI and NSTEMI patients, after accounting for age and sex differences. We used the models to assess interaction between age and sex. We also calculated the age- and sex-standardized prevalences of each atherosclerotic disease subtype and risk factor for STEMI and NSTEMI patients, using the age and sex distribution of the study population as the standard.
To investigate the timing of disease prior to AMI, we calculated rates of new coronary, cerebrovascular, and peripheral arterial disease in 1-year time bands in the period before AMI, and rates of new coronary diagnoses and chest pain consultations in 1-month time bands in the period before AMI. We used Poisson regression to calculate rate ratios and 95% confidence intervals, comparing the rate of coronary diagnosis in the year before AMI to the rate in the previous 9 years, and also to test for linear trend in the rate of diagnosis in the years leading to AMI. All analyses were performed in STATA. The study details are registered online at clinicaltrials.gov (NCT01379131) and a time-stamped detailed analytic protocol is available on request. CALIBER has received ethics approval (ref. 09/H0810/16) for creation of linked pseudoanonymized data encompassing GPRD and MINAP.
Results
We identified 8174 first AMI patients who met the eligibility criteria. Their median age was 71 years (IQR 59–80), 2946 (36%) were women, and 3780 (46%) had STEMI. The median duration of follow up before AMI was 8.7 years (overall 77,228 person-years of follow up). Table 1 shows the demographic and hospital admission characteristics of patients by AMI type.
Table 1.
Demographics and hospital admission characteristics of STEMI and NSTEMI patients at the time of hospital admission.
| Characteristic | STEMI (n=3780) | NSTEMI (n=4394) |
|---|---|---|
| Age (years) | 67.0 (57.0–77.0) | 74.0 (63.0–82.0) |
| Female | 1172 (31.0) | 1774 (40.4) |
| Ethnicity | ||
| White | 3146 (83.2) | 3739 (85.1) |
| South Asian | 13 (0.3) | 18 (0.4) |
| Other | 59 (1.6) | 63 (1.4) |
| Unknown | 562 (14.9) | 574 (13.1) |
| ECG at admission | ||
| ST-segment elevation | 3552 (94.0) | 0 (0) |
| Left bundle branch block | 87 (2.3) | 246 (5.6) |
| ST-segment depression | 0 (0.0) | 1144 (26) |
| T-wave changes only | 0 (0.0) | 1024 (23.3) |
| Other abnormality | 0 (0.0) | 836 (19) |
| Normal ECG | 0 (0.0) | 473 (10.8) |
| Unknown | 141 (3.7) | 671 (15.3) |
| Peak troponin at admission (µg/l)a | 5.2 (1.2–25.0) | 1.0 (0.3–3.9) |
| Heart rate at admission (bpm)a | 76.0 (63.0–90.0) | 80.0 (68.0–98.0) |
| Systolic BP at admission (mmHg)a | 138.0 (120.0–157.0) | 140.0 (121.0–160.0) |
Values are median (interquartile range) or n (%). As shown in Table 3, previous treatment with cardiovascular medication was different in STEMI and NSTEMI.
Completeness in peak troponin, heart rate, and systolic BP was 85, 77, and 77%, respectively.
BP, blood pressure.
Acute myocardial infarction occurring with and without heralding
As shown in Figure 1, among patients with STEMI, 29% had prior atherosclerotic disease, 56% had no prior atherosclerotic disease diagnosis but at least one cardiovascular risk factor, and 0.6% experienced only chest pain, leaving 14% (95% CI 13–16%) unheralded by these factors. In NSTEMI patients, 50% had previous disease, 40% had no previous disease but at least one cardiovascular risk factor, 0.7% reported only chest pain, and 9% (95% CI 9–10%) experienced AMI unheralded by these factors. Thus NSTEMIs were more often heralded by prior atherosclerotic disease rather than other risk factors only. STEMIs were more likely to be unheralded than NSTEMIs, but the absolute proportions of AMIs unheralded by these factors were low for both types.
Figure 1.
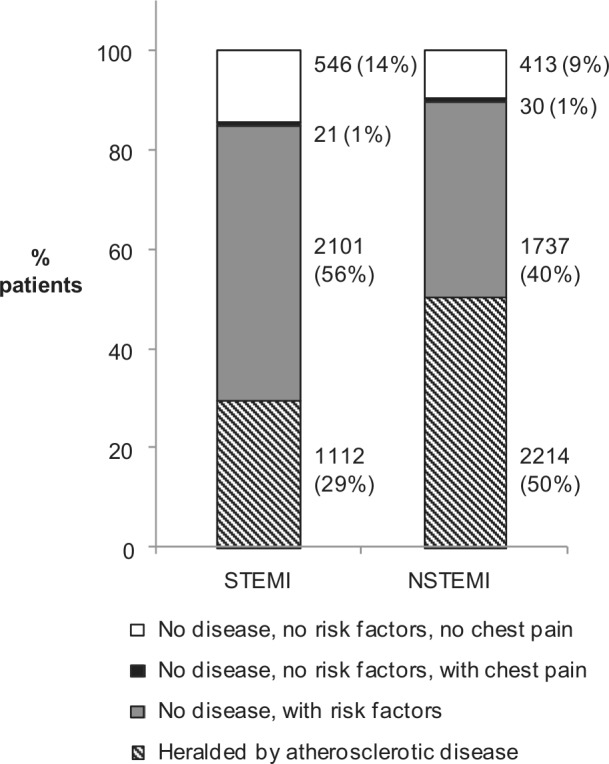
Previous atherosclerotic disease and risk factors in patients with first ST-elevation myocardial infarction (STEMI, n=3780) and non-ST-elevation myocardial infarction (NSTEMI, n=4394).
Diagnosed atherosclerotic disease before first AMI
As shown in Table 2, 3326 (41%) of patients had previously diagnosed atherosclerotic disease. Patients with NSTEMI experienced more disease (STEMI 29%, NSTEMI 50%, age- and sex-standardized values 32% and 47%, respectively, p<0.001) and this pattern was consistent across age groups, for men and women and for different atherosclerotic disease manifestations, even after standardizing for age and sex. There was no age–sex interaction. Coronary disease was the most common presentation before AMI, diagnosed in 21% of STEMI patients and 41% of NSTEMI; most of these patients had stable angina (16% in STEMI and 33% in NSTEMI). Although most patients with previous disease had a coronary diagnosis, 9% of STEMIs and 10% of NSTEMIs were heralded only by PAD and/or atherosclerotic cerebrovascular disease.
Table 2.
Prevalence (unstandardized and age- and sex-standardized) and duration of diagnosed atherosclerotic disease in patients with first STEMI and NSTEMI, recorded over a median 8.7 years follow up before myocardial infarction, including patients with atherosclerotic disease at more than one site.
| STEMI (N=3780) |
NSTEMI (N=4394) |
p-value | |||||
|---|---|---|---|---|---|---|---|
| n (%) | Standardized prevalence (95% CI) | Median disease duration (IQR) | n (%) | Standardized prevalence (95% CI) | Median disease duration (IQR) | ||
| Any atherosclerotic disease | 1112 (29.4) | 32.0 (30.5–33.5) | 6.2 (2.2–11.7) | 2214 (50.4) | 47.2 (45.8–48.5) | 7.6 (3.2–13.4) | <0.001 |
| Coronary disease | 788 (20.8) | 22.7 (21.3–24) | 4.5 (1–8.9) | 1795 (40.9) | 38.2 (36.8–39.5) | 4.2 (1.1–9.3) | <0.001 |
| Stable angina | 587 (15.5) | 16.9 (15.7–18.2) | 6.3 (1.4–11.4) | 1442 (32.8) | 30.8 (29.5–32.1) | 7.2 (2.5–13.2) | <0.001 |
| Unstable angina | 46 (1.2) | 1.4 (1.0–1.9) | 4.6 (1.8–7.9) | 172 (3.9) | 3.8 (3.2–4.3) | 2.7 (0.3–6.9) | <0.001 |
| PCI or CABG | 99 (2.6) | 2.6 (2.1–3.1) | 6.5 (1.5–10.7) | 281 (6.4) | 6.4 (5.7–7.2) | 7.4 (2.0–13.1) | <0.001 |
| CHD not otherwise specified | 404 (10.7) | 11.7 (10.6–12.7) | 7.3 (2.8–12.2) | 969 (22.1) | 20.5 (19.4–21.7) | 8.1 (3.5–13.7) | <0.001 |
| Heart failure | 142 (3.8) | 4.6 (3.9–5.4) | 4.5 (1.5–9.5) | 498 (11.3) | 9.9 (9.1–10.7) | 4.1 (1.2–7.9) | <0.001 |
| Cardiac arrest | 3 (0.1) | 0.1 (0–0.1) | 0.1 (0–8.3) | 7 (0.2) | 0.2 (0–0.3) | 2.3 (0.4–18.8) | 0.277 |
| Other atherosclerotic disease | 537 (13.9) | 15.6 (14.4–16.8) | 4.8 (1.8–9.3) | 1036 (23.6) | 21.7 (20.5–22.8) | 5.6 (2.6–9.7) | <0.001 |
| Cerebrovascular disease | 276 (7.3) | 9.5 (8.5–10.5) | 5.3 (2.2–11.3) | 554 (12.6) | 12.6 (11.7–13.5) | 6.1 (2.8–10.9) | <0.001 |
| Peripheral arterial disease | 261 (6.9) | 7.7 (6.8–8.6) | 4.4 (1.7–8.4) | 565 (12.9) | 12.0 (11.1–13.0) | 6.1 (2.9–10.5) | <0.001 |
| Unknown initial presentationa | 6 (0.2) | 0.2 (0–0.3) | 15.8 (12.7–17.6) | 23 (0.5) | 0.5 (0.3–0.8) | 4.4 (2.1–8.1) | 0.009 |
p-values for the association between MI subtype and each presentation (adjusted for age and sex).
CABG, coronary artery bypass graft; CHD, coronary heart disease; PCI, percutaneous coronary intervention.
Where the only code indicating atherosclerotic disease was unspecific.
As shown in Figure 2, 30% of patients were diagnosed with disease in only one arterial bed, 9% in two, and 2% in three. The extent of disease differed by AMI type (age- and sex-adjusted logistic regression p<0.001); overall, 15% of patients with NSTEMI had disease at more than one site, compared to 6% in STEMI.
Figure 2.
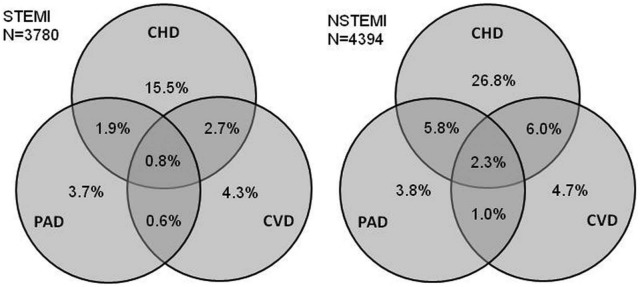
Proportions of patients with ST-elevation myocardial infarction (STEMI, n=3780) and non-ST-elevation myocardial infarction (NSTEMI, n=4394) with different combinations of disease in one, two, or three arterial beds; 71% of STEMI patients and 50% of NSTEMI patients were unheralded by atherosclerotic disease at any site. CHD, coronary heart disease; CVD, cerebrovascular disease; PAD, peripheral arterial disease.
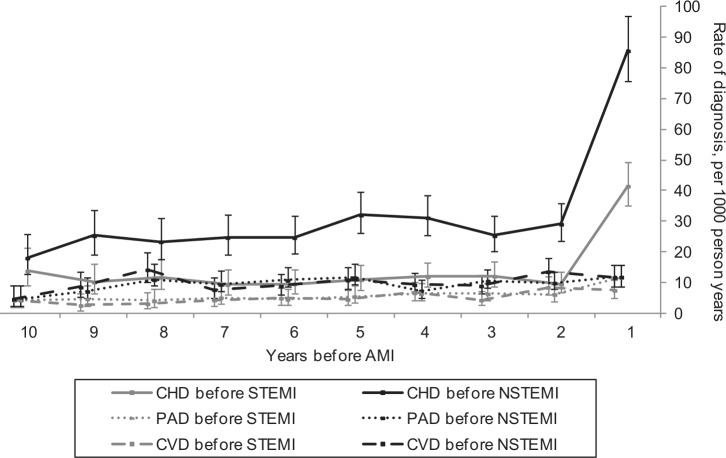
Of the 3326 patients with atherosclerotic disease diagnoses, we were able to estimate a date of disease onset for 2891 (87%; 84% STEMI, 89% NSTEMI). Throughout the 10 years preceding infarction, the rates of diagnosis of coronary, cerebrovascular, and peripheral disease were higher in NSTEMI than STEMI (Figure 3). The rates of cerebrovascular disease and PAD remained stable throughout follow up, with an upward trend towards AMI over time (average increase in rate per 1-year time band: 1.06, 95% CI 1.03–1.10; p<0.001). Rates of coronary disease were higher than rates of peripheral or cerebrovascular disease throughout follow up, consistent with the higher prevalence of coronary disease at the time of AMI.
Figure 3.
Rates of coronary heart disease (CHD), peripheral arterial disease (PAD) and cerebrovascular disease (CVD) in the 10 years before diagnosis of ST-elevation myocardial infarction (STEMI) and non-ST-elevation myocardial infarction (NSTEMI), with 95% confidence intervals. Each time point covers a 1-year time band (1=0–1 years before AMI, 2=1–2 years before AMI, etc).
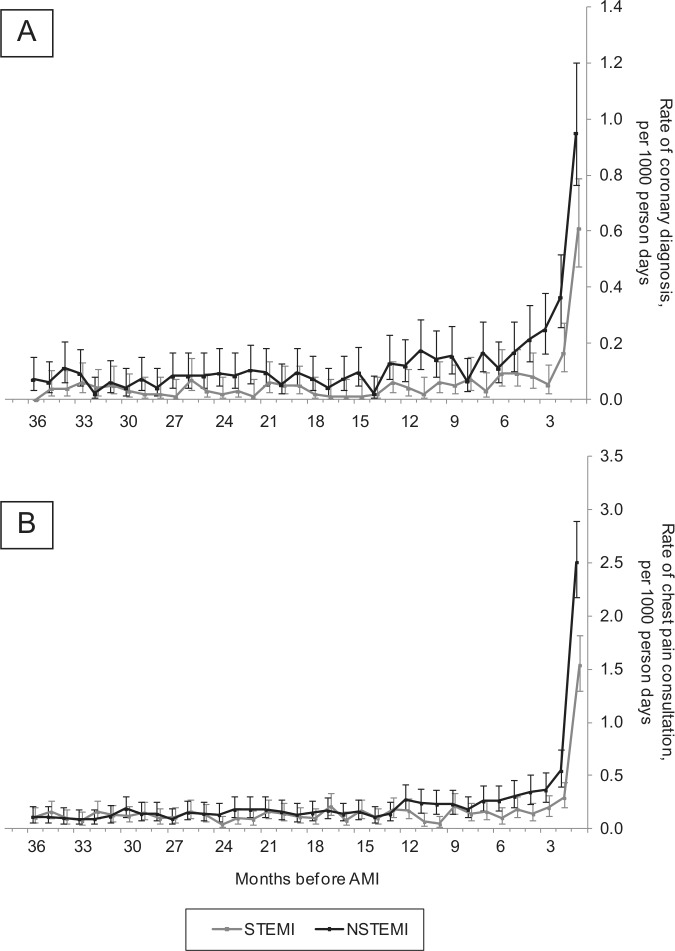
In contrast to the patterns observed in cerebrovascular and peripheral diseases, the rate of coronary disease diagnosis rose rapidly in the year before AMI (Figures 3 and 4). Compared to the rate in the previous 9 years, the rate of coronary diagnosis was 4.1-times higher (95% CI 3.3–5.0) in the year before STEMI and 3.6-times higher (3.1–4.2) in the year before NSTEMI. Figure 4A shows that these increases were largely restricted to the 3 months before infarct, during which 159 (2%) patients were first diagnosed with coronary disease or received a coronary intervention (102 stable angina, 17 unstable angina, 26 CHD of unspecified type, 14 PCI/CABG). A similar pattern was observed in the rate of chest pain consultations in patients without diagnosed atherosclerotic disease (Figure 4B).
Figure 4.
Rates of coronary diagnosis (A) and chest pain consultations (B) in the months leading to ST-elevation myocardial infarction (STEMI) and non-ST-elevation myocardial infarction (NSTEMI), with 95% confidence intervals. Consultations for chest pain are only in those without diagnosed atherosclerotic disease. Each time point covers a 1-month time band (0–1 months, 1–2 months, etc). NB: Figure 4B describes chest pain consultations in only patients without previously diagnosed atherosclerotic disease, therefore describing that although these patients have not received a coronary disease diagnosis, they may well be heralded by possible coronary symptoms.
Among patients with prior atherosclerotic disease, the median duration between first diagnosis and STEMI was 6.2 years (IQR 2.2–11.7) and in NSTEMI 7.6 years (3.2–13.4) (Table 2). The median duration of all atherosclerotic diseases combined was longer in NSTEMI at all age groups and for men and women (Supplementary Table 2). Importantly, the duration of diagnosed disease tended to be long: 26% of atherosclerotic disease heralding in STEMI and 35% in NSTEMI was 10 or more years’ duration (48% and 57% 5 or more years, respectively).
Use of cardiovascular medications in patients with atherosclerotic disease
Of those with previously diagnosed atherosclerotic disease, 87% were being prescribed one or more of aspirin, statins, and blood-pressure-lowering treatment in the 6 months before AMI, but only 34% were receiving all three.
Cardiovascular risk factors and medications in patients without previous atherosclerotic disease
Fifty-nine per cent of AMIs were unheralded by previously diagnosed atherosclerotic disease (71% STEMI, 95% CI 69–72%; 50% NSTEMI, 95% CI 48–51%). Overall, 79% of these patients had at least one elevated or treated risk factor (ever had a record of diabetes, hypertension, dyslipidaemia, current smoking, or a prescription for statins, blood pressure-lowering, or antiplatelets in the 6 months before AMI). This was the same in STEMI (79%) and NSTEMI (80%) (Table 3). The most common risk factors were diagnosed hypertension or recent use of blood pressure-lowering drugs (42% of STEMI patients and 53% of NSTEMI) and current smoking (39.8% STEMI, 28.3% NSTEMI); one or both of these risk factors was present in 70% of STEMI and 80% of NSTEMI patients. STEMI patients tended to have a slightly lower burden of cardiovascular risk factors than NSTEMI (median two risk factors in STEMI patients, three in NSTEMI).
Table 3.
Prospectively collected evaluation of prevalence (standardized and unstandardized) of cardiovascular risk factors and cardiovascular medications in STEMI (n=2268) and NSTEMI (n=2180) patients without previously diagnosed atherosclerotic disease.
| STEMI (n=2668) |
NSTEMI (n=2180) |
p-value | |||
|---|---|---|---|---|---|
| n (%) | Age- and sex-standardized prevalence (95% CI) | n (%) | Age- and sex-standardized prevalence (95% CI) | ||
| Smoking | |||||
| Non | 339 (12.7) | 13.4 (12.1–14.6) | 338 (15.5) | 15.0 (13.5–16.5) | <0.001 |
| Former | 1261 (47.3) | 48.8 (47–50.5) | 1219 (55.9) | 54.0 (52–56) | |
| Current | 1063 (39.8) | 37.4 (35.7–39) | 616 (28.3) | 30.5 (28.6–32.3) | |
| Unknown | 5 (0.2) | 0.2 (0–0.4) | 7 (0.3) | 0.3 (0.1–0.5) | |
| Hypertension | 1083 (40.6) | 41.9 (40.1–43.7) | 1082 (49.6) | 47.7 (45.7–49.7) | <0.001 |
| Dyslipidaemia | 569 (21.3) | 21.4 (19.9–22.9) | 459 (21.1) | 21.4 (19.7–23.1) | 0.913 |
| Diabetes | 276 (10.3) | 10.6 (9.4–11.7) | 302 (13.9) | 13.4 (12–14.7) | 0.002 |
| Blood pressure-loweringa | 806 (30.2) | 31.8 (30.1–33.5) | 880 (40.4) | 38.3 (36.4–40.2) | <0.001 |
| Statinsa | 430 (16.1) | 16.3 (14.9–17.6) | 404 (18.5) | 18.4 (16.8–20) | 0.043 |
| Antiplateletsa | 420 (15.7) | 16.3 (14.9–17.7) | 509 (23.3) | 22.9 (21.1–24.6) | <0.001 |
| Chest pain consultationb in 90 days before MI | 122 (4.6) | 4.7 (3.9–5.4) | 158 (7.2) | 7.4 (6.3–8.5) | <0.001 |
| Without any of these risk factors or cardiovascular medications (% of unheralded MI)a | 567 (21.3) | 21.4 (19.8–22.9) | 443 (20.3) | 20.3 (18.6–22) | 0.318 |
| Without any of these risk factors or cardiovascular medications (% of all MI)a | 567 (15) | 14.7 (13.6–15.8) | 443 (10.1) | 10.8 (9.8–11.7) | <0.001 |
p-values for the association of risk factor with MI subtype (adjusted for age and sex).
Prescribed in the 6 months before MI.
Excluding consultations recorded for administrative and prescription purposes only.
Patients without heralding by previous atherosclerotic disease, risk factors, medications, or chest pain
In STEMI patients, 546 (14%) were unheralded by all of the factors discussed and in NSTEMI patients, 413 (9%). These patients were more likely to be younger and men than those who were heralded (heralded median age 71 years (IQR 60–80), 63% men; unheralded median age 67 years (IQR 58–77), 72% men). They were also likely to have a lower rate of consultation with the GP in the period leading to AMI (median 7 consultations per year in patients heralded by anything, compared to 4 per year in those unheralded). The proportions of patients by age group are shown in Supplementary Figure 2.
We conducted a sensitivity analysis including family history of cardiovascular disease and obesity as cardiovascular risk factors. This reduced the proportion of STEMIs unheralded by disease, risk factors, or chest pain from 14% to 9% and NSTEMIs from 9% to 7%.
Discussion
We found that heart attack without previous clinically ascertained and recorded atherosclerotic disease was common, but due to the high prevalence of elevated cardiovascular disease risk factors, it was rare for heart attack to occur without warning by disease, risk factors, or chest pain. In the first large-scale evaluation of coronary, cerebral, and peripheral atherosclerotic disease manifestations, risk factors, and symptoms prior to STEMI and NSTEMI using prospectively collected data, we found large differences in the pattern of diagnosed atherosclerotic disease by AMI type in the period leading up to AMI. While the proportion of AMI that occurs without disease, cardiovascular risk factors, medications, or chest pain (i.e. ‘out of the blue’) was slightly higher in STEMI, an important proportion of unheralded AMIs are NSTEMI (14% vs. 9%, respectively). We also found that there was a premonitory period for both AMI types during which the rates of both coronary disease diagnosis and chest pain consultation were raised, but there was no equivalent increase in the rate of peripheral artery or cerebrovascular disease diagnoses.
Previous atherosclerotic disease and risk factors
Uniquely, our study provided prospective data on the rate of onset of different subtypes of atherosclerotic disease in the years leading to AMI. Patients with NSTEMI had a consistently higher rate of coronary, cerebrovascular and peripheral disease diagnosis throughout follow up compared to STEMI. This is in line with other studies showing that patients with NSTEMI are more likely to have prior atherosclerotic disease than STEMI patients (Supplementary Table 1).6–10,17,18
Our results describing the extent of disease across vascular territories are also similar to published findings for NSTEMI,5 and we have shown that in STEMI patients, disease in two or more sites is less common. This is consistent with the idea that NSTEMI patients tend to be a sicker group overall. The widely different pattern in the prevalence and rate of onset of atherosclerotic disease between AMI types lends support to the hypothesis that STEMI and NSTEMI are two different pathophysiological entities (NSTEMI is more often caused by a non-occlusive thrombus and STEMI is more often caused by a complete occlusion19).
If AMI occurs without prior symptomatic atherosclerotic disease, to what extent can it be considered to occur ‘out of the blue’? Although a substantial proportion of infarcts were unheralded by diagnosed atherosclerotic disease, the majority of these had at least one cardiovascular risk factor (smoking, hypertension, dyslipidaemia, diabetes) or were being treated with a cardiovascular medication. Our sensitivity analysis indicated that inclusion of a family history of cardiovascular disease and obesity as risk factors increased this majority. The relatively high prescription of antiplatelets before both STEMI and NSTEMI indicates that GPs suspected a high risk of atherosclerotic disease in many of these patients. Our findings are consistent with other prospective studies showing high risk factor burdens in AMI patients overall (Supplementary Table 3).20,21 To our knowledge, there are no other estimates for the proportion of STEMI and NSTEMI occurring without heralding. We have shown that unheralded AMI is uncommon, occurring in roughly one in 10 patients in our study, and more often in younger men. The true prevalence of unheralded AMI is likely to be lower than our data suggest because our data were from general practice where risk factors are recorded opportunistically during patient consultations.
Premonitory period
Clinical experience and retrospective studies have long suggested that AMI might be preceded by premonitory symptoms of chest pain presenting to a family physician or ambulatory care.22,23 Our study extends knowledge in several respects. First, we confirmed this association with prospective data. Second, we found that there were increases in coronary disease diagnoses and chest pain consultations in both STEMI and NSTEMI. This is in contrast to the widely held view that STEMI is usually of sudden onset. Third, we showed that the increases were specific to coronary diagnoses and chest pain, rather than disease in cerebral or peripheral circulations, suggesting a local rather than systemic pro-thrombotic state.
Clinical implications and missed opportunities for care?
In patients with previously diagnosed atherosclerotic disease or risk factors, AMI represents the unmet potential of secondary or primary prevention, respectively. Despite a clear premonitory period where many patients were diagnosed with coronary disease shortly before AMI, the majority of disease was diagnosed long in advance of both AMI types. Therefore, there is an extended period during which secondary prevention could be implemented. Our data describing the use of secondary prevention measures in the 6 months before AMI showed that most patients with diagnosed atherosclerotic disease were receiving one of either statins, aspirin, or blood pressure-lowering drugs, but only a third were in receipt of all three, indicating that there are likely to be missed opportunities for secondary prevention in this group.
Interestingly, while coronary disease was the most common pre-AMI presentation, 10% of both STEMIs and NSTEMIs were heralded by peripheral artery disease and/or cerebrovascular disease alone. This emphasizes the importance of further efforts to improve secondary prevention following diagnoses in the cerebral and peripheral arteries in order to prevent an important proportion of AMI. The high prevalence of risk factors in both STEMI and NSTEMI suggests the importance of tackling the widely reported missed opportunities for implementation of existing interventions known to be effective.24–27 Additionally, the categorization of continuous measures in this analysis may have been an over-simplification of cardiovascular risk. Although a binary indicator is simple to interpret in studies and a useful basis on which to prescribe treatment, it does not reflect the continuum of risk over the full range of measurements. A more detailed investigation of these risk factors might reveal borderline raised risk in many patients and lowering blood pressure and lipids in those not diagnosed as hypertensive or dyslipidaemic may also prevent AMI. However, the implications of our analysis are limited by a lack of comparison to AMI-free controls. Such a comparison may allow further conclusions to be drawn from these data.
Strengths
The main strength of this study is the quality of data from the linked MINAP and GPRD records. MINAP collects data from all hospitals in England and undergoes annual assessments to ensure the data are of research quality.28 ECG and cardiac marker results are recorded and our STEMI and NSTEMI case definitions were based on the international definition of AMI.16 The recording of admission date in MINAP allowed us to interpret the timing of previous atherosclerotic disease diagnoses in relation to AMI. The GPRD is representative of the UK population13 and roughly half of GPRD practices consented to linkage with MINAP; patients in practices that participated in the linkage were representative of the GPRD as a whole.14
The primary care GPRD data are collected prospectively as part of usual clinical care and therefore are not subject to recall bias or differential error related to outcome. Data regarding new diagnoses and treatment of disease were available for a median of 8.7 years before AMI, allowing sufficient time for incident diagnoses to arise and be recorded. The GPRD closely monitors data quality and the recording of a wide range of atherosclerotic disease outcomes have undergone validation in GPRD studies, which, for example, have compared the electronic data to paper-based medical records or compared the rate of a condition in the GPRD to an external source. These have shown most diagnoses to be of high quality.29–32
For the recording of cardiovascular disease, concordance between GPRD and MINAP was over 90%, and for risk factors and medications was over 80% (assuming missingness in MINAP was concordant with a complete GPRD record); these values represent further evidence of data quality.
Our analysis was based on patients with ‘definite’ atherosclerotic disease diagnoses, using diagnostic codes which had been rated by two clinicians as being indicative of disease. If ‘possible’ diagnoses were included, the proportion with previous disease rose from 41% to 44%; this small change indicates that our ‘definite’ atherosclerotic disease definition had high sensitivity.
Weaknesses
This study included only patients hospitalized with their first AMI recorded in MINAP data and therefore our results cannot be generalized to patients who die outside hospital or those with recurrent AMI, in whom the prevalence of heralding factors is likely to differ. Data were not available in this study to describe the numbers of patients who had out of hospital fatal AMI, but data from a further CALIBER study33 suggest that MINAP captures 30% of patients with AMI recorded as their cause of death.
Because our analyses of heralding are based largely on general practice data, symptomatic atherosclerotic disease may be undiagnosed if patients do not consult their GP. We excluded only fourteen patients without any consultations as these patients never had an opportunity for measurement of risk factors or morbidity. However, introducing a minimum consultation rate could introduce a bias towards sicker patients. The data available for this analysis did not allow us to differentiate between patients with a low consultation rate because of good health and those that did not consult despite symptomatic disease. Excluding patients with less than one year of follow up prior to AMI may also have introduced a selection bias if patients who tend to move practices more frequently are different to patients who stay in a practice for longer periods. However, shortening this time period would likely lead to misclassification of disease and an absence of cardiovascular risk factor records as the GP would not have sufficient time to record these.
Implications for research
A small but important proportion of STEMI and NSTEMI do appear to occur with no recognized heralding signs and further research is warranted to better characterize these phenotypes, their causes and their prognosis. For patients with different forms of heralding the challenge remains to better characterize short term risk of coronary events in order to identify for which patients this represents a (potentially remediable) premonitory period.
A research priority would be an analysis comparing AMI patients to a control group without AMI, including a comparison of missed opportunities for care in measuring and controlling elevated risk.
Patients with NSTEMI were more likely to have been in receipt of cardiovascular medications but the causal relationship between medications and severity of AMI is unclear. CALIBER data present a new opportunity to study the effects of medications in a population for whom detailed information regarding risk factors and prescription of medications are collected.
Conclusion
The majority of STEMIs and NSTEMI were heralded by prior disease or at least one other risk factor, suggesting that opportunities for prevention may be being missed.
Acknowledgments
AT acknowledges the support of Barts and the London Cardiovascular Biomedical Research Unit, which is funded by the National Institute for Health Research.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
Funding: This work was supported by the UK National Institute for Health Research (grant number RP-PG-0407-10314) and the Wellcome Trust (086091/Z/08/Z). EH is supported by a Medical Research Council studentship. LS is supported by a senior clinical fellowship from the Wellcome Trust. KB is supported by a post-doctoral fellowship from the National Institute for Health Research. JG is supported by a doctoral fellowship from the National Institute for Health Research (DRF-2009-02-50). The work of SD, AT, HH, and LS is supported by CHAPTER (Centre for Health service and Academic Partnership in Translational E-Health Research), part of Health eResearch Centre Network (HeRC-UK), funded by the Medical Research Council, in partnership with Arthritis Research UK, the British Heart Foundation, Cancer Research UK, the Economic and Social Research Council, the Engineering and Physical Sciences Research Council, the National Institute of Health Research, the National Institute for Social Care and Health Research (Welsh Assembly Government), the Chief Scientist Office (Scottish Government Health Directorates), and the Wellcome Trust.
Department of Health disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR PHR Programme or the Department of Health.
References
- 1. Yawn BP, Wollan PC, Jacobsen SJ, et al. Identification of women’s coronary heart disease and risk factors prior to first myocardial infarction. J Women’s Health 2004; 13: 1087–1100 [DOI] [PubMed] [Google Scholar]
- 2. Pierard LA, Dubois C, Smeets JP, et al. Prognostic significance of angina pectoris before first acute myocardial infarction. Am J Cardiol 1988; 61: 984–987 [DOI] [PubMed] [Google Scholar]
- 3. Cupples LA, Gagnon DR, Wong ND, et al. Preexisting cardiovascular conditions and long-term prognosis after initial myocardial infarction – the Framingham Study. Am Heart J 1993; 125: 863–872 [DOI] [PubMed] [Google Scholar]
- 4. Kobayashi Y, Miyazaki S, Itoh A, et al. Previous angina reduces in-hospital death in patients with acute myocardial infarction. Am J Cardiol 1998; 81: 117–122 [DOI] [PubMed] [Google Scholar]
- 5. Bhatt DL, Peterson ED, Harrington RA, et al. Prior polyvascular disease: risk factor for adverse ischaemic outcomes in acute coronary syndromes. Eur Heart J 2009; 30: 1195–1202 [DOI] [PubMed] [Google Scholar]
- 6. Terkelsen CJ, Lassen JF, Norgaard BL, et al. Mortality rates in patients with ST-elevation vs. non-ST-elevation acute myocardial infarction: observations from an unselected cohort. Eur Heart J 2005; 26: 18–26 [DOI] [PubMed] [Google Scholar]
- 7. Abbott JD, Ahmed HN, Vlachos HA, et al. Comparison of outcome in patients with ST-elevation versus non-ST-elevation acute myocardial infarction treated with percutaneous coronary intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol 2007; 100: 190–195 [DOI] [PubMed] [Google Scholar]
- 8. Dziewierz A, Siudak Z, Dykla D, et al. Management and mortality in patients with non-ST-segmentelevation vs. ST-segment elevation myocardial infarction. Data from the Malopolska Registry of Acute Coronary Syndromes. Kardiol Pol 2009; 67: 115–120; discussion 21–22 [PubMed] [Google Scholar]
- 9. Bin Song Y, Hahn JY, Kim JH, et al. Comparison of angiographic and other findings and mortality in non ST-segment elevation versus ST-segment elevation myocardial infarction in patients undergoing early invasive intervention. Am J Cardiol 2010; 106: 1397–1403 [DOI] [PubMed] [Google Scholar]
- 10. Abbas AE, Boura JA, Brewington SD, et al. Acute angiographic analysis of non-ST-segment elevation acute myocardial infarction. Am J Cardiol 2004; 94: 907–909 [DOI] [PubMed] [Google Scholar]
- 11. Denaxas S, George J, Herrett E, et al. Data resource profile: Cardiovascular disease research using Linked Bespoke studies and Electronic health Records (CALIBER). Int J Epidemiol 2012; 41: 1625–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herrett E, Smeeth L, Walker L, et al. The Myocardial Ischaemia National Audit Project (MINAP). Heart 2010; 96: 1264–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. General Practice Research Database General Practice Research Database (GPRD). Available at: www.gprd.com (consulted October 2011).
- 14. Gallagher AM, Puri S, Van Staa T. Linkage of the General Practice Research Database (GPRD) with other data sources. Pharmacoepidemiol Drug Saf 2011; 20: S1–S36421837638 [Google Scholar]
- 15. CALIBER Group CArdiovascular disease research using Linked Bespoke studies and Electronic Records (CALIBER) Available at: www.caliberresearch.org (consulted October 2010). [DOI] [PMC free article] [PubMed]
- 16. Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation 2007; 116: 2634–2653 [DOI] [PubMed] [Google Scholar]
- 17. Simpson CR, Buckley BS, McLernon DJ, et al. Five-year prognosis in an incident cohort of people presenting with acute myocardial infarction. PLoS ONE 2011; 6: e26573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steg PG, Goldberg RJ, Gore JM, et al. Baseline characteristics, management practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE). Am J Cardiol 2002; 90: 358–363 [DOI] [PubMed] [Google Scholar]
- 19. Rott D, Leibowitz D. STEMI and NSTEMI are two distinct pathophysiological entities. Eur Heart J 2007; 28: 2685. [DOI] [PubMed] [Google Scholar]
- 20. Greenland P, Knoll MD, Stamler J, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA 2003; 290: 891–897 [DOI] [PubMed] [Google Scholar]
- 21. Mensah GA, Brown DW, Croft JB, et al. Major coronary risk factors and death from coronary heart disease – baseline and follow-up mortality health and nutrition examination data from the second national survey (NHANES II). Am J Prev Med 2005; 29: 68–74 [DOI] [PubMed] [Google Scholar]
- 22. Harper RW, Kennedy G, Desanctis RW, et al. Incidence and pattern of angina prior to acute myocardial infarction – study of 577 cases. Am Heart J 1979; 97: 178–183 [DOI] [PubMed] [Google Scholar]
- 23. Stowers M, Short D. Warning symptoms before major myocardial infarction. BMJ 1970; 32: 833–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Primatesta P, Poulter NR. Lipid concentrations and the use of lipid lowering drugs: evidence from a national cross sectional survey. BMJ 2000; 321: 1322–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Primatesta P, Poulter NR. Levels of dyslipidaemia and improvement in its management in England: results from the Health Survey for England 2003. Clin Endocrinol (Oxf) 2006; 64: 292–298 [DOI] [PubMed] [Google Scholar]
- 26. Foss FA, Dickinson E, Hills M, et al. Missed opportunities for the prevention of cardiovascular disease among British hypertensives in primary care. Br J Gen Practice 1996; 46: 571–575 [PMC free article] [PubMed] [Google Scholar]
- 27. Teeling M, Bennett K, Feely J. The influence of guidelines on the use of statins: analysis of prescribing trends. Br J Clin Pharmacol 1998; 59: 227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Royal College of Physicians MINAP data quality. Available at: www.ucl.ac.uk/nicor/audits/minap/datacollection (consulted February 2012).
- 29. Herrett E, Thomas SL, Schoonen WM, et al. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010; 69: 4–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maru S, Koch GG, Stender M, et al. Antidiabetic drugs and heart failure risk in patients with type 2 diabetes in the U.K. primary care setting. Diabetes Care 2005; 28: 20–26 [DOI] [PubMed] [Google Scholar]
- 31. Hammad TA, McAdams MA, Feight A, et al. Determining the predictive value of Read/OXMIS codes to identify incident acute myocardial infarction in the General Practice Research Database. Pharmacoepidemiol Drug Saf 2008; 17: 1197–1201 [DOI] [PubMed] [Google Scholar]
- 32. Mulnier HE, Seaman HE, Raleigh VS, et al. Risk of stroke in people with type 2 diabetes in the UK: a study using the General Practice Research Database. Diabetologia 2006; 49: 2859–2865 [DOI] [PubMed] [Google Scholar]
- 33. Herrett E, Shah AD, Boggon R, et al. Completeness and diagnostic validity of recording acute myocardial infarction events in primary care, hospital care, disease registry, and national mortality records: cohort study. BMJ 2013; 346: f2350. [DOI] [PMC free article] [PubMed] [Google Scholar]