Abstract
Aims:
Rapid heart rate lowering may be attractive in acute ST-segment elevation myocardial infarction (STEMI). Accordingly we studied the effect of intravenous ivabradine on heart rate in this setting.
Methods and results:
This was a multicenter randomized double-blind placebo-controlled trial: patients aged 40–80 years were randomized after successful primary percutaneous coronary intervention (PCI) performed within 6 h of STEMI symptom onset. Patients were in sinus rhythm and with heart rate >80 bpm and systolic blood pressure >90 mm Hg. They were randomly assigned (2:1 ratio) to intravenous ivabradine (n=82) (5 mg bolus over 30 s, followed by 5 mg infusion over 8 h) or matching placebo (n=42). The primary outcome measure was heart rate and blood pressure. In both groups, heart rate was reduced over 8 h, with a faster and more marked decrease on ivabradine than placebo (22.2±1.3 vs 8.9±1.8 bpm, p<0.0001). After treatment discontinuation, heart rate was similar in both groups. Throughout the study, there was no difference in blood pressure between groups. There was no difference in cardiac biomarkers (creatine kinase (CK-MB), troponin T and troponin I). On echocardiography performed at baseline and post treatment (median 1.16 days), final left ventricular volumes were lower in the ivabradine group both for left ventricular end-diastolic volume (LVEDV) (87.1±28.2 vs 117.8±21.4 ml, p=0.01) and left ventricular end-systolic volume (LVESV) (42.5±19.0 versus 59.1±11.3 ml, p=0.03) without differences in volume change or left ventricular ejection fraction.
Conclusion:
This pilot study shows that intravenous ivabradine may be used safely to slow the heart rate in STEMI. Further studies are needed to characterize its effect on infarct size, left ventricular function and clinical outcomes in this population.
Keywords: Acute Coronary Syndromes (ACS), heart rate, ivabradine, percutaneous coronary intervention, revascularization, ST-segment elevation myocardial infarction
Introduction
Tachycardia is common in the acute stage of ST-elevation myocardial infarction (STEMI), whether related to the sympathetic nervous system activation caused by pain or as a compensatory phenomenon to acute heart failure complicating STEMI. It increases the imbalance between the oxygen supply to the area at risk (which is limited by occlusion of the infarct-related artery) and myocardial oxygen demand, in which it plays a critical role.1 Therefore, use of heart rate lowering agents is, in theory, attractive in this setting2 and, indeed, prior studies have demonstrated a benefit of beta-blockers in acute myocardial infarction (AMI), limiting myocardial infarct size3 and reducing cardiovascular mortality in some,4 but not all,5,6 clinical trials. However, these studies antedate the widespread use of reperfusion therapy, and the more recent large scale Clopidogrel and Metoprolol in Myocardial Infarction (COMMIT) randomized trial did not find an overall reduction in mortality.7 In contrast, it suggested that early use of intravenous (IV) beta-blockers was associated with hazard of an early mortality, particularly in patients with acute heart failure, with an increased risk of cardiogenic shock followed by a subsequent clinical benefit beyond day 1.7 This hazard appears likely to be related to the negative inotropic effect of beta-blockers. For this reason, the current guidelines for the management of STEMI do not support the early use of IV beta-blockers during acute evolving AMI.8 A heart-rate lowering agent devoid of effects on blood pressure and ventricular function such as ivabradine9–12 may be useful in this setting. An IV formulation may allow us to start and titrate therapy in the acute setting of AMI without the potential adverse effects related to hypotension or negative inotropy.
The primary objective of the VIVIFY (eValuation of the IntraVenous If inhibitor ivabradine after ST-segment elevation mYocardial infarction) pilot trial was to describe the effects of IV ivabradine on heart rate and hemodynamic parameters after percutaneous coronary intervention (PCI) for STEMI. The secondary objectives were to assess the safety and tolerability of ivabradine in this setting. An ancillary objective was to assess the effect of heart rate reduction with ivabradine on infarct size measured by magnetic resonance imaging (MRI) in a subset of patients at four months post-STEMI.
Methods
This international, multicenter, randomized, placebo-controlled, blinded, pilot trial included two unbalanced parallel groups of patients undergoing PCI following STEMI.
Study population
Eligible patients were men or women of non-childbearing potential, 40–80 years old, and weighing between 50–100 kg. They had been diagnosed with a STEMI in the previous 9 h and were undergoing PCI <6 h after the onset of chest pain. They were in sinus rhythm with heart rate >80 bpm and systolic blood pressure (SBP) >90 mm Hg. In selected centers, all patients who were eligible for the main study were invited to participate in the MRI substudy.
Exclusion criteria included second- or third-degree atrioventricular block, trifascicular block and PR interval >240 ms; atrial fibrillation or flutter; ventricular tachycardia (duration >30 s and heart rate >100 bpm); QT interval >450 ms; unstable vital signs; known aortic dissection; Killip class IV heart failure; need for IV inotropic agents, urgent need for cardiac surgery; and moderate or severe liver disease. Additional exclusion criteria for patients participating in the MRI substudy included glomerular filtration rate (GFR) <60 ml/min/1.73 m2 (calculated using the Cockcroft-Gault formula), claustrophobia and devices not compatible with magnetic fields.
Drugs with known or suspected interactions with ivabradine (strong CYP3A4 inhibitors and QT-prolonging agents) were prohibited within five half-lives prior to inclusion and during the first 24 h of the study. Amiodarone, verapamil, or diltiazem could be initiated if necessary but only after stopping study drug administration and with close cardiac monitoring. IV beta-blockers were forbidden; oral beta-blockers were allowed, as was heparin according to local practice. All drugs usually administered to patients with an AMI and PCI were authorized, including statins, aspirin, angiotensin-converting enzyme (ACE) inhibitors and antiplatelet agents.
The study was performed in accordance with the ethical principles stated in the Declaration of Helsinki (1964) and its revisions. The protocol was approved by the independent Ethics Committees in the countries concerned. All patients gave written informed consent. The study is registered on www.controlled-trials.com (ISRCTN number 66067800).
Study protocol
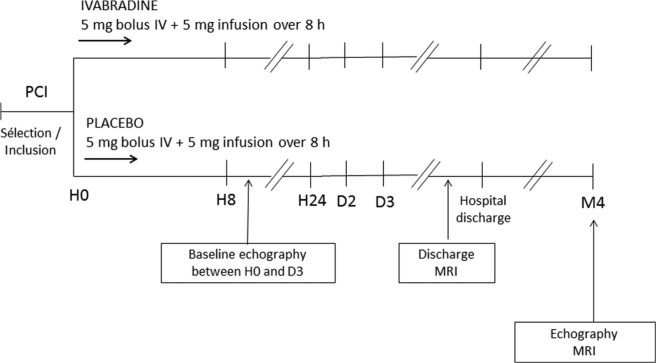
The study design is presented in Figure 1. Treatment was initiated (H0) at least 1 h after the end of PCI. During the course of the trial, a protocol amendment allowed initiation between the start of the PCI and 1 h after the end of the procedure. An IV bolus of study treatment (5 mg ivabradine or placebo) was administered over 30 s, immediately followed by an IV infusion over 8 h (5 mg ivabradine or placebo). The study treatment was prepared by diluting 2.5 ml of a solution containing 2 mg/ml ivabradine or placebo with a 0.9% sodium chloride solution, and was infused without any other IV medication. The infusion was stopped if heart rate was <60 bpm. No dose adjustment was made for body weight or baseline heart rate. The total fluid volume did not exceed 50 ml over 8 h. Treatment was allocated in chronological order of inclusion in each centre using an unbalanced (2 ivabradine/1 placebo) permuted-block randomization. An unbalanced randomization (2:1) was chosen in order to limit the number of patients exposed to placebo and acquire information on the safety of IV ivabradine in this setting. Both patients and investigators were blinded to treatment allocation, and treatment vials were identical.
Figure 1.
VIVIFY trial design.
IV: intravenous; MRI: magnetic resonance imaging; PCI: percutaneous coronary intervention.
The main assessment criterion was heart rate. At inclusion, 12-lead resting electrocardiography (ECG) was used to measure heart rate (two measurements 10 min apart, before treatment administration), on day 1 (at 1, 3, 4, and 8 h), on day 3, and at hospital discharge. Continuous ECG monitoring was performed over the first 24 h. ECG measurement was performed at baseline (if possible) and repeated up to three days after the intervention, to record left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV) and left ventricular ejection fraction (LVEF). Echocardiograms were analyzed at each site, according to local methods, by observers blinded to treatment assignment. Blood samples were collected at baseline, and at 20 min and 1, 5, 8, 12, and 24 h, to measure plasma concentrations of the cardiac markers creatine kinase (CK-MB) and troponin I and T, which were assayed in a central laboratory (Eurofins Medinat, Breda, The Netherlands).
In an MRI substudy, MRI was performed at hospital discharge and four months after STEMI with the patient in a supine position in a 1.5-tesla scanner. Image capture was ECG-gated, breath-hold, and k-space segmented, with T1-weighted inversion recovery gradient-echo sequencing. The MRI images were sent to a central laboratory (Leuven Coordinating Centre, Belgium), where they were interpreted by an expert radiologist blinded to treatment assignment, with measurement of infarct size (estimated at hospital discharge as the area of delayed hyperenhancement); area at risk (i.e. the area of delayed hyperenhancement and the surrounding edematous area); and microvascular obstruction (i.e. no reflow or incompletely restored perfusion).
Safety assessments included vital signs (SBP and diastolic blood pressure (DBP)) at baseline, every hour during infusion, at 12 and 24 h, after three days and at discharge, as well as any abnormalities on the 12-lead ECG. Adverse events were collected during the study period from selection to the end of follow-up at four months.
Statistical methods
Descriptive statistics are presented for baseline characteristics and cardiac markers. Between-group differences in heart rate (12-lead ECG), echocardiography variables and MRI parameters were estimated on the change between baseline and last value. Estimate (E), standard errors (SEs), 95% confidence intervals (CIs) and p-value for testing the difference between groups were provided from a parametric method based on a Student distribution and Student t test for independent samples13 and also from a nonparametric method as sensitivity analysis. Based on the same approaches, 95% CIs adapted for paired samples were also computed to estimate intra-group changes in heart rates. A p value of less than 0.05 was considered as being statistically significant.
No formal sample size calculation was performed for this exploratory study or its MRI substudy. However, in view of the pathology involved and the severity of patients in this pilot study, it was estimated that 120 randomized patients (80 ivabradine and 40 placebo) would be clinically relevant for a proper assessment of the effects of ivabradine on heart rate and echocardiographic parameters. It was also anticipated that 30 ivabradine patients and 15 placebo patients would be sufficient to evaluate the effect of ivabradine on infarct size in the MRI substudy. Efficacy analyses were carried out on patients of the full analysis set (FAS) (as well as on the FAS-MRI for the substudy), in respect of the intention to treat principle, and then on patients of a per-protocol set as a sensitivity analysis regarding any deviation that could interfere the efficacy assessment. Umanis (France) were responsible for data management and statistical analysis, using SAS version 9.1 software.
Results
Patients
Patients were screened in 24 centers in five countries (France, Germany, Spain, Belgium and Australia), of which 10 centers took part in the MRI substudy. The flow of patients in the main study and in the MRI substudy is presented in Figure 2. A total of 124 patients were randomized to receive ivabradine (n=82) or placebo (n=42). In the placebo group, one randomized patient did not receive drug due to concomitant beta-blocker infusion). Two additional patients were not evaluable (n=1, ivabradine group, unreliable timings of ECG; and n=1, placebo group, withdrawal for childbearing potential). Therefore the evaluable population (full analysis set) comprises 121 patients (n=81 ivabradine, n=40 placebo). Of the main study population, 39% (n=48) participated in the MRI substudy (n=32, ivabradine; n=16, placebo). In eight patients, no MRI data were collected and so were not available for analysis for the following reasons: claustrophobia (two ivabradine patients, one placebo patient); inadequate renal function (three ivabradine patients); refusal of MRI (one placebo patient); and unreliable timings of ECG (one ivabradine patient). Therefore the evaluable MRI substudy population (full analysis set) comprises 40 patients (26 ivabradine, 14 placebo).
Figure 2.
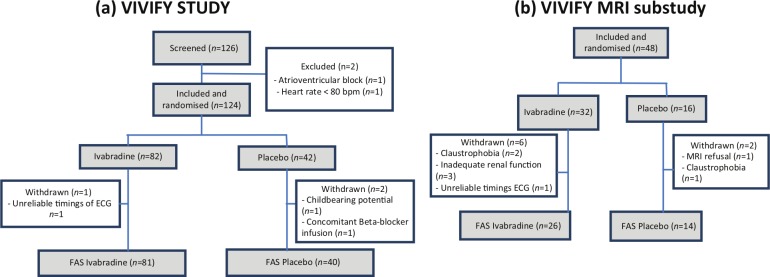
Trial profile for (a) the main eValuation of the IntraVenous If inhibitor ivabradine after ST-segment elevation mYocardial infarction (VIVIFY) study and (b) the magnetic resonance imaging (MRI) substudy.
ECG: electrocardiography; FAS: full analysis set.
The main baseline characteristics of the VIVIFY population are presented in Table 1. The mean age of the population was 59.4±11.0 years (range 40–81 years) and there was a majority of men (78%). There were no major differences between groups in demographic characteristics. A history of previous MI was more frequent in the ivabradine than among placebo patients (10% versus 2%). Nine (7%) patients had had previous percutaneous transluminal coronary angioplasty (PTCA), but none had had coronary artery bypass grafting. The mean heart rate at baseline was 87.8±9.3 bpm. SBP was slightly lower at baseline in the ivabradine group (122.8 vs 132.0 mm Hg). The patients participating in the MRI substudy had similar demographic and disease characteristics at baseline to the main population. The majority of the population underwent angioplasty during the study with placement of at least one stent (119 patients (96%)) (Table 1). The mean time between STEMI symptom onset and start of PCI was 215±105 min (range 37–709 min) and the mean time between start of PCI and the bolus was 112±69 min (range −15 to 472 min; one patient received the bolus before the procedure).
Table 1.
Characteristics of the population at baseline and details of current coronary event and intervention.
| Ivabradine n=82 |
Placebo n=42 |
p | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 60.1±10.8 | 58.0±11.6 | 0.2971 |
| Men, n (%) | 64 (78%) | 33 (79%) | 0.9468 |
| Weight (kg) | 77.9±13.1 | 80.5±13.0 | 0.2979 |
| Body mass index (kg/m2) | 27.0±3.5 | 27.6±3.5 | 0.3385 |
| Smoking habits | |||
| Nonsmoker, n (%) | 27 (33%) | 10 (24%) | |
| Ex-smoker, n (%) | 24 (29%) | 15 (36%) | |
| Smoker, n (%) | 31 (38%) | 17 (41%) | 0.5510 |
| Cardiac parameters | |||
| Heart rate (bpm) | 88.0±10.0 | 87.4±8.0 | 0.7689 |
| Systolic blood pressure (mm Hg) | 122.8±22.1 | 132.0±22.0 | 0.4258 |
| Diastolic blood pressure (mm Hg) | 78.4±12.1 | 78.9±14.0 | 0.9989 |
| Medical history | |||
| Previous myocardial infarction, n (%) | 8 (10%) | 1 (2%) | 0.1341 |
| Previous PTCA, n (%) | 5 (6%) | 4 (10%) | 0.4864 |
| Lipid metabolism disorders, n (%) | 31 (38%) | 21 (50 %) | 0.1508 |
| Diabetes mellitus, n (%) | 13 (16%) | 10 (24%) | 0.2887 |
| Hypertension, n (%) | 33 (40%) | 26 (62%) | 0.223 |
| Details of current coronary event | |||
| Angioplasty with at least one stent, n (%) | 78 (95%) | 41 (98%) | 0.5035 |
| Patients with single-vessel disease, n (%) | 51 (62%) | 26 (62%) | 0.4864 |
| Patient with two-vessel disease, n (%) | 20 (24%) | 8 (19%) | |
| Patients with three-vessel disease, n (%) | 11 (13%) | 8 (19%) | 0.6292 |
| Patients with left main coronary disease, n (%) | 3 (4%) | 2 (5%) | 0.7675 |
| Time between STEMI and PCI Median (Q1;Q3) |
209.0 (140.0;270.0) | 186.5 (140.0;290.0) | 0.6489 |
| Time between STEMI and PCI Median (Q1;Q3) |
87.5 (64.0;130.0) | 113.0 (91.0;132.0) | 0.1052 |
| Concomitant treatments | |||
| Beta-blockers | |||
| At infusion | 22 (27%) | 18 (43%) | 0.1978 |
| After infusion | 71 (87%) | 32 (76%) | 0.1441 |
| Nitrates | |||
| At infusion | 48 (59%) | 25 (60 %) | 0.8817 |
| After infusion | 32 (39%) | 12 (29%) | 0.2496 |
| Antithrombotic agents | |||
| At infusion | 75 (92%) | 38 (91%) | 0.717 |
| After infusion | 78 (95%) | 36 (86%) | 0.686 |
| RAS inhibitors | |||
| At infusion | 46 (56%) | 22 (52%) | 0.6939 |
| After infusion | 61 (74%) | 30 (71%) | 0.7240 |
| Lipid-lowering agents | |||
| At infusion | 52 (63%) | 25 (60%) | 0.4974 |
| After infusion | 42 (51%) | 28 (67%) | 0.1006 |
PCI: percutaneous coronary intervention; PTCA: percutaneous transluminal coronary angioplasty; RAS: renin-angiotensin system; STEMI: ST-segment elevation myocardial infarction.
At inclusion, concomitant medications were similar for the two groups (59% nitrates, 91% antithrombotic agents, 55% renin-angiotensin system (RAS) inhibitors, and 62% lipid-lowering agents), except for beta-blockers which were used by fewer patients in the ivabradine group (27% versus 43%) (Table 1). There were considerable increases in use of RAS inhibitors (73%) and beta-blockers (83%) after the infusion. All PCIs were performed on aspirin and clopidogrel and heparin (with dosing according to local practice). There was no use of bivalirudin, ticagrelor or prasugrel in the study. With respect to glycoprotein IIb/IIIa blockers, 59% (48/82) of the patients received GpIIb/IIIa blockers in the ivabradine group (41 abciximab, 4 tirofiban and 3 eptifibatide) and the proportion was 70% (25/41) in the placebo group (19 abciximab, 4 tirofiban, 2 eptifibatide). The majority of patients received bare metal stents, with 28% of the ivabradine patients and 37% of the placebo patients receiving at least one drug-eluting stent.
Effect on heart rate
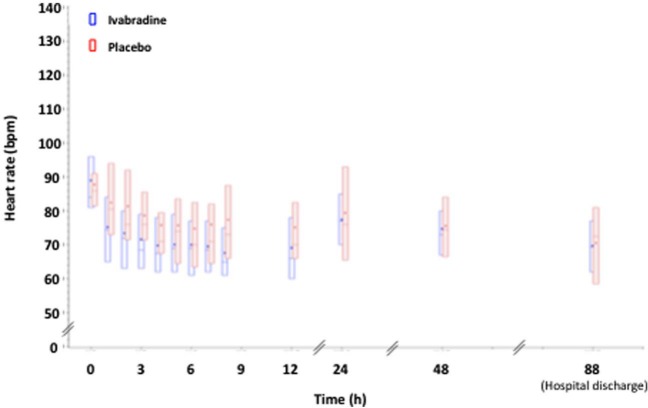
The changes in heart rate over the treatment period and up to hospital discharge are shown in Figure 3. Ivabradine reduced heart rate from 88.2±9.8 bpm at baseline to 66.2±10.1 bpm at last value over the 8 h treatment period (E(SE), −22.2 (1.3) bpm, 95% CI, −24.6 to −19.4). Most of the heart rate reduction was achieved by 4 h after starting therapy. Heart rate in the placebo group was reduced from 87.2±8.1 to 78.3±14.6 bpm over the same time period (E(SE), −8.9 (1.8) bpm, 95% CI, −12.6 to −5.2). This difference at 8 h between groups was significant (p<0.001). Similar changes in heart rate were found by continuous heart rate monitoring (–19.3±10.9 bpm from baseline to last value over 12 h with ivabradine versus −8.4±11.6 bpm with placebo (p<0.001). After the infusion, heart rate returned to placebo levels by 48 h, and remained similar in both groups at hospital discharge.
Figure 3.
Heart rate from bolus to discharge (12-lead electrocardiographic recordings).
As expected in STEMI patients, there were increases in plasma concentrations of all cardiac markers (troponin I, troponin T, and CK-MB) up to 5 h after bolus, declining towards normal values at 24 h (Figure 4). Throughout the study period, there was no difference between groups in the levels of biomarkers.
Figure 4.
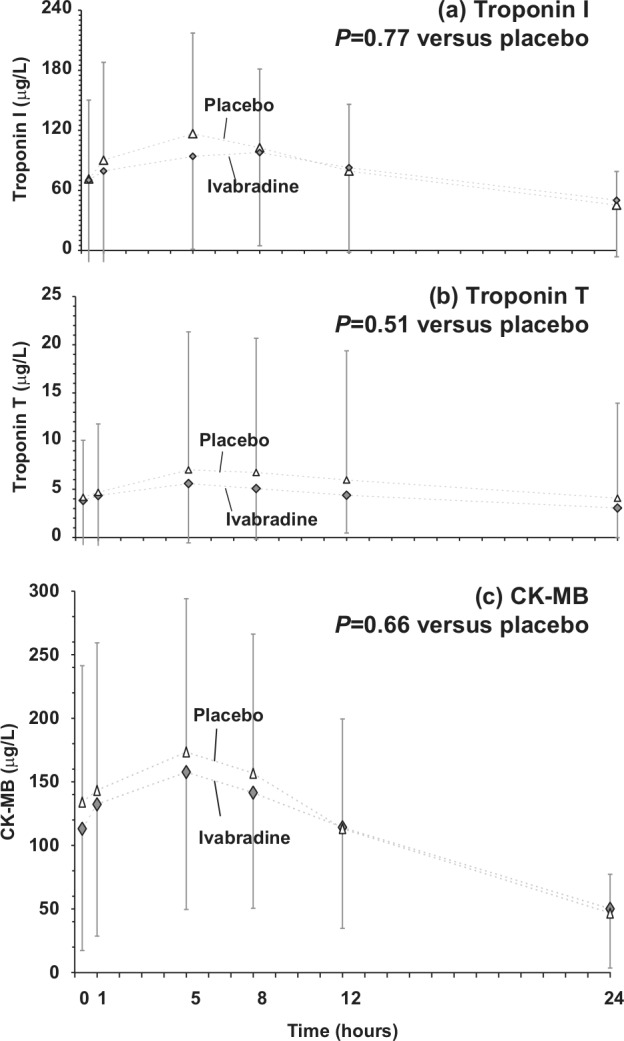
Changes in plasma concentrations of cardiac markers over the 24 hours following the bolus: (a) troponin I; (b) troponin I; and (c) creatine kinase (CK)-MB.
Values are mean±standard deviation (SD).
Echocardiographic results
Echocardiographic measurements were made at baseline and last post-treatment (mean: 1.16±0.98 days) for 23 patients (28%) in the ivabradine group versus 11 patients (28%) in the placebo group (Table 2). There were no differences between ivabradine and placebo in baseline left ventricular volumes. However, final volumes were lower in the ivabradine group both for LVEDV (87.1±28.2 versus 117.8±21.4 ml for ivabradine versus placebo, p=0.01) and LVESV (42.5±19.0 versus 59.1±11.3 ml, p=0.03). There were no significant differences in the changes in volumes between groups, nor was there a difference in baseline or final LVEF (Table 2).
Table 2.
Echocardiographic results. Change in left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), and left ventricular ejection fraction (LVEF) from baseline to last value.
| Ivabradine | Placebo | p | |
|---|---|---|---|
| LVEDV results | n=18 | n=8 | |
| Baseline LVEDV (ml) | 96.0 (79;124.0) | 121.5 (74.5;147.0) | 0.65 |
| Final LVEDV (ml) | 79.0 (70.0;99.0) | 113.5 (103.5;139.0) | 0.01 |
| Change in LVEDV from baseline to last value (ml) | −8.5 (−52.0;7.9) | 30 (−22.0;24.5) | 0.12 |
| LVESV results | n=17 | n=8 | |
| Baseline LVESV (ml) | 50.0 (38.0;65.6) | 52.9 (45.0;83.4) | 0.82 |
| Final LVESV (ml) | 36.0 (32.0;51.0) | 63.5 (49.5;67.3) | 0.03 |
| Change in LVESV from baseline to last value (ml) | −12.0 (−28.0;0.0) | −2.9 (−18.7;17.3) | 0.18 |
| LV ejection fraction | n=23 | n=11 | |
| Baseline LVEF (%) | 50.0 (39.0;57.0) | 45 (40.0;60.0) | 0.95 |
| Final LVEF (%) | 53.0 (42.0;61.0) | 54.0 (43.0;57.0) | 0.74 |
| Change in LVEF from baseline to last value (ml) | 5.0 (−2.0;6.0) | 0.0 (−4.0;4.0) | 0.55 |
Results are presented as median (Q1;Q3).
MRI results
Based upon feasibility in the AMI setting, a subset of 37 patients underwent MRI. The MRI results at hospital discharge and at four months are presented in Table 3. The area of delayed hyperenhancement, which is indicative of the infarcted volume as a percentage of LV mass, was 12.7% with ivabradine versus 17.2% placebo at hospital discharge. The inter–group difference was −4.6% (95% CI −11.4 to 2.3, p=0.190). Microvascular obstruction was found in 8/26 patients in the ivabradine group (31%) and 6/14 patients in the placebo group (43%) at discharge. The inter-group difference (in grams of myocardium with obstruction) was 2.6 (95% CI −8.8 to 3.7, p=0.315). In patients with obstruction, the mean size of the area of microvascular obstruction was similar in both groups (3.6% vs 4.6% of the LV mass at discharge, for the ivabradine and placebo groups respectively, with a between-group difference of −0.9% (95% CI, −5.1 to 3.3, p=0.6170). Infarct size at four months was similar in both groups.
Table 3.
Magnetic resonance imaging (MRI) results at hospital discharge and at four months.
| Ivabradine | Placebo | p | |
|---|---|---|---|
| MRI at hospital discharge | |||
| Infarct size (g) | 13.7 (5.1;24.3) | 22.1 (12.0;29.9) | |
| Number of patients | 24 | 13 | |
| E (SE) (95% CI) | −7.5 (4.4) (−16.4–1.5) | 0.103 | |
| Relative infarct size (% of LV mass) | 11.7 (4.3;16.5) | 18.1 (12.5;21.1) | |
| Number of patients | 24 | 13 | |
| E (SE) (95% CI) | −4.6 (3.4) (−11.4–2.3) | 0.190 | |
| Area at risk (% of LV mass) | 24.4 (18.5;38.8) | 31.4 (23.0;39.4) | |
| Number of patients | 15 | 10 | |
| E (SE) (95% CI) | 1.0 (6.5) (−12.4–14.4) | 0.879 | |
| Microvascular obstruction (g) | 4.1 (1.6;6.1) | 5.2 (1.8;7.1) | |
| Number of patients | 8 | 6 | |
| E (SE) (95% CI) | −2.6 (2.9) (−8.8–3.7) | 0.315 | |
| Microvascular obstruction (% of LV mass) | 2.8 (1.3;5.8) | 4.0 (1.7;4.6) | |
| Number of patients | 8 | 6 | |
| E (SE) (95% CI) | −0.9 (1.9) (−5.1–3.3) | 0.617 | |
| MRI at four months follow up | |||
| Infarct size (g) | 8.7 (2.7;16.8) | 6.3 (5.1;18.7) | |
| Number of patients | 24 | 12 | |
| E (SE) (95% CI) | −1.2 (3.1) (−7.5–5.2) | 0.726 | |
| Relative infarct size (% of LV mass) | 7.5 (2.5;17.6) | 5.8 (4.9;14.6) | |
| Number of patients | 24 | 12 | |
| E (SE) (95% CI) | 0.2 (2.9) (−5.8–6.2) | 0.945 | |
CI: confidence interval; E: estimate; LV: left ventricular; SE: standard error.
Values are median (Q1;Q3) unless otherwise indicated.
Safety and tolerability
Mean SBP and DBP decreased slightly from baseline to hospital discharge and in parallel and to a similar extent in both treatment groups. There were five cases of hypotension in the ivabradine group (one mild, three moderate, and one severe) vs none in the placebo group. None of these cases was considered as serious or related to the study drug: they were related to bleeding from catheter site, excessive diuresis, or the qualifying AMI, and all recovered completely. ECG assessment revealed no clinically relevant changes.
Sixty-three patients reported at least one emergent adverse event over the first 48 h: 46 patients (56%) in the ivabradine group versus 17 patients (42%) in the placebo group. The most frequent events were tachyarrhythmias (16% versus 12%) including ventricular tachycardia (11% versus 9%), headache (both 5%), and hypotension (6% versus 0%). The incidence of heart failure–related events was similar in both groups (6% versus 7%). The most frequent drug-related event was bradycardia (4% of the ivabradine group, none on placebo). There was one accidental overdose in the ivabradine group which led to transient severe bradycardia (40 bpm), from which the patient recovered uneventfully. Two patients died (both in the ivabradine group) during follow-up, one from worsening chronic obstructive pulmonary disease (five days after the bolus) and one from mesenteric infarction (seven days after the bolus). Neither case was considered by the investigator as being related to study treatment.
Discussion
This pilot study indicates that the use of IV ivabradine after PCI for STEMI produced a rapid and sustained reduction in heart rate, which was safe and well tolerated. Specifically, the heart rate reduction produced by ivabradine was not associated with any impact on SBP or DBP but was associated with lower LVESV and LVEDV at ECG performed 1.13 days after starting therapy.
Ivabradine is an If channel blocker, which produces pure heart rate reduction in patients in sinus rhythm.9 As a bradycardic agent, it has anti-anginal properties, which have been well established and are, in double blind studies, of the same order of magnitude as those produced by atenolol11 or amlodipine. The antianginal benefits have also been established on top of beta-blocker therapy.12 In the recent SHIFT trial, ivabradine, on top of standard therapy, was associated with a reduction in the composite of death or heart failure admission in patients with heart failure.14
This first study of IV ivabradine in the context of STEMI suggests that it produces a rapid and reversible slowing of heart rate, which is not associated with changes in blood pressure or major side effects. The signal of reduced left ventricular end systolic and end-diastolic volumes on ECG performed during in hospital stay should be interpreted conservatively given the limited study size, the number of patients with incomplete datasets, the multiplicity of endpoints and the absence of improvement in LVEF. Likewise, the small size of this pilot trial and the limited number of patients undergoing MRI does not allow a sound assessment of the impact of ivabradine on infarct size. However, measurement of left ventricular volumes by ECG was a pre-specified endpoint and we certainly cannot rule out a positive impact of heart rate reduction on left ventricular remodeling. Although it should be interpreted cautiously given the small sample size, there was no apparent effect on infarct size as derived from biomarker release and MRI measurements, which suggests that there may be benefits on left ventricular function independently of infarct size.
Tolerability of IV ivabradine was excellent: there were five patients with hypotension but in all of these clearcut causes were identified and ivabradine does not affect blood pressure, as judged from its mechanism of action, and its assessment in large scale clinical trials.9–12, 14–16 The rate of drug-induced bradycardia was low (4%) and one accidental overdose led to transient bradycardia which recovered uneventfully. Interestingly, the use of IV ivabradine did not affect the use of beta-blockers, as the proportion of patients who underwent beta-blocker initiation during treatment was 60% in the ivabradine arm and 33% in the placebo arm.
Limitations of this study
There are important limitations to these observations: given the size and exploratory nature of this pilot study, the study lacks the power to demonstrate a reduction in biomarker release, infarct size or clinical outcomes. Only a small number of patients had complete paired ECG or MRI studies. It was merely designed as the first pilot experience of IV ivabradine in the setting of primary PCI for STEMI. Further larger trials are required to determine whether early heart rate reduction with IV ivabradine added to standard care translates into benefit on clinical outcomes.
Conclusion
IV ivabradine may be of potential value in STEMI, by allowing rapid heart rate control without affecting blood pressure or hemodynamics. However, to characterize its effect, further controlled trials are required to assess its impact on infarct size, left ventricular function and, ultimately, clinical outcomes.
Acknowledgments
This study was presented in part at the American Heart Association Scientific Sessions, Chicago, USA, in November 2010. The Vivify investigators include international coordinators: P-G Steg (Paris, France), JL Lopez-Sendon (Madrid, Spain) and investigators: P-G Steg, M Slama, P Coste, P Garot, J Puel/M Elbaz, F Albert, B Charbonnier, M Hamon, F Schiele (France); L Missault, F Van de Werf (Belgium); E Lopez-de-Sa, C Macaya, FJ Goicolea, H Buen (Spain); CW Hamm (National Coordinator), M Bohm, T Meinertz, G Richardt, H Schunkert, K Werdan, A Gitt (Germany); P Aylward, J Horowitz (Australia).
Footnotes
Funding: This study was supported by Servier.
Conflict of interest: PGS has received research grants from Servier; fees for consultancy or participation in advisory board meetings from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo/Eli Lilly alliance, GlaxoSmithKline, Medtronic, Merck Sharpe and Dohme, Roche, sanofi-aventis, Servier, and The Medicines Company; payment for development of educational presentations from AstraZeneca, Boehringer Ingelheim, and The Medicines Company; and has equity ownership in Aterovax.
ELdS has received research grants from Anthera Pharmaceuticals, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo/Lilly, Ferrer, Pfizer and Servier.
FS has received research grants from GlaxoSmithKline, St Jude Medical, Sanofi, Servier, Daiichi-Sankyo/Lilly; has been a speaker for Boehringer-Ingelheim, Daiichi-Sankyo/Lilly, Novartis, Sanofi, Servier, The Medicines Company, AstraZeneca; and a consultant for Sanofi, Astra Zeneca, Lilly.
MH has received fees for lectures, advisory board or consulting services over the last two years from Cordis, Terumo, The Medicines Company, Biotronik, and Medtronic; and research grants from The Medicines Company, GlaxoSmithKline and Eli Lilly.
TM has no disclosures.
JG has no disclosures.
KW has received honoraria for lectures from Abbott, Bayer, Biogen, Biotest, Boehringer-Ingelheim, Boston Scientific, Datascope, Maquet, MSD, Novartis, Roche, Servier; honorariums for advisory board activities: Abbott, Bayer, Baxter, Biotest,,Datascope, Novartis, Servier; grants for participation in clinical trials: Arrows, Biotest, Datascope, MSD, Novartis, Servier; and research funding: Biotest, Bayer, Datascope, Novartis, Roche, Servier.
JLS has received research grants from Servier, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo/Lilly, GlaxoSmithKline and Pfizer.
References
- 1. Collins P, Fox KM. Pathophysiology of angina. Lancet 1990; 1: 94–96 [DOI] [PubMed] [Google Scholar]
- 2. Fox K, Borer JS, Camm AJ, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol 2007; 50: 823–830 [DOI] [PubMed] [Google Scholar]
- 3. Ibanez B, Prat-Gonzàlez S, Speidl WS, et al. Early metoprolol administration before coronary reperfusion results in increased myocardial salvage: Analysis of ischemic myocardium at risk using cardiac magnetic resonance. Circulation 2007; 115: 2909–2916 [DOI] [PubMed] [Google Scholar]
- 4. First International Study of Infarct Survival Collaborative Group Randomized trial of intravenous atenolol among 16,027 cases of suspected acute myocardial infarction: ISIS 1. Lancet 1986; 2: 57–66 [PubMed] [Google Scholar]
- 5. Roberts R, Rogers WJ, Mueller HS, et al. Immediate versus deferred beta-blockade following thrombolytic therapy in myocardial infarction (TIMI) II-B Study. Circulation 1991; 83: 422–437 [DOI] [PubMed] [Google Scholar]
- 6. The MIAMI Trial Research Group Metroprolol in acute myocardial infarction (MIAMI). A randomised placebo –controlled international trial. Eur Heart J 1985; 6: 199–226 [PubMed] [Google Scholar]
- 7. Chen ZM, Pan HC, Chen YP, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: Randomised placebo-controlled trial. Lancet 2005; 366: 1622–1632 [DOI] [PubMed] [Google Scholar]
- 8. Steg PG, James SK, Atar DA, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33: 2569–2619 [DOI] [PubMed] [Google Scholar]
- 9. Borer JS, Le Heuzey JY. Characterization of the heart rate-lowering action of ivabradine, a selective If current inhibitor. Am J Ther 2008; 15: 461–473 [DOI] [PubMed] [Google Scholar]
- 10. Manz M, Reuter M, Lauck G, et al. A single dose of ivabradine, a novel I(f) inhibitor, lowers heart rate but does not depress left ventricular function in patients with left ventricular dysfunction. Cardiology 2003; 100: 149–155 [DOI] [PubMed] [Google Scholar]
- 11. Tardif JC, Ford I, Tendera M, et al. Efficacy of ivabradine, a new selective If inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J 2005; 26: 2529–2536 [DOI] [PubMed] [Google Scholar]
- 12. Tardif JC, Ponikowski P, Kahan T. for the ASSOCIATE study investigators Efficacy of the If current inhibitor ivabradine in patients with chronic stable angina receiving beta-blockers therapy: A 4-month, randomized placebo-controlled trial. Eur Heart J 2009; 30: 540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hettmansperger TP, McKean JW. Robust non-parametric statistical methods. Kendall’s library of statistics. Arnold: London, 1998 [Google Scholar]
- 14. Swedberg K, Komajda M, Böhm M, et al. , on behalf of the SHIFT investigators Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo-controlled study. Lancet 2010; 376: 875–885 [DOI] [PubMed] [Google Scholar]
- 15. Pathak A, Berdeaux A, Mulder A, et al. Ivabradine dans la maladie coronaire: Pharmacologie expérimentale et clinique. Thérapie 2010; 65: 483–489 [DOI] [PubMed] [Google Scholar]
- 16. Fox K, Ford I, Steg PG, et al. On behalf of the BEAUTIFUL Investigators Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): A randomised, double-blind, placebo-controlled trial. Lancet 2008; 372: 807–816 [DOI] [PubMed] [Google Scholar]