Abstract
Aim:
Poorer outcomes in women with ST-elevation myocardial infarction (STEMI) are often attributed to gender differences in baseline characteristics. However, these may be age dependent. We examined the importance of gender in separate age groups of patients with STEMI undergoing primary percutaneous coronary intervention (PPCI).
Methods and results:
Data of 6746 consecutive patients with STEMI admitted for PPCI between 1998 and 2008 in our hospital were evaluated. Age was stratified into two groups, <65 years (young group) and ≥65 years (elderly). Endpoints were enzymic infarct size as well as 30-day and 1 year mortality. We studied a total of 4991 (74.0%) men and 1755 (26.0%) women; 40% of women were <65 years and 60% of men were <65 years of age. In the elderly group (≥65 years), women had more frequently diabetes and hypertension while they smoked less frequently than men. Younger women smoked more often than similarly aged men and had more hypertension. At angiography, single-vessel disease and TIMI 3 flow before PPCI was more present in younger women than men, whereas these differences were not found in the older age group. Patient delay before admission was shorter in men at all ages, while women had lower creatine kinase levels. Younger women had a higher mortality after 30 days (HR 2.1, 95% CI 1.3−3.4) and at 1 year (HR 1.7, 95% CI 1.2−2.6), whereas in the older age group women mortality rates were higher at 30 days (HR 1.5, 95% CI 1.1−2.0) but not at 1 year (HR 1.2, 95% CI 0.9−1.5). After multivariate analysis, 1-year mortality remained significantly higher in women at younger age (HR 1.7, 95% CI 1.1−2.5). Patient delay before admission was shorter in men in both age groups. Creatine kinase levels were in both age groups higher in men.
Conclusions:
Differences in mortality between men and women with STEMI treated with PPCI are age dependent. Although young women have less obstructive coronary artery disease and more often TIMI 3 flow before PCI (suggesting a lower risk), survival was worse compared to similarly aged men. Women had a longer patient delay compared to men, but this was not related to gender-specific mortality.
Keywords: Acute coronary syndrome, age, gender, STEMI, women
Introduction
Over the past decade, several reports on gender differences in prognosis after primary percutaneous coronary intervention (PPCI) in ST-segment elevation acute myocardial infarction (STEMI) have revealed conflicting results.1–6 Higher in-hospital mortality in women was often attributed to a longer patient delay before admission, older age, a higher clustering of cardiovascular risk factors, lower use of invasive and medical treatment, and more bleeding complications after interventions.6–13 Remarkably, especially in the younger age groups, women had a worse outcome compared with age-matched men.14–18 This may be related to a variety of factors such as gender differences in plaque composition, differences in thrombotic activity, and a higher prevalence of microvascular disease in younger women.19–22 However, as most previous studies did not stratify into age groups, it is less clear whether these gender differences in prognosis after STEMI are age dependent. Some studies did stratify on age but included patients with both STEMI and non-STEMI, representing a heterogenous population with acute coronary syndrome.6,23 In our present study from the Zwolle Myocardial Infarction Study Registry, we compare outcomes between women and men with STEMI, all referred for PPCI, within two different age groups.
Methods
Population
From January 1998 to January 2008, individual data from all STEMI patients who were considered for PPCI at our centre, were prospectively recorded in a dedicated database. Patients were diagnosed with STEMI if they had chest pain longer than 30 minutes and ECG changes with ST elevation greater than 2 mm in at least two precordial leads or greater than 1 mm in the limb leads. Cardiac biomarkers were elevated in all patients. According to the protocol, all patients received 500 mg aspirin and 5000 IU heparin intravenously before the PPCI procedure. Primary PCI was performed using standard techniques, if the coronary anatomy was suitable for intervention. Success rate of the procedure was assessed according to TIMI24 classification, in which a grade 3 blood flow within the infarct related artery and a myocardial blush grade25 2 or 3 were considered to be adequate. The classification for bleeding (major and minor) was used according to the definition of the TIMI study group. Where appropriate, patients were treated with drug therapy, including aspirin, clopidogrel, heparin, beta-blockers, ACE, angiotensin-converting enzyme (ACE) inhibitors, and lipid-lowering medication. Glycoprotein IIb/IIIa inhibitors were used according to the discretion of the treating cardiologist.
Laboratory measurements
According to the hospital protocol, blood sampling for creatine kinase (CK) and CK-MB levels was done at baseline and 8, 16, and 24 hours after PPCI. Infarct size was also estimated according to measurements of cumulative enzyme activities using lactate dehydrogenase (LDH) as the reference enzyme, as we have previously described in detail.26
Data collection
Data were divided into four groups according to gender and age <65 years and ≥65 years. Information on demographic parameters, risk factors, laboratory values, angiographic variables, and medication was derived from the patient files. Follow-up information was obtained from the outpatient files, the general physicians, or by direct telephone interview with the patients and was prospectively obtained using pre-defined time intervals of 30 days and 1 year with telephone interviews performed by independent research nurses, who were not involved in patient treatment. MACE was defined as the combination of death, myocardial infarction (MI), percutaneous coronary intervention (PCI), and/or coronary artery bypass surgery (CABG). Recurrent MI was diagnosed when there was 50% increase of CKMB from a previous peak value, followed by a subsequent rise to a level exceeding the upper limit during hospital stay or recurrent hospitalization with the diagnosis MI. Bleeding was defined as intracranial or overt bleeding with a decrease of haemoglobin ≥3 g/dl (≥1.9 mmol/l) or >10% decrease in haematocrit within 48 hours, and, if a bleeding site was not identified >4 g/dl decrease in haemoglobin or >12% decrease in haematocrit within 48 hours.
Statistical analysis
Statistical analysis was performed using SPSS version 18.0 (SPSS, Chicago, IL, USA). Continuous data were expressed as median and interquartile range and categorical data as number and percentage, unless otherwise indicated. Tests for significance were two-sided, with an α of 0.05. Cox proportional hazard regression was performed to estimate hazard ratios for mortality. Proportional hazard assumption was evaluated both graphically as with the Schoenfeld residual.27 As proportional hazard assumption was not respected for gender, Killip class >1 and multivessel disease in the older age group, we therefore employed a time-dependent covariate. Mortality was reported with hazard ratio (HR) and 95% confidence intervals (CI). Baseline characteristics with a p-value of <0.1 and significantly values in the different age and gender groups with univariate analysis were analysed with stepwise regression and included in the final multivariate model. We limited the variables to four per group to prevent overfitting. Variables in the multivariate model were age, multivessel disease, Killip class, and hypertension. Medication use was analysed using landmark analysis at discharge and 30 days. Gray’s test was used for analysis of competing risks to analyse re-PCI, re-CABG, and re-MI with mortality as a competing event.28
Results
Data from 6746 patients with STEMI who underwent a PPCI between 1998 and 2008 in our hospital were evaluated, consisting of 4991 (74%) men and 1755 (26%) women. Baseline characteristics for men and women in age groups of <65 years (young) and ≥65 years (elderly) are presented in Table 1; 40% of the women were <65 years old and 60% of the men were <65 years old.
Table 1.
Baseline characteristics according to gender and age.
| Variable | Women <65 years (n=708) | Men <65 years (n=3006) | p-value | Women ≥65 years (n=1047) | Men ≥65 years (n=1985) | p-value |
|---|---|---|---|---|---|---|
| Age (years) | 55 (48−61) | 54 (48−60) | 0.25 | 75 (70−80) | 72 (68−77) | <0.001 |
| History of: | ||||||
| MI | 38 (5) | 224 (8) | 0.05 | 77 (7) | 301 (15) | <0.001 |
| CABG | 11 (3) | 56 (2) | 0.58 | 35 (3) | 115 (6) | 0.003 |
| PCI | 19 (3) | 162 (5) | 0.003 | 57 (6) | 195 (10) | <0.001 |
| Stroke | 17 (2) | 50 (2) | 0.18 | 33 (3) | 110 (6) | 0.003 |
| Risk factors | ||||||
| History of hypertension | 245 (35) | 799 (27) | <0.001 | 495 (48) | 690 (35) | <0.001 |
| History of DM | 70 (10) | 235 (8) | 0.069 | 214 (21) | 251 (13) | <0.001 |
| Hyperlipidaemia | 137 (20) | 701 (25) | 0.023 | 204 (21) | 372 (20) | 0.69 |
| Positive family history | 375 (55) | 1400 (48) | 0.001 | 314 (32) | 554 (29) | 0.22 |
| Current smoking | 468 (67) | 1766 (60) | <0.001 | 236 (23) | 565 (30) | <0.001 |
| Admission data | ||||||
| Killip class >1 | 49 (7) | 162 (5) | 0.10 | 116 (11) | 179 (9) | 0.068 |
| Ischaemic time (min) | 218 (160−339) | 200 (148−296) | 0.001 | 237 (178−364) | 220 (164−315) | <0.001 |
| Patient delay (min) | 165 (110−285) | 150 (100−240) | <0.001 | 180 (120−291) | 165 (110−254) | <0.001 |
| Door-to-balloon time (min) | 45 (30−64) | 44 (30−66) | 0.32 | 48 (33−73) | 46 (33−73) | 0.12 |
Values are median (IQR) or n (%).
CABG, coronary artery bypass surgery; DM, diabetes mellitus; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Hypertension was more prevalent in women within both age groups than in men. In younger women, a positive family history and current smoking were significantly more present, while at older age women had more hypertension and diabetes. In both age groups, total ischaemic time and patient delay before hospital admission were significantly longer in women, whereas there was no gender difference in in-hospital delay from admission to first balloon inflation. Angiographic data showed less obstructive coronary artery disease in younger women compared with younger men, with a higher TIMI 3 flow at angiography and a lower CK release (Table 2). In the older age group, the occurrence of multivessel disease and TIMI-3 flow before PPCI were not significantly different between men and women. The TIMI flow and blush grade 3 post PPCI was not significantly different between men and women.
Table 2.
Angiographic findings and treatment strategies according to gender and age.
| Variable | Women <65 years (n=708) | Men <65 years (n=3006) | p-value | Women ≥65 years (n=1047) | Men ≥65 years (n=1985) | p-value |
|---|---|---|---|---|---|---|
| Multivessel disease | 33.2 | 44.7 | <0.001 | 59.7 | 61.6 | 0.31 |
| Initial treatment | 0.36 | 0.24 | ||||
| Conservative | 5.6 | 5.4 | 6.5 | 5.3 | ||
| PPCI | 91.9 | 91.1 | 89.0 | 89.4 | ||
| CABG | 2.5 | 3.5 | 4.5 | 5.3 | ||
| Stent placement | 75.2 | 74.8 | 0.85 | 66.4 | 69.7 | 0.07 |
| Infarct related vessel | ||||||
| Left main | 1.2 | 0.8 | 0.34 | 0.9 | 1.3 | 0.58 |
| Graft | 0.7 | 0.8 | 0.76 | 1.8 | 3.3 | 0.02 |
| LAD | 43.9 | 43.9 | 0.97 | 45.2 | 45.4 | 0.91 |
| RCA | 41.3 | 38.9 | 0.25 | 40.7 | 36.7 | 0.04 |
| Cx | 12.9 | 15.6 | 0.07 | 11.4 | 13.4 | 0.12 |
| TIMI-3 before PPCI | 24.6 | 19.9 | 0.008 | 19.7 | 18.4 | 0.42 |
| TIMI-3 after PPCI | 92.1 | 91.7 | 0.72 | 87.3 | 87.8 | 0.73 |
| TIMI 0−1 after PPCI | 3.7 | 3.2 | 0.48 | 4.9 | 4.3 | 0.51 |
| Blush grade 3 post PPCI | 51.9 | 49.7 | 0.38 | 40.8 | 41.3 | 0.85 |
| CK max. | 1400, 581−2854 | 1691, 685−3398 | 0.001 | 1268, 549−2500 | 1514, 640−3008 | 0.001 |
| LDH max. | 489, 297−827 | 485 272−837 | 0.80 | 503 305−872 | 491 299−792 | 0.24 |
Values are median (IQR) or n (%).
CK, creatine kinase; Cx, circumflex; LAD, left anterior descending; LDH, lactate dehydrogenase; PPCI, primary percutaneous coronary intervention; RCA, right coronary artery; TIMI, Thrombolysis in Myocardial Infarction.
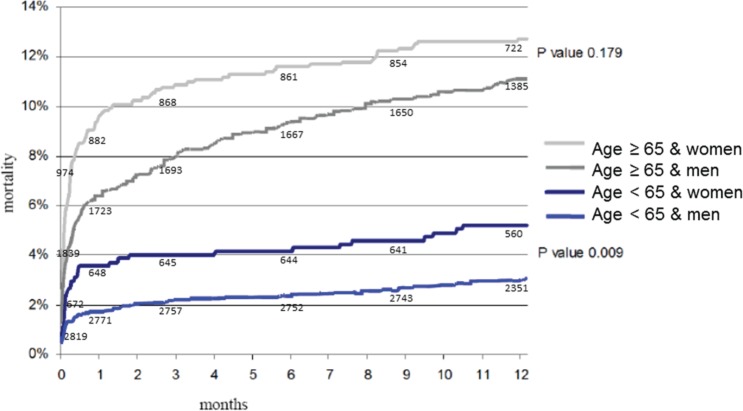
Overall, mortality at 30 days (HR 2.1, 95% CI 1.6−2.5) and at 1 year (HR 1.6, 95% CI 1.3−1.9) was higher in women than in men. The median duration of follow up in the younger group was 403 (396−409) days and in the older group 395 (389−400) days. The missing patients at 1 year in the younger group were 36 women and 187 men and in the older group 73 women and 146 men. At 1 year, 454 patients deceased in the total population. In the younger group 36 women and 87 men died and in the older group 124 women and 207 men died. At univariate analyses, women compared to men in the younger age group had a significantly increased risk of mortality both 30 days (HR 2.1, 95% CI 1.3−3.4) and at 1 year (HR 1.7, 95% CI 1.2−2.6). Mortality at 30 days was also higher in women in the older age group (HR 1.5, 95% CI 1.1−2.0). There was no difference, however, in mortality between both genders at 1 year (HR 1.2, 95% CI 0.9−1.5) (Table 3, Figure 1). At univariate analysis common predictors for mortality in elderly men and woman were age, Killip class, and previous history of cerebrovascular accident. In the younger age group, adverse predictors were age, Killip class, and the presence of multivessel disease. Hypertension was a common predictor for mortality in elderly women and in young men. Multivariate analyses, adjusting for multivessel disease, Killip class, age, and hypertension confirmed these findings in the younger age group (HR 1.7, 95% CI1.1−2.6). In the older age group, the hazard ratio for 1-year mortality was comparable in women and men (HR 1.0, 95% CI 0.8−1.4) (Figure 2). In the model with age and gender as an interaction term, 1-year mortality remained significant (HR 0.97, 95% CI 0.95−0.99). Re-MI, re-PCI, and re-CABG were analysed with the Gray’s test for comparison of cumulative incidence curves between men and women for 30-day and 1-year mortality. Mortality remained significant between men and women in both age groups at 30 days and in the younger group at 1 year.
Table 3.
Clinical outcomes according to gender and age.
| Variable | Women <65 years (n=708) | Men <65 years (n=3006) | p-value | Women ≥65 years (n=1047) | Men ≥65 years (n=1985) | p-value |
|---|---|---|---|---|---|---|
| Bleeding (<48 hour) | 5.0 | 3.8 | 0.125 | 6.6 | 9.3 | 0.008 |
| At 30 days | ||||||
| Death | 3.6 | 1.7 | 0.002 | 9.6 | 6.4 | 0.005 |
| Re-MI | 1.3 | 2.1 | 0.18 | 2.0 | 2.8 | 0.16 |
| Re-PCI | 4.3 | 6.7 | 0.02 | 4.3 | 6.4 | 0.02 |
| Re-CABG | 2.3 | 3.1 | 0.25 | 3.6 | 4.6 | 0.23 |
| Death and/or re-MI | 4.6 | 3.7 | 0.25 | 20.3 | 8.7 | 0.05 |
| MACE | 11.8 | 3.7 | 0.10 | 20.3 | 8.7 | 0.41 |
| At 1 year | ||||||
| Death | 5.3 | 3.1 | 0.004 | 12.7 | 11.2 | 0.24 |
| Re-MI | 2.8 | 3.5 | 0.38 | 3.3 | 5.0 | 0.04 |
| Re-PCI | 12.7 | 13.6 | 0.55 | 10.1 | 12.2 | 0.12 |
| Re-CABG | 2.3 | 2.9 | 0.40 | 3.9 | 4.7 | 0.39 |
| Death and/or re-MI | 7.6 | 6.4 | 0.26 | 15.4 | 15.0 | 0.81 |
| MACE | 23.9 | 24.8 | 0.64 | 33 | 35.2 | 0.27 |
Values are %. Death is depicted as cumulative mortality.
MACE, combination of death, myocardial infarction, PCI, and/or CABG; MI, myocardial infarction.
Figure 1.
Kaplan−Meier curves for gender and age groups in patients with ST-elevation myocardial infarction.
Figure 2.
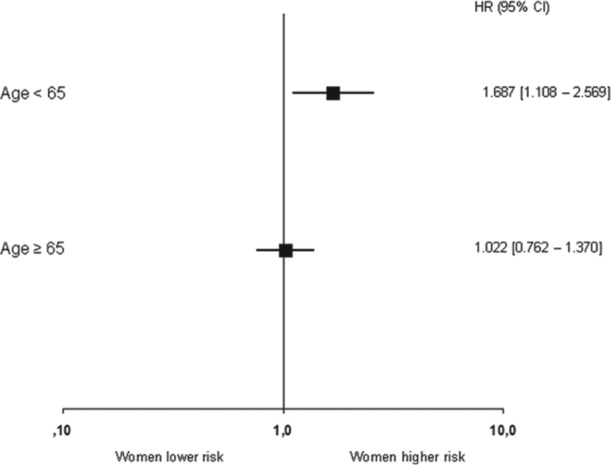
Multivariate analysis of 1-year mortality in women as compared to men, stratified to age.
Medication use at various time intervals is depicted in Table 4. Women were using more often insulin and diuretics at discharge, except in the younger age group. The use of aspirin, beta-blockers, statins, and ACE inhibitors was comparable in all 12 subgroups, except for higher rates of ACE inhibitors use at discharge in elderly men. Clopidogrel has been routinely used in our hospital since 2004. Its use in the acute phase was comparable between women and men in both age groups. Also glycoprotein IIb/IIIa inhibitor use in the acute phase was comparable between women and men in both age groups (Table 4).
Table 4.
Medication use at discharge by gender and age.
| Medication use | Women <65 years (n=708) | Men <65 years (n=3006) | p-value | Women ≥65 years (n=1047) | Men ≥65 years (n=1985) | p-value |
|---|---|---|---|---|---|---|
| In the acute phase (since 2004) | ||||||
| Clopidogrel | 75 | 77 | 0.48 | 71 | 70 | 0.56 |
| GP IIbIIIa inhibitor * | 27 | 28 | 0.69 | 25 | 25 | 0.79 |
| At discharge | ||||||
| Aspirin | 92 | 94 | 0.09 | 91 | 92 | 0.43 |
| Beta-blocker | 93 | 90 | 0.06 | 88 | 87 | 0.43 |
| Statins | 85 | 84 | 0.44 | 76 | 77 | 0.23 |
| ACE inhibitor | 55 | 57 | 0.36 | 54 | 59 | 0.01 |
| Diuretics | 8 | 8 | 0.87 | 21 | 16 | <0.001 |
| Insulin | 5 | 3 | 0.02 | 6 | 4 | <0.001 |
| At 30 days | ||||||
| Aspirin | 91 | 91 | 0.53 | 87 | 87 | 0.98 |
| Beta-blocker | 93 | 90 | 0.08 | 89 | 88 | 0.29 |
| Statins | 88 | 89 | 0.51 | 81 | 82 | 0.36 |
| ACE inhibitor | 60 | 61 | 0.64 | 59 | 63 | 0.06 |
| Diuretics | 8 | 5 | 0.002 | 17 | 12 | <0.001 |
| Insulin | 2 | 4 | 0.006 | 6 | 3 | <0.001 |
| Oral antidiabetics | 3 | 3 | 0.50 | 6 | 3 | 0.003 |
| At 1 year | ||||||
| Aspirin | 90 | 89 | 0.51 | 83 | 82 | 0.35 |
| Beta-blocker | 83 | 79 | 0.05 | 81 | 81 | 0.70 |
| Statins | 91 | 90 | 0.37 | 83 | 83 | 0.70 |
| ACE inhibitor | 49 | 53 | 0.05 | 53 | 56 | 0.31 |
| Diuretics | 10 | 5 | <0.001 | 19 | 13 | <0.001 |
| Insulin | 4 | 2 | 0.014 | 6 | 3 | 0.004 |
| Oral antidiabetics | 4 | 3 | 0.33 | 7 | 5 | 0.09 |
ACE, angiotensin-converting enzyme; GP, glycoprotein IIbIIIa inhibitor.
Of the 367 patients in our study who initially received conservative treatment, 102 patients had non-obstructive coronary artery disease and 23 patients were treated with PCI during the same hospitalization. In two patients it was not possible to pass the wire through the stenosis whereas in three patients, the PCI was performed after several days, after an initially conservative approach. One patient had an older infarction and PCI was performed after demonstrating residual ischaemia by non-invasive testing. Two patients were referred from other hospitals after conservative treatment for unknown reasons. In 10 patients no culprit vessel was treatable due to too small vessel size and in the remaining five patients no details were available, and 243 patients were treated conservatively because PCI was not indicated.
Discussion
In our present study in patients with STEMI, treated with PPCI, we found that 1-year mortality was higher in women than in men in the age group <65 years. Whereas in the older age group mortality was higher at 30 days in women, there was no gender difference at 1 year. Several other single-centre and multicentre studies have shown higher in-hospital mortality rates in women compared to men. These differences are often attributed to their higher age and clustering of more CV risk factors.9–11 In some studies, gender differences in treatment and higher rates of in-hospital complications in women are considered as possible causes for their higher mortality rates.5,10 In our study, we found that differences in mortality between women and men persisted after correction for confounders by multivariate analysis. Treatment strategies in the acute setting were standardized and therefore comparable between both genders. It is alarming, however, that, although younger women had a lower risk profile at baseline, with more TIMI 3 flow before PCI and less multivessel disease, they had a higher mortality than similarly aged men. There may be several explanations for this adverse prognosis in women. First, despite their increased mortality rates at 30 days and at 1 year, younger women undergo less often a re-PCI than men (Table 3). Almost half of all re-PCI procedures were performed within the first 30 days, in which time most gender-related mortality differences are also seen. With our data, we cannot demonstrate whether the lower number of re-PCIs in younger women has had prognostic importance, and this should be examined carefully in future studies.
The total enzymatic infarct size (as measured by CK) was lower in young women compared to similarly aged men. We found no gender differences in LDH values within both age groups. This may partly be related to the usually smaller body weight in women, although detailed information on body mass index is lacking in our study. Further, since women have a longer patient delay in both age groups compared to men and therefore hypothetically present more often after the peak of the CK levels (2−12 hours), this may possibly have resulted in lower CK levels. Secondly, because men have a shorter patient delay, they are more likely to be discharged before the peak LDH levels (24−48 hours) as most patients were discharged shortly after the CK peak. It is noteworthy that longer patient delay in women may be related to the absence of chest pain. However, patient delay was not a strong predictor for gender-specific mortality neither in a recent large study29 nor in our study. In our study cohort, we have no data on type of chest pain/discomfort.
Our present findings confirm the existence of the so called ‘gender paradox’ in young women with STEMI.13,14,30 Several mechanisms may be involved to explain this phenomenon. First, we found important gender differences in baseline characteristics within the younger age group with a higher percentage of current smokers in women (67%) in comparison with men (60%). Smoking increases the risk of an acute MI relatively more in young women than in young men.31,32 Cigarette smoking increases oxidative stress and promotes the release of vascular inflammatory markers leading to a decrease in endothelial function.33 This counteracts the protective vasodilating effects of endogenous oestrogens in women before menopause.34 Although data are lacking in our cohort on the percentage of women that were using oral contraceptives, its use is common in premenopausal women in the Netherlands and also enhances the risk of arterial thrombosis and MI.35,36 In contrast, the use of hormone replacement therapy after menopause is rare in our country. In addition, data on previous gynaecology operations, endogenous oestrogen status, or menopausal status are lacking in our patients while this has shown to be relevant in women.37 Evidence is increasing that hysterectomy, especially before the age of 50 years, interferes with an increased risk of cardiovascular disease.38
Gender-related differences in the pathophysiology of STEMI in women at younger age may also increase mortality. At younger age, the occurrence of plaque rupture with subsequent thrombosis is more common in men, while plaque erosions with microvascular embolization are relatively more frequently reported in women.19–21 In pathology studies, it is shown that erosive plaques have a lower degree of critical stenosis with a greater maturation of thrombus material compared to ruptured plaques, especially in younger women.19,39 Furthermore, if plaque ruptures occur, they are more related to thrombus formation in women than in men.40 Women exhibit acute coronary syndrome with open coronary arteries more frequently than men.41,42 Non-obstructive coronary artery disease with microvascular dysfunction and abnormal coronary reactivity may also be relatively more important in women with STEMI and affect prognosis negatively.43
In our study, TIMI–flow post PPCI and myocardial blush grades, as indicators of reperfusion, were comparable between women and men in both age groups. This is in concordance with data from previous studies.44
We found that main gender differences in medication use after STEMI were higher rates of insulin and diuretics use in women. This can be explained by the higher prevalence of hypertension and diabetes mellitus. Limitations are that our patients were included during a 10-year time period in which PPCI procedures and medication given during this procedure were due to some changes. Also, although clopidogrel was prescribed for 1 year in all patients, we do not have detailed information about eventually discontinuation of clopidogrel, and we can not exclude that discontinuation may have affected late stent thrombosis and re-MI.45,46 In conclusion, in our single-centre cohort with STEMI patients, we found that younger women have a higher mortality than similarly aged men, despite the presence of less obstructive coronary artery disease and better TIMI 3 flow before PCI. More data are needed to explain these differences to improve prognosis in younger women.
Acknowledgments
We thank Vera Derks for excellent editorial assistance.
Footnotes
Conflict of interest: None.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Vakili BA, Kaplan RC, Brown DL. Sex-based differences in early mortality of patients undergoing primary angioplasty for first myocardial infarction. Circulation 2001; 104: 3034–3038 [DOI] [PubMed] [Google Scholar]
- 2. Peterson ED, Lansky AJ, Kramer J, et al. ; National Cardiovascular Network Clinical Investigators Effect of gender on the outcomes of contemporary percutaneous coronary intervention. Am J Cardiol 2001; 88: 359–364 [DOI] [PubMed] [Google Scholar]
- 3. Duvernoy CS, Smith DE, Manohar P, et al. Gender differences in adverse outcomes after contemporary percutaneous coronary intervention: an analysis from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) percutaneous coronary intervention registry. Am Heart J 2010; 159: 677–683 [DOI] [PubMed] [Google Scholar]
- 4. Singh M, Rihal CS, Gersh BJ, et al. Mortality between men and women after percutaneous coronary interventions: 25-year, single-center experience. J Am Coll Cardiol 2008; 51: 2313–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akhter N, Milford-Beland S, Roe MT, et al. Gender differences among patients with acute coronary syndromes undergoing percutaneous coronary intervention in the American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR). Am Heart J 2009; 157: 141–148 [DOI] [PubMed] [Google Scholar]
- 6. Radovanovic D, Erne P, Urban P, et al. ; AMIS Plus Investigators Gender differences in management and outcomes in patients with acute coronary syndromes: results on 20290 patients from the AMIS plus registry. Heart 2007; 93: 1369–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diercks DB, Owen KP, Kontos MC, et al. Gender differences in time to presentation for myocardial infarction before and after a national women’s cardiovascular awareness campaign: a temporal analysis from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress ADverse Outcomes with Early Implementation (CRUSADE) and the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network−Get with the Guidelines (NCDR ACTION Registry−GWTG). Am Heart J 2010; 160: 80–87 [DOI] [PubMed] [Google Scholar]
- 8. Kaul P, Armstrong PW, Sookram S, et al. Temporal trends in patient and treatment delay among men and women presenting with ST-elevation myocardial infarction. Am Heart J 2011; 161: 91–97 [DOI] [PubMed] [Google Scholar]
- 9. Cheng CI, Yeh KH, Chang HW, et al. Comparison of baseline characteristics, clinical features, angiographic results, and early outcomes in men vs women with acute myocardial infarction undergoing primary coronary intervention. Chest 2004; 126: 47–53 [DOI] [PubMed] [Google Scholar]
- 10. Benamer H, Tafflet M, Bataille S, et al. Female gender is an independent predictor of in-hospital mortality after STEMI in the era of primary PCI: insights from the greater Paris area PCI registry. EuroIntervention 2011; 6: 1029–1031 [DOI] [PubMed] [Google Scholar]
- 11. Milcent C, Dormont B, Durand-Zaleski I, et al. Gender differences in hospital mortality and use of percutaneous coronary intervention in acute myocardial infarction. Microsimulation analysis of the 1999 nationwide French hospitals database. Circulation 2007; 115: 833–839 [DOI] [PubMed] [Google Scholar]
- 12. Jneid H, Fonarow GC, Cannon CP, et al. ; Get With the Guidelines Steering Committee and Investigators Sex differences in medical care and early death after acute myocardial infarction. Circulation 2008; 118: 2803–2810 [DOI] [PubMed] [Google Scholar]
- 13. Alexander KP, Chen AY, Newby LK, et al. ; CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines) Investigators Sex differences in major bleeding with glycoprotein IIb/IIIa inhibitors. Results from the CRUSADE initiative. Circulation 2006; 114: 1380–1387 [DOI] [PubMed] [Google Scholar]
- 14. Hochman JS, Tamis JE, Thompson TD, et al. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. N Engl J Med 1999; 341: 226–232 [DOI] [PubMed] [Google Scholar]
- 15. Vaccarino V, Parsons L, Every NR, et al. Sex-based differences in early mortality after acute myocardial infarction. N Engl J Med 1999; 341:217–225 [DOI] [PubMed] [Google Scholar]
- 16. Berger JS, Brown DL. Gender−age interaction in early mortality following primary angioplasty for acute myocardial infarction. Am J Cardiol 2006; 98: 1140–1143 [DOI] [PubMed] [Google Scholar]
- 17. Berger JS, Elliott L, Gallup D, et al. Sex differences in mortality after acute coronary syndromes. JAMA 2009; 302: 874–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lawesson SS, Stenestrand U, Lagerqvist B, et al. Gender perspective on risk factors, coronary lesions and long-term outcome in young patients with ST-elevation myocardial infarction. Heart 2010; 96: 453–459 [DOI] [PubMed] [Google Scholar]
- 19. Arbustini E, Dal Bello B, Morbini P, et al. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart 1999; 82: 269–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Farb A, Burke AP, Tang AL, et al. Coronary plaque erosion without rupture into a lipid core: a frequent cause of coronary thrombosis in sudden coronary death. Circulation 1996; 93: 1354–1363 [DOI] [PubMed] [Google Scholar]
- 21. Frink RJ. Gender gap, inflammation and acute coronary disease: are women resistant to atheroma growth? Observations at autopsy. J Invasive Cardiol 2009; 21: 270–277 [PubMed] [Google Scholar]
- 22. Shaw LJ, Bugiardini R, Bairey Merz CN. Women and ischemic heart disease. J Am Coll Cardiol 2009; 54: 1561–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Champney KP, Frederick PD, Bueno H, et al. ; NRMI Investigators The joint contribution of sex, age and type of myocardial infarction on hospital mortality following acute myocardial infarction. Heart 2009; 95: 895–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. The Thrombolysis in Myocardial Infarction (TIMI) trial: phase I findings: TIMI Study Group. N Engl J Med 1985; 312: 932–936 [DOI] [PubMed] [Google Scholar]
- 25. Van ’t Hof AWJ, Liem A, Suryapranata H, et al. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation 1998; 97: 2302–2306 [DOI] [PubMed] [Google Scholar]
- 26. De Boer MJ, Suryapranata H, Hoorntje JCA, et al. Limitation of infarct size and preservation of left ventricular function after primary coronary angioplasty compared with intravenous streptokinase in acute myocardial infarction. Circulation 1994; 90: 753–761 [DOI] [PubMed] [Google Scholar]
- 27. Schoenfeld D. Residuals for the proportional hazards regression model. Biometrika 1982; 69: 239–241 [Google Scholar]
- 28. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Statist 1988; 16: 1141–1154 [Google Scholar]
- 29. Canto JG, Rogers WJ, Goldberg RJ, et al. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA 2012; 307: 813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bairey Merz CN. The Yentl syndrome is alive and well. Eur Heart J 2011; 32: 1313–1315 [DOI] [PubMed] [Google Scholar]
- 31. Prescott E, Hippe M, Schnohr P, et al. Smoking and risk of myocardial infarction in women and men: longitudinal population study. BMJ 1998; 316: 1043–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grundtvig M, Hagen TP, German M, et al. Sex-based differences in premature first myocardial infarction caused by smoking: twice as many years lost by women as by men. Eur J Cardiovasc Prev Rehabil 2009; 16: 174–179 [DOI] [PubMed] [Google Scholar]
- 33. Barbieri SS, Zacchi E, Amadio P, et al. Cytokines present in smokers’ serum interact with smoke components to enhance endothelial dysfunction. Cardiovascular Research 2011; 90: 475–483 [DOI] [PubMed] [Google Scholar]
- 34. Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 1999; 340: 1801–1811 [DOI] [PubMed] [Google Scholar]
- 35. Tanis BC, van den Bosch MAAJ, Kemmeren JM, et al. Oral contraceptives and the risk of myocardial infarction. N Engl J Med 2001; 345: 1787–1793 [DOI] [PubMed] [Google Scholar]
- 36. Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med 2012; 366: 2257–2266 [DOI] [PubMed] [Google Scholar]
- 37. Bairey MCN, Johnson BD, Sharaf BL, et al. ; WISE Study Group Hypoestrogenemia of hypothalamic origin and coronary artery disease in premenopausal women: a report from the NHLBI-sponsored WISE study. J Am Coll Cardiol 2003; 41: 413–419 [DOI] [PubMed] [Google Scholar]
- 38. Ingelsson E, Lundholm C, Johansson ALV, et al. Hysterectomy and the risk of cardiovascular disease: a population based cohort study. Eur Heart J 2011; 32: 745–750 [DOI] [PubMed] [Google Scholar]
- 39. Kramer MCA, Rittersma SZH, de Winter RJ, et al. Relationship of thrombus healing to underlying plaque morphology in sudden coronary death. J Am Coll Cardiol 2010; 55: 122–132 [DOI] [PubMed] [Google Scholar]
- 40. Kruk M, Pregowski J, Mintz GS, et al. Intravascular ultrasonic study of gender differences in ruptured coronary plaque morphology and its associated clinical presentation. Am J Cardiol 2007; 100: 185–189 [DOI] [PubMed] [Google Scholar]
- 41. Bugiardini R, Bairey Merz CN. Angina with ‘normal’ coronary arteries: a changing philosophy. JAMA 2005; 293: 477–484 [DOI] [PubMed] [Google Scholar]
- 42. Smilowitz NR, Sampson BA, Abtrecht CR, et al. Women have less severe and extensive coronary atherosclerosis in fatal cases of ischemic heart disease: an autopsy study. Am Heart J 2011; 161: 681–688 [DOI] [PubMed] [Google Scholar]
- 43. Ong P, Athanasiadis A, Hill S, et al. Coronary artery spasm as a frequent cause of acute coronary syndrome. J Am Coll Cardiol 2008; 52: 523–527 [DOI] [PubMed] [Google Scholar]
- 44. Ndrepepa G, Tiroch K, Keta D, et al. Predictive factors and impact of no reflow after primary percutaneous coronary intervention in patients with acute myocardial infarction. Circ Cardiovasc Interv 2010; 3: 27–33 [DOI] [PubMed] [Google Scholar]
- 45. McFadden P, Stabile E, Regar E, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet 2004; 364: 1519–1921 [DOI] [PubMed] [Google Scholar]
- 46. Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005; 293: 2126–2130 [DOI] [PubMed] [Google Scholar]