Abstract
Aims:
Criteria for diagnosing myocardial infarction (MI) after coronary artery bypass grafting (CABG) are controversial. Uncertainties remain around the optimal threshold for biomarker elevation and the need for associated criteria. There are no studies of high-sensitivity troponin (hs-TnT) after CABG. We assessed whether using hs-TnT to define MI after CABG was associated with 30-day and medium-term mortality and evaluated the utility of adding to the troponin criteria new Q-waves or imaging evidence of new wall motion abnormality as suggested in the Universal Definition of MI.
Methods:
Isolated CABG was performed in 818 patients from July 2010 to June 2012 and hs-TnT was measured 12–24 hours after CABG. Patients with rising baseline or missing troponins (n=258) were excluded. Thresholds of 140 ng/l (10-times 99th percentile upper reference limit) and 500 ng/l (10-times coefficient of variation of 10% for fourth-generation troponin T applied to hs-TnT) were prespecified.
Results:
Mean follow up was 1.8±0.6 years. On multivariate analyses, isolated hs-TnT rise >140 ng/l (n=360) or >500 ng/l (n=162) were not associated with mortality. Additional ECG and/or echocardiographic criteria plus hs-TnT >140 ng/l was associated with 30-day mortality (hazard ratio, HR, 4.92, 95% CI 1.34–18.1; p=0.017) and medium-term mortality (HR 3.44, 95% CI 1.13–10.5; p=0.030), whereas ECG and/or echocardiographic abnormalities with hs-TnT >500 ng/l was not (p=0.281 and p=0.123 for 30-day and medium-term mortality, respectively).
Conclusions:
A definition for MI following CABG using hs-TnT with a cut point of 10-times 99th percentile upper reference limit and ECG and/or echocardiographic criteria predicts 30-day and medium-term mortality. These findings validate the Third Universal Definition of type 5 MI.
Keywords: Coronary artery bypass surgery, high-sensitivity troponin, myocardial infarction, Universal Definition
Introduction
Cardiac troponins have been recommended by the Universal Definition of myocardial infarction (MI) as the preferred biomarkers for diagnosing MI after coronary artery bypass grafting (CABG),1 due to superior sensitivity and specificity compared to traditional biomarkers such as creatine kinase MB.2,3 Several studies have found that isolated troponin rise after CABG, measured with contemporary assays, independently predicts adverse outcomes.3–7
High-sensitivity troponin assays have recently been developed,8 being more sensitive than contemporary assays at detecting lower troponin levels.1,9 The properties and utility of these assay may be different after CABG, and there are no data about their use for the diagnosis of MI. The 2012 Third Universal Definition defined MI (type 5) after CABG as requiring two criteria: (1) cardiac biomarkers (with troponins preferred) rise >10-times 99% upper reference limit (URL) from a normal preoperative level; and (2) new pathological Q-waves or new left bundle branch block (LBBB) and/or imaging or angiographic evidence of new occlusion of native vessels or grafts, new regional wall motion abnormality, or loss of viable myocardium.1
We therefore assessed the ability of high-sensitivity troponin T (hs-TnT) to diagnose MI following CABG using several prespecified criteria including the Universal Definition and assessed its associations with mortality and morbidity.
Methods
Patient selection and data collection
Ethical approval of this study was obtained from our ethics review committee.
Patients undergoing isolated CABG without other concomitant cardiac surgery from the commencement of hs-TnT use from July 2010 to June 2012 were identified retrospectively from the cardiothoracic surgical unit database. Logistic EuroScore II, which predicts operative risk, was calculated.10 Buckberg cold blood cardioplegia was used for on-pump CABG.
Electrocardiograms (ECGs) were performed multiple times until discharge. Transthoracic echocardiograms were performed as indicated clinically. New Q-waves or LBBB on the ECG or new regional wall motion abnormality on echocardiography were independently interpreted by two authors blinded to outcomes (TKMW and HDW), in accordance with the criteria of the Universal Definition.1
Mortality data were checked against New Zealand’s national registry up until 31 March 2013. Thirty-day and medium-term mortality were prespecified as the primary outcomes. A composite of post-operative complications (renal failure, stroke, prolonged ventilation >24 hours, deep sternal wound infection, and return to theatre) were the secondary outcomes as determined according to the Society of Thoracic Surgeons definitions.11
Troponin assays and classification
The hs-TnT assay (Roche Elecsys), which is guideline compliant,8 was introduced in July 2010. The 99th percentile URL of this assay is 14 ng/l.8 The cut point commonly used clinically for the fourth-generation troponin T with a 10% coefficient of variation (0.03 ng/ml) when applied to the hs-TnT assay is 50 ng/l.9 Patients routinely had hs-TnT measured 12–24 hours after surgery. The highest hs-TnT level was used for analyses.
Pre-operative troponin assays used at our hospital were the same hs-TnT. Two referral hospitals used the Abbott Architect troponin I assay with URL of 28 ng/l or the Siemens Dimension RxL troponin I assay with URL of 70 ng/l.8 Baseline troponin levels measured >2 weeks before CABG were disregarded.
Patients were divided into three prespecified categories based on preoperative troponin levels: (1) stable baseline troponins: patients with preoperative levels below the 99th percentile URL for the assay used or with no troponin measurement and undergoing elective CABG; (2) elevated stable baseline troponins: patients with elevated preoperative troponins above the 99th percentile URL, with at least two measurements within 48 hours of each other, and the final preoperative level either lower or <20% higher than the earlier measurement; and (3) excluded: patients with elevated and rising, or no baseline troponin levels.
Two cut points for hs-TnT rise were prespecified: (1) 10-times 99th percentile URL1 of the hs-TnT assay (i.e. 140 ng/l); and (2) 10-times the coefficient of variation of 10% for the previous cut point of the fourth-generation troponin assay of 50 ng/l9 (i.e. 500 ng/l).
For patients with stable baseline troponins, a significant hs-TnT rise was defined as the post-operative hs-TnT being above the cut point. In patients with elevated stable baseline troponins, a significant hs-TnT rise required the post-operative hs-TnT to be above the cut point, and also a 20% rise from the preoperative level.
The prognostic significance of five prespecified potential criteria for the diagnosis of MI were: (1) hs-TnT rise >140 ng/l alone; (2) hs-TnT rise >500 ng/l alone; (3) new signs of MI on ECG and/or echocardiogram alone; (4) hs-TnT >140 ng/l plus ECG and/or echocardiographic criteria; and (5) hs-TnT rise >500 ng/l plus ECG and/or echocardiographic criteria.
Statistical analyses
Continuous and categorical variables are presented as mean±standard deviation or median (interquartile range, IQR) and n (%), respectively. Student t-test and Fisher’s Exact test for two groups or analysis of variance (ANOVA) and chi-squared for three or more groups were used for univariate analyses. Kaplan–Meier curves and log-rank (Mantel-Cox) test were performed for longitudinal survival analysis.
Receiver operating characteristics (ROC) analysis was also used to assess how well combinations of post-operative hs-TnT levels and ECG and/or echocardiographic criteria correlated with mortality and morbidity. The areas under ROC curve (AUC) and corresponding 95% confidence intervals (CI) and p-values were calculated using the Wald Test for pairwise comparison with chance.
Baseline and operative variables with p<0.10 in univariate analyses were entered into multivariate analyses. Logistic regression was used to calculate odds ratios (OR) or Cox proportional hazards regression for hazard ratio (HR) and 95% CI. Independent predictors of five potential MI criteria as well as 30-day mortality, medium-term mortality, and composite morbidity were determined. Each potential MI criteria was individually added to the models of post-operative outcomes to see whether they predicted these outcomes.
All tests were two tailed and p-values less than 0.05 were deemed statistically significant. SAS version 9.1 (SAS Institute, Cary, NC, USA) and Prism version 5 (GraphPad Software, San Diego, CA, USA) were used for analyses.
Results
Study population
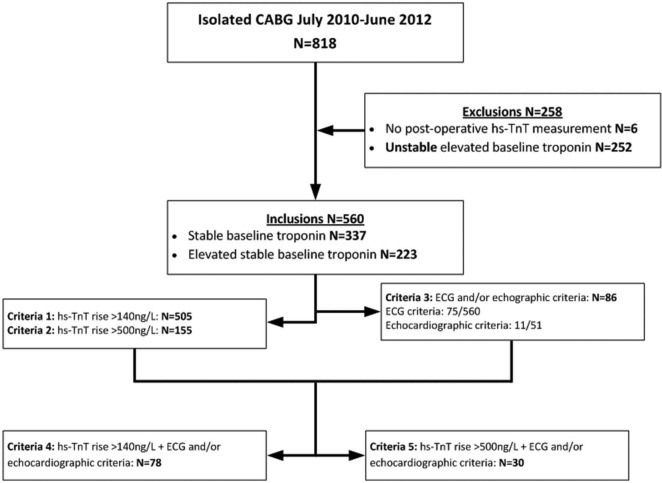
Figure 1 describes the study population. A total of 818 patients underwent isolated CABG during the 2-year study period. There were 258 patients excluded: six without post-operative hs-TnT measurements and 252 with elevated and rising preoperative troponins or lack of troponin measurements. There were 560 patients included: 337 having stable baseline troponins and 223 having elevated stable baseline troponins. Hs-TnT was measured on average 18.4±3.5 hours after beginning surgery. Mean follow up was 1.8±0.6 years, with all patients having at least 9 months follow up. New Q-waves or new LBBB were documented in 75 patients. Echocardiograms were performed in 51 patients, 11 of which showed new regional wall motion abnormalities. Only one post-operative coronary angiogram was performed, showing patent grafts and unchanged native vessels.
Figure 1.
Study population.
Clinical characteristics
Table 1 shows the baseline characteristics categorized by post-operative hs-TnT levels of ≤140, 141–500, and >500 ng/l. The medians (IQR) of hs-TnT in these groups were 116 (99–129), 282 (225–375), and 730 (603, 1100) ng/l respectively. There was no association between the time from operation to post-operative hs-TnT measurement and hs-TnT level (Pearson coefficient r=0.01, p=0.37).
Table 1.
Baseline characteristics.
| Post-operative high-sensitivity troponin T levels (ng/L) | ≤140 (n=38) | 141-500 (n=360) | >500 (n=162) | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 61.4 (9.9) | 64.2 (9.9) | 65.1 (9.6) | 0.105 |
| Male (%) | 76.3% (29) | 80.8% (291) | 80.2% (130) | 0.800 |
| Ethnicity (%) | 0.150 | |||
| Caucasian | 57.9% (22) | 58.3% (210) | 46.3% (75) | |
| Maori/Pacific Islander | 23.7% (9) | 20.8% (75) | 25.3% (41) | |
| Other | 23.7% (9) | 20.8% (75) | 28.4% (46) | |
| Body mass index (kg/m2) | 29.6 (5.8) | 29.4 (5.4) | 28.4 (4.9) | 0.102 |
| Presentation | ||||
| Canadian Cardiovascular Society Class IV angina (%) | 39.5% (15) | 35.0% (126) | 39.5% (64) | 0.571 |
| New York Heart Association Class IV dyspnoea (%) | 5.3% (2) | 2.5% (9) | 6.2% (10) | 0.109 |
| Recent myocardial infarction within 6 weeks (%) | 36.8% (14) | 43.1% (155) | 50.6% (82) | 0.163 |
| Pre-operative intra-aortic balloon pump (%) | 7.9% (3) | 5.8% (21) | 10.5% (17) | 0.166 |
| Urgent operation (%) | 71.1% (27) | 70.3% (253) | 73.5% (119) | 0.759 |
| Past medical history | ||||
| Myocardial infarction (%) | 55.3% (21) | 63.3% (228) | 66.7% (108) | 0.405 |
| Percutaneous coronary intervention (%) | 13.2% (5) | 9.2% (33) | 14.2% (23) | 0.209 |
| Coronary artery bypass grafting (%) | 2.6% (1) | 0.6% (2) | 3.7% (6) | 0.026 |
| Congestive heart failure (%) | 5.3% (2) | 4.1% (15) | 4.9% (8) | 0.897 |
| Atrial fibrillation (%) | 2.6% (1) | 6.7% (24) | 7.4% (12) | 0.565 |
| Diabetes (%) | 36.8% (14) | 35.0% (126) | 43.2% (70) | 0.200 |
| Diabetes on insulin (%) | 10.5% (4) | 7.5% (27) | 14.2% (23) | 0.055 |
| Hypercholesterolemia | 92.1% (35) | 91.7% (330) | 90.7% (147) | 0.930 |
| Hypertension | 63.2% (24) | 71.1% (256) | 59.9% (109) | 0.464 |
| Current smoker (%) | 15.8% (6) | 13.6% (49) | 12.3% (20) | 0.837 |
| Stroke (%) | 5.3% (2) | 5.6% (20) | 4.9% (8) | 0.959 |
| Peripheral vascular disease (%) | 0.0% (0) | 10.3% (37) | 13.0% (21) | 0.062 |
| Chronic respiratory disease (%) | 13.2% (5) | 17.8% (64) | 19.8% (32) | 0.622 |
| Dialysis (%) | 0.0% (0) | 0.6% (2) | 8.0% (13) | <0.001 |
| Investigations | ||||
| Left main stem stenosis >50% (%) | 55.3% (21) | 39.2% (141) | 48.1% (78) | 0.044 |
| Three-vessel disease (%) | 71.1% (27) | 77.2% (278) | 87.0% (141) | 0.014 |
| Ejection fraction (%) | 0.432 | |||
| Normal (>50%) | 73.7% (28) | 74.7% (269) | 67.3% (109) | |
| Mild impairment (40-50%) | 13.2% (5) | 14.2% (51) | 14.8% (24) | |
| Moderate impairment (30-40%) | 7.9% (3) | 6.4% (23) | 12.3% (20) | |
| Severe impairment (<30%) | 5.3% (2) | 4.6% (17) | 5.6% (9) | |
| Estimated glomerular filtration rate (mL/min) | 95 (34) | 82 (25) | 74 (35) | <0.001 |
| Pre-operative troponin groups | <0.001 | |||
| Normal (%) | 84.2% (32) | 62.2% (224) | 50.0% (81) | |
| Stable elevated (%) | 15.8% (6) | 37.8% (136) | 50.0% (81) | |
| EuroScore II(10) | 1.8% (1.0%) | 2.2% (3.1%) | 2.7% (3.5%) | 0.046 |
Post-operative outcomes
Table 2 shows operative and post-operative variables according to post-operative hs-TnT levels. Thirty-day mortality, medium-term mortality, and composite morbidity were 1.8, 2.9, and 16.3% respectively.
Table 2.
Operative and post-operative variables.
| Post-operative high-sensitivity troponin T levels (ng/L) | ≤140 (n=38) | 141-500 (n=360) | >500 (n=162) | P-value |
|---|---|---|---|---|
| Operation details | ||||
| Off-pump (%) | 13.2% (5) | 1.7% (6) | 2.5% (4) | <0.001 |
| Number of distal anastomoses | 0.002 | |||
| 1 | 5.3% (2) | 1.1% (4) | 0.6% (1) | |
| 2 | 28.9% (11) | 16.1% (58) | 10.5% (17) | |
| 3 | 44.7% (17) | 47.2% (170) | 55.6% (90) | |
| 4 | 18.4% (7) | 31.9% (115) | 23.5% (38) | |
| 5 | 2.6% (1) | 3.3% (12) | 9.3% (15) | |
| 6 | 0.0% (0) | 0.3% (1) | 0.6% (1) | |
| Left internal mammary artery graft (%) | 94.7% (36) | 98.3% (354) | 96.3% (156) | 0.204 |
| Right internal mammary artery graft (%) | 2.6% (1) | 3.9% (14) | 10.5% (17) | 0.008 |
| Radial artery graft (%) | 18.4% (7) | 26.1% (94) | 19.1% (31) | 0.164 |
| Saphenous vein grafts (%) | 86.8% (33) | 93.3% (336) | 95.7% (155) | 0.129 |
| Cardiopulmonary bypass time (minutes) | 85 (32) | 89 (23) | 97 (33) | 0.004 |
| Aortic cross-clamp time (minutes) | 54 (25) | 59 (19) | 63 (23) | 0.080 |
| Post-operative Outcomes | ||||
| ECG (new Q wave or left bundle branch block %) | 18.4% (7/38) | 13.1% (47/358) | 13.3% (21/158) | 0.630 |
| Echocardiogram (new regional wall motion abnormalities %) | 0.0% (0/1) | 8.7% (2/23) | 33.3% (9/27) | 0.094 |
| ECG and/or echocardiographic criteria (%) | 18.4% (7/38) | 13.7% (49/358) | 19.0% (30/158) | 0.271 |
| Composite morbidity (%) | 15.8% (6) | 10.6% (38) | 29.0% (47) | <0.001 |
| Stroke (%) | 0.0% (0) | 1.1% (4) | 1.9% (3) | 0.603 |
| Renal failure (%) | 5.3% (2) | 1.1% (4) | 1.9% (3) | 0.147 |
| Prolonged ventilation >24hours (%) | 10.5% (4) | 6.7% (24) | 22.8% (37) | <0.001 |
| Deep sternal wound infection (%) | 0.0% (0) | 0.3% (1) | 0.0% (0) | 0.757 |
| Reoperation (%) | 2.6% (1) | 3.9% (14) | 6.2% (10) | 0.430 |
| Operation to discharge (days) | 6.7 (2.8) | 7.8 (5.6) | 8.9 (5.7) | 0.028 |
| Re-admit to hospital within 30 days (%) | 26.3% (10) | 19.7% (71) | 20.4% (33) | 0.631 |
| 30-day mortality (%) | 2.6% (1) | 1.1% (4) | 3.1% (5) | 0.266 |
| Discharge medications | ||||
| Aspirin (%) | 97.3% (36/37) | 98.9% (353/357) | 98.7% (155/157) | 0.716 |
| Statin (%) | 94.6% (35/37) | 89.9% (321/357) | 86.0% (135/157) | 0.227 |
| Beta-blocker (%) | 89.2% (33/37) | 78.7% (281/357) | 73.9% (116/157) | 0.113 |
| Angiotensin converting enzyme blockers or Angiotensin II receptor blocker (%) | 32.4% (12/37) | 30.0% (107/357) | 29.3% (46/157) | 0.932 |
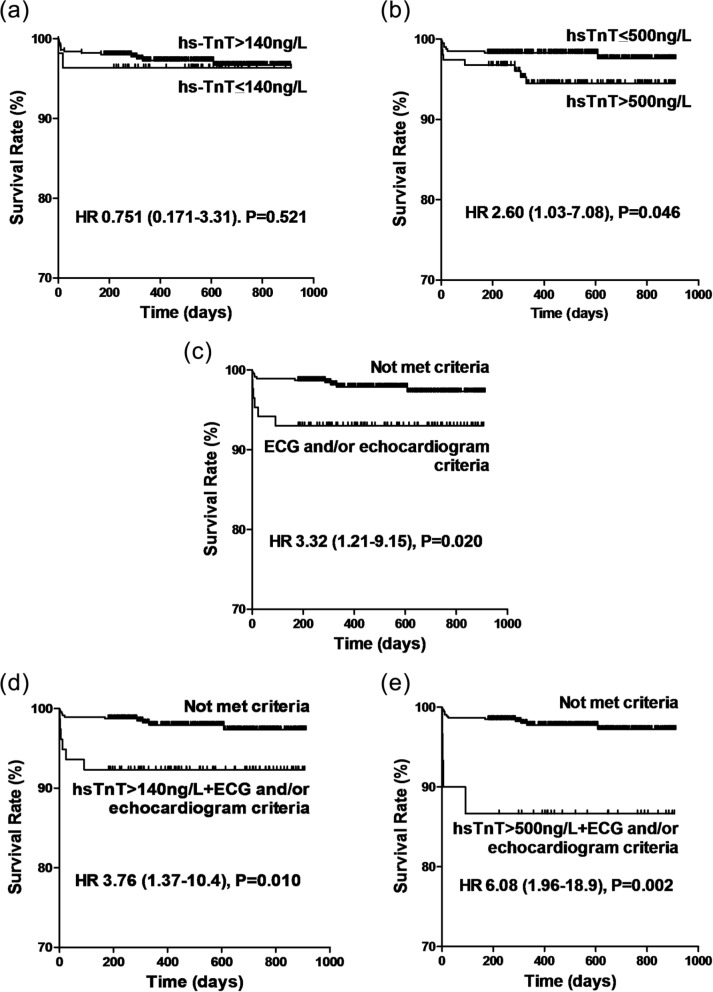
Figure 2 shows the Kaplan–Meier survival curves for the five potential MI criteria. Isolated hs-TnT >140 ng/l was not associated with mortality (Figure 2a; p=0.521). Isolated hs-TnT >500 ng/l was associated with mortality (HR 2.60, 95% CI 1.03–7.08; p=0.046; Figure 2b). ECG and/or echocardiographic criteria alone were also associated with mortality (HR 3.32, 95% CI 1.21–9.15; p=0.020; Figure 2c). When the ECG and/or echocardiographic criteria for MI were added to hs-TnT rise, the associations with mortality were strengthened. One-year survival rates for those with and without hs-TnT >140 ng/l and ECG and/or echocardiographic criteria were 92.% and 97.9% (HR 3.76, 95% CI 1.37–10.4; p=0.010; Figure 2d) and for hs-TnT >500 ng/l and ECG and/or echocardiographic criteria were 86.7 and 97.7% (HR 6.08, 95% CI 1.96–18.9; p=0.002; Figure 2e).
Figure 2.
Kaplan–Meier survival curves for five MI criteria: (a) hs-TnT >140 ng/l; (b) hs-TnT >500 ng/l; (c) ECG and/or echocardiographic criteria; (d) hs-TnT >140 ng/l and ECG and/or echocardiographic criteria; (e) hs-TnT >500 ng/l and ECG and/or echocardiographic criteria.
Results of log-rank test are shown.
Receiver operating characteristics analyses
Table 3 lists the results from the ROC analyses. Dual criteria were best at detecting 30-day mortality (AUC 0.693, 95% CI 0.521–0.865; p=0.036) and medium-term mortality (AUC 0.682, 95% CI 0.521–0.844; p=0.027). Hs-TnT >140 ng/l and ECG and/or echocardiographic data were the only criteria predicting 30-day mortality (AUC 0.683, 95% CI 0.519–0.857; p=0.029) and medium-term mortality (AUC 0.621, 95% CI 0.507–0.743; p=0.045). For predicting composite morbidity, hs-TnT alone had the highest AUC (AUC 0.658, 95% CI 0.589–0.726; p<0.001), although dual criteria were nearly as good (AUC 0.645, 95% CI 0.577–0.714; p=0.002). Of the single criteria, the cut point of hs-TnT >500 ng/l had the highest AUC (AUC 0.603, 95% CI 0.548–0.658; p>0.001).
Table 3.
Receiver-operating characteristics analysis and areas under curve with corresponding 95% confidence interval.
| Criteria | Operative mortality | Medium-term mortality | Composite morbidity |
|---|---|---|---|
| hs-TnT (continues) | 0.606 (0.379–0.832) | 0.634 (0.465–0.804) | 0.658 (0.589–0.726) |
| ECG and/or echocardiographic criteria | 0.676 (0.512–0.840) | 0.613 (0.490–0.737) | 0.540 (0.494–0.586) |
| hs-TnT (continues) + ECG and/or echocardiographic criteria | 0.693 (0.521–0.865) | 0.682 (0.521–0.844) | 0.645 (0.577–0.714) |
| hs-TnT >140ng/L | 0.551 (0.420–0.683) | 0.513 (0.429–0.598) | 0.520 (0.483–0.558) |
| hs-TnT >500ng/L | 0.565 (0.404–0.726) | 0.617 (0.489–0.745) | 0.603 (0.548–0.658) |
| hs-TnT >140ng/L + ECG and/or echocardiographic criteria | 0.683 (0.519–0.857) | 0.641 (0.527–0.763) | 0.549 (0.503–0.594) |
| hs-TnT>500ng/L + ECG and/or echocardiographic criteria | 0.625 (0.475–0.775) | 0.601 (0.491–0.711) | 0.561 (0.522–0.599) |
All figures are areas under receiver-operative characteristics curve and 95% confidence intervals, hs-TnT = high-sensitivity troponin T.
The hs-TnT levels with maximum sensitivity × specificity for detecting 30-day and medium-term mortality were 580 ng/l alone or 200 ng/l as dual criteria. Corresponding figures for detecting composite morbidity were 410 and 200 ng/l.
Multivariate analyses
Table 4 shows on multivariate analyses that the features associated with patients having hs-TnT >140 ng/l and ECG and/or echocardiographic criteria for MI (n=78/560, 13.9%) were Canadian Cardiovascular Society class 4 angina (p=0.017), and longer cardiopulmonary bypass time (p=0.048). The features associated with patients having hs-TnT >500 ng/l and ECG and/or echocardiographic criteria for MI (n=30/560, 5.4%) were dialysis (p=0.032) and longer cardiopulmonary bypass time (p=0.006).
Table 4.
Multivariate predictors of troponin rise and criteria for myocardial infarction.
| Predictors | OR | 95%CI | P-value |
|---|---|---|---|
| hs-TnT >140ng/L (n=505) | |||
| Peripheral vascular disease | 11.5 | 0.74–179 | 0.082 |
| Estimated glomerular filtration rate (per 10ml/min decrease) | 1.15 | 1.04–1.27 | 0.024 |
| Off-pump | 0.10 | 0.04–0.32 | <0.001 |
| hs-TnT >500ng/L (n=155) | |||
| Body mass index (per 1 kg/m2) | 0.96 | 0.92–1.00 | 0.075 |
| Dialysis | 8.78 | 1.79–43.1 | 0.008 |
| Previous coronary artery bypass grafting | 4.37 | 1.02–18.6 | 0.047 |
| Three-vessel disease | 1.75 | 0.97–3.15 | 0.063 |
| Cardiopulmonary bypass time (per 10 minutes) | 1.08 | 1.00–1.17 | 0.038 |
| ECG and/or echocardiographic criteria (n=86) | |||
| None | |||
| hs-TnT >140ng/L + ECG and/or echocardiographic criteria (n=78) | |||
| Canadian Cardiovascular Society Class IV angina | 1.94 | 1.13–3.33 | 0.017 |
| Cardiopulmonary bypass time (per 10 minutes) | 1.09 | 1.00–1.18 | 0.048 |
| hs-TnT >500ng/L + ECG and/or echocardiographic criteria (n=30) | |||
| Diabetes on insulin | 2.46 | 0.91–6.71 | 0.078 |
| Dialysis | 4.58 | 1.14–18.4 | 0.032 |
| EuroScore II(10) | 1.11 | 1.00–1.22 | 0.061 |
| Cardiopulmonary bypass time (per 10 minutes) | 1.17 | 1.05–1.31 | 0.006 |
OR = odds ratio, 95% CI = 95% confidence interval, hs-TnT high sensitivity troponin T.
Table 5 shows the variables predictive for 30-day and medium-term mortality. Both hs-TnT >140 ng/l with ECG and/or echocardiographic criteria and ECG and/or echocardiographic criteria alone predicted 30-day mortality (OR 6.12, 95% CI 1.50–25.0, p=0.012; OR 5.93, 95% CI 1.46–24.2, p=0.013, respectively). Other predictors of 30-day mortality were NYHA class 4 dyspnoea (p=0.016), not using left internal mammary grafts, (p=0.008), and return to theatre (p=0.049).
Table 5.
Multivariate predictors of mortality.
| Predictors | OR or HR | 95%CI | P-value |
|---|---|---|---|
| 30-day mortality | |||
| Female | 3.74 | 1.03–13.6 | 0.045 |
| Maori/Pacific Islander | 3.25 | 0.82–13.0 | 0.095 |
| New York Heart Association Class IV dyspnoea | 11.4 | 2.58–50.8 | 0.001 |
| Left internal mammary artery graft | 0.06 | 0.01–0.63 | 0.02 |
| Post-operative hs-TnT (per 100ng/L) | 1.07 | 0.93–1.23 | 0.36 |
| hs-TnT >140ng/L | 0.37 | 0.06–2.23 | 0.277 |
| hs-TnT >500ng/L | 0.84 | 0.21–3.34 | 0.803 |
| ECG and/or echocardiographic criteria | 4.68 | 1.28–17.2 | 0.020 |
| hs-TnT >140ng/L + ECG and/or echocardiographic criteria | 4.92 | 1.34–18.1 | 0.017 |
| hs-TnT>500ng/L + ECG and/or echocardiographic criteria | 2.59 | 0.46–14.6 | 0.281 |
| Medium-term mortality | HR | ||
| Estimated glomerular filtration rate (per 10ml/min decrease) | 1.34 | 1.03–1.73 | 0.021 |
| Left internal mammary artery graft | 0.11 | 0.02–0.53 | 0.006 |
| Post-operative hs-TnT (per 100ng/L) | 1.04 | 1.00–1.08 | 0.045 |
| hs-TnT >140ng/L | 1.03 | 0.20–5.41 | 0.970 |
| hs-TnT >500ng/L | 1.51 | 0.51–4.42 | 0.456 |
| ECG and/or echocardiographic criteria | 3.28 | 1.08–9.98 | 0.036 |
| hs-TnT >140ng/L + ECG and/or echocardiographic criteria | 3.44 | 1.13–10.5 | 0.030 |
| hs-TnT >500ng/L + ECG and/or echocardiographic criteria | 2.73 | 0.76–9.80 | 0.123 |
OR = odds ratio, HR = hazards ratio, 95%CI = 95% confidence interval, hs-TnT = high-sensitivity troponin T.
Factors predicting medium-term mortality were estimated glomerular filtration rate (p=0.021), not using left internal mammary grafts (p=0.006), and level of post-operative hs-TnT (p=0.045). Levels of hs-TnT >140 ng/l with ECG and/or echocardiographic criteria also predicted medium-term survival (HR 3.44, 95% CI 1.13–10.5; p=0.031) but hs-TnT >500 ng/l with ECG and/or echocardiographic criteria did not (p=0.125).
Table 6 shows the predictors of post-operative composite morbidity. These included Maori or Pacific Island ethnicity (p=0.021) use of an intra-aortic balloon pump (p<0.001), previous CABG (p=0.037), and cardiopulmonary bypass time (p=0.002).
Table 6.
Multivariate predictors of composite morbidity.
| Predictor | OR | 95%CI | P-value |
|---|---|---|---|
| Maori/Pacific Islander | 1.86 | 1.02–3.40 | 0.043 |
| Pre-operative intra-aortic balloon pump | 5.39 | 2.29–12.7 | <0.001 |
| Previous coronary artery bypass grafting | 7.12 | 1.13–44.7 | 0.037 |
| Ejection fraction (<40%) | 1.95 | 0.95–4.01 | 0.072 |
| EuroScore II(10) | 1.09 | 1.01–1.16 | 0.035 |
| Cardiopulmonary bypass time (per 10 minutes) | 1.16 | 1.06–1.27 | 0.002 |
| Post-operative hs-TnT (per 100ng/L) | 1.06 | 1.01–1.10 | 0.014 |
| hs-TnT >140ng/L | 0.91 | 0.36–2.32 | 0.846 |
| hs-TnT >500ng/L | 2.20 | 1.27–3.82 | 0.005 |
| ECG and/or echocardiographic criteria | 1.64 | 0.84–3.21 | 0.151 |
| hs-TnT >140ng/L + ECG and/or echocardiographic criteria | 1.75 | 0.89–3.46 | 0.098 |
| hs-TnT >500ng/L + ECG and/or echocardiographic criteria | 3.02 | 1.18–7.76 | 0.022 |
OR = odds ratio, 95%CI = 95% confidence interval, hs-TnT = high-sensitivity troponin.
The level of post-operative troponins was also predictive of composite morbidity (p=0.014). Isolated levels of hs-TnT >500 ng/l were predictive (p=0.005) as well as hs-TnT levels >500 ng/l and ECG and/or echocardiographic criteria for MI (p=0.022).
Discussion
There are several novel findings of this study. Firstly, using a guideline-compliant high-sensitivity assay,8 dual criteria of hs-TnT levels >140 ng/l associated with ECG and/or echocardiographic criteria were predictive of 30-day and medium-term mortality after CABG. A higher cut point of >500 ng/l associated with ECG and/or echocardiographic changes was not associated with mortality.
Secondly, the finding of elevated hs-TnT >10-times the URL and associated ECG and/or echocardiographic evidence of MI validates the recommendation of Third Universal Definition of MI with hs-TnT.1 Thirdly, higher hs-TnT levels predicted post-operative morbidity.
Prognostic value of isolated high-sensitivity troponin T rise
Post-operative hs-TnT alone as a continuous parameter independently predicted medium-term mortality and composite morbidity. Previous studies with various contemporary troponin T and troponin I assays have reported similar findings.3–6,12 The level of post-operative hs-TnT >140 ng/l (10-times the URL) was found in 90% of patients, whereas hs-TnT >500 ng/l (36-times the URL) were found in 29% of patients.
Isolated hs-TnT >140 ng/l was not associated with mortality or composite morbidity. Isolated hs-TnT >500 ng/l was associated with medium-term mortality on univariate analysis and predicted composite morbidity. These findings of a requirement of a high cut point are similar to the findings of other studies with various assays, including point of care, none of which are guideline compliant,13 using cut points 7.8–170-times URL.4,7,14,15 However, we found on multivariate analysis that isolated hs-TnT >500 ng/l was not related to mortality.
Hs-TnT >500 ng/l was an independent predictor of composite morbidity, consistent with elevation of hs-TnT being a marker of myocardial injury due to multiple causes and not restricted to ischaemia (e.g. heart failure, sepsis, renal failure).16
ECG and/or echocardiographic features of MI
This study shows that new signs of infarction on the ECG and/or evidence of new wall motion abnormality on echocardiography are important predictors of post-CABG mortality. The prevalence of ECG signs of infarction was relatively uniform across the three thresholds of hs-TnT rise, including below 140 ng/l. This suggests that if an isolated hs-TnT threshold is set too high, some patients with poor prognosis will be missed. ECG and/or echocardiographic criteria however did not predict composite post-operative morbidity, showing their specificity for MI.
There were 11 patients with new regional wall motion abnormalities out of 51 patients who had post-operative echocardiograms, all of whom had no new ECG changes but met the Universal Definition criteria for MI. The diagnosis of MI would have been missed if an echocardiogram was not performed in these patients.
Dual elevation of high-sensitivity troponin plus ECG and/or echocardiographic criteria
Our results showed that dual criteria are more prognostic of mortality than single criteria. Fourteen percent (78/560) of patients had an MI as defined by the dual criteria corresponding to the Universal Definition in our cohort, where the hs-TnT threshold was 140 ng/l (10-times 99th percentile URL). This criteria was the strongest predictor of both 30-day and medium-term mortality in both the ROC and multivariate analyses amongst the five criteria assessed. It utilises the strengths of both hs-TnT’s sensitivity for myocardial injury with the specificity of new Q-waves and new LBBB on ECG for MI.
A higher threshold for hs-TnT >500 ng/l with ECG and/or echocardiographic criteria did not independently predict mortality at 30 days or medium term. This may be because the dual criteria, although very specific for adverse outcomes, compromise its sensitivity and therefore fails to capture the majority of post-operative mortality throughout the follow-up period. As the ECG and/or echocardiogram are very specific for MI after CABG, a lower threshold for hs-TnT rise for dual criteria than single criteria has better prognostic value, as shown by our results for hs-TnT >140 ng/l with ECG and/or echocardiogram criteria.
The highest AUCs for mortality we found with dual criteria were 0.64–0.70, while previous studies have reported area under ROC curves of 0.73–0.82 using single criteria with contemporary I or T troponin assays.3,7 A potential reason for our finding of a lower area under the ROC curve may be that the superior sensitivity of the hs-TnT assay may have compromised its specificity and positive predictive value
The biomarker cut point for the Universal Definition for MI associated with CABG in the 2007 guidelines was 5-times the URL.17 The increase to 10-times the URL threshold for biomarker elevation in the 2012 Universal Definition of MI URL was arbitrary.1 Troponins are preferred because of their sensitivity and specificity.1,9 Creatine kinase MB, although a proven predictor of mortality after CABG, has been shown to be inferior to troponins in predicting adverse cardiovascular outcomes.2,3
The importance of stable baseline troponin levels
To diagnose MI appropriately, a stable baseline of troponin is required. We thus included patients with ‘normal stable baseline’ and ‘elevated stable baseline’ troponins. Patients with elevated but rising troponin levels were excluded because post-operative troponin rises cannot be accurately distinguished from an index MI and be attributed to an MI after CABG.18
The Universal Definition for MI after CABG does not define the amount of rise required in patients with ‘elevated stable baseline troponins’. We used the 20% elevation from baseline criteria, defined by the Universal Definition for MI with PCI.1
Independent predictors of MI and mortality
Unstable angina and cardiopulmonary bypass time were identified as independent predictors of MI (defined as hs-TnT >140 ng/l with ECG and/or echocardiographic criteria), both of which have been previously reported with other definitions of MI.19
In addition to MI, predictors of 30-day or medium term mortality were female sex, NYHA class IV dyspnoea, and reduced renal function, all of which are incorporated in the EuroScore20 and the Society of Thoracic Surgeons score.11 We also found, as reported by others,21 that revascularization with the left internal mammary artery was significantly associated with lower mortality.
Implications
The presence of predictors of MI should alert clinicians to the importance of their management. As the occurrence of MI is associated with mortality and other adverse outcomes such as inotropic requirement5 and longer ventilation time,19 treatments proven to improve survival after CABG, including aspirin,22 statins,23 and beta-blockers24 should be administered. A lower threshold for angiography and return to theatre, in patients requiring inotropes or with prolonged ventilation should be considered.4
The 30-day mortality rate is similar to that reported for 2009 by the STS database; 1.8 vs. 1.9% STS.25 However, the rate of MI we found (14%) is higher than previously reported using conservative definitions. This means that more patients will be labelled with the diagnosis. However, there is an opportunity to improve outcomes in these patients. More research is required to assess which therapies may reduce the occurrence of MI, and adverse outcomes after MI.
Study limitations
This is a retrospective observational study from one centre. About 20% of patients were excluded because of rising or missing baseline troponin levels. The timing of troponin measurements post-operatively was at 12–24 hours and not at a fixed time. The moderate cohort size means some of the outcomes might have been underpowered for analyses. A small number of patients had post-operative cardiac imaging such as echocardiography, based on clinical indications.
Conclusions
This study shows the utility of hs-TnT in detecting MI using a cut point of 10-times 99th percentile URL with ECG and/or echocardiographic findings. Diagnosis of MI with dual criteria was related to 30-day and medium-term mortality. These results validate the Third Universal Definition of MI using hs-TnT as the biomarker.
Acknowledgments
We would like to thank all our colleagues affiliated with the Green Lane Cardiovascular Service for their contributions. We also thank Charlene Nell, for excellent secretarial assistance.
Footnotes
Conflict of interest: HW has received research grants from Sanofi Aventis, Eli Lilly, Medicines Company, Pfizer, Roche, Johnson & Johnson, Schering Plough, Merck Sharpe & Dohme, Astra Zeneca, GlaxoSmithKline, Daiichi Sankyo Pharma Development, and Bristol-Myers Squibb and has served on advisory boards for Merck Sharpe & Dohme, Roche, and Regado Biosciences. TW, RS, TR, NK and GG declare that they have no conflicts of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Thygesen K, Alpert JS, Jaffe AS, et al. ; for the Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction Third universal definition of myocardial infarction. Eur Heart J 2012; 33: 2551–2567 [DOI] [PubMed] [Google Scholar]
- 2. Januzzi JL, Lewandrowski K, MacGillivray TE, et al. A comparison of cardiac troponin T and creatine kinase-MB for patient evaluation after cardiac surgery. J Am Coll Cardiol 2002; 39: 1518–1523 [DOI] [PubMed] [Google Scholar]
- 3. Muehlschlegel JD, Perry TE, Liu KY, et al. Troponin is superior to electrocardiogram and creatinine kinase MB for predicting clinically significant myocardial injury after coronary artery bypass grafting. Eur Heart J 2009; 30: 1574–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Croal BL, Hillis GS, Gibson PH, et al. Relationship between postoperative cardiac troponin I levels and outcome of cardiac surgery. Circulation 2006; 114: 1468–1475 [DOI] [PubMed] [Google Scholar]
- 5. Martin CB, Shaw AD, Gal J, et al. The comparison and validity of troponin I assay systems in diagnosing myocardial ischemic injury after surgical coronary revascularization. J Cardiothorac Vasc Anesth 2005; 19: 288–293 [DOI] [PubMed] [Google Scholar]
- 6. Pegg TJ, Maunsell Z, Karamitsos TD, et al. Utility of cardiac biomarkers for the diagnosis of type V myocardial infarction after coronary artery bypass grafting: insights from serial cardiac MRI. Heart 2011; 97: 810–816 [DOI] [PubMed] [Google Scholar]
- 7. Mohammed AA, Agnihotri AK, van Kimmenade RR, et al. Prospective, comprehensive assessment of cardiac troponin T testing after coronary artery bypass graft surgery. Circulation 2009; 120: 843–850 [DOI] [PubMed] [Google Scholar]
- 8. Apple FS, Collinson PO. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012; 58: 54–61 [DOI] [PubMed] [Google Scholar]
- 9. Giannitsis E, Kurz K, Hallermayer K, et al. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem 2010; 56: 254–261 [DOI] [PubMed] [Google Scholar]
- 10. Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg 2012; 41: 734–744; discussion 744–745. [DOI] [PubMed] [Google Scholar]
- 11. Shahian DM, O’Brien SM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1 – coronary artery bypass grafting surgery. Ann Thorac Surg 2009; 88: S2–S22 [DOI] [PubMed] [Google Scholar]
- 12. Domanski MJ, Mahaffey K, Hasselblad V, et al. Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. JAMA 2011; 305: 585–591 [DOI] [PubMed] [Google Scholar]
- 13. Apple FS. A new season for cardiac troponin assays: it’s time to keep a scorecard. Clin Chem 2009; 55: 1303–1306 [DOI] [PubMed] [Google Scholar]
- 14. Carrier M, Pellerin M, Perrault L, et al. Troponin levels in patients with myocardial infarction after coronary artery bypass grafting. Ann Thorac Surg 2000; 69: 435–440 [DOI] [PubMed] [Google Scholar]
- 15. Lim CC, Cuculi F, van Gaal WJ, et al. Early diagnosis of perioperative myocardial infarction after coronary bypass grafting: a study using biomarkers and cardiac magnetic resonance imaging. Ann Thorac Surg 2011; 92: 2046–2053 [DOI] [PubMed] [Google Scholar]
- 16. White HD. Pathobiology of troponin elevations: do elevations occur with myocardial ischemia as well as necrosis? J Am Coll Cardiol 2011; 57: 2406–2408 [DOI] [PubMed] [Google Scholar]
- 17. Thygesen K, Alpert JS, White HD; on behalf of the Joint ESC-ACC-AHA-WHF Task Force for the Redefinition of Myocardial Infarction Universal definition of myocardial infarction. Eur Heart J 2007; 28: 2525–2538 [DOI] [PubMed] [Google Scholar]
- 18. Miller WL, Garratt KN, Burritt MF, et al. Baseline troponin level: key to understanding the importance of post-PCI troponin elevations. Eur Heart J 2006; 27: 1061–1069 [DOI] [PubMed] [Google Scholar]
- 19. Onorati F, De Feo M, Mastroroberto P, et al. Determinants and prognosis of myocardial damage after coronary artery bypass grafting. Ann Thorac Surg 2005; 79: 837–845 [DOI] [PubMed] [Google Scholar]
- 20. Nashef SA, Roques F, Michel P, et al. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 1999; 16: 9–13 [DOI] [PubMed] [Google Scholar]
- 21. Cameron A, Davis KB, Green G, et al. Coronary bypass surgery with internal-thoracic-artery grafts - effects on survival over a 15-year period. N Engl J Med 1996; 334: 216–219 [DOI] [PubMed] [Google Scholar]
- 22. Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy – II: Maintenance of vascular graft or arterial patency by antiplatelet therapy. BMJ 1994; 308: 159–168 [PMC free article] [PubMed] [Google Scholar]
- 23. Liakopoulos OJ, Choi YH, Haldenwang PL, et al. Impact of preoperative statin therapy on adverse postoperative outcomes in patients undergoing cardiac surgery: a meta-analysis of over 30,000 patients. Eur Heart J 2008; 29: 1548–1559 [DOI] [PubMed] [Google Scholar]
- 24. Ferguson TB Jr, Coombs LP, Peterson ED. Preoperative beta-blocker use and mortality and morbidity following CABG surgery in North America. JAMA 2002; 287: 2221–2227 [DOI] [PubMed] [Google Scholar]
- 25. ElBardissi AW, Aranki SF, Sheng S, et al. Trends in isolated coronary artery bypass grafting: an analysis of the Society of Thoracic Surgeons adult cardiac surgery database. J Thorac Cardiovasc Surg 2012; 143: 273–281 [DOI] [PubMed] [Google Scholar]