Abstract
Background:
Current ESC guidelines for the diagnosis of myocardial infarction consider a rise and/or fall of cardiac biomarkers. However, whether rising or falling patterns of high-sensitivity cardiac troponin T (hs-cTnT) improve the discrimination of ST-elevation myocardial infarction (non-STEMI) from non-acute coronary syndromes (ACS) has not been evaluated yet.
Methods:
We compared protocols of rising and falling absolute and relative hs-cTnT changes in an unselected emergency department population.
Results:
A total of 635 patients with unstable angina pectoris (UAP), non-STEMI, or acute symptoms and increased hs-cTnT (>99th percentile) were enrolled. Of these, 572 patients met the inclusion criteria of consistently rising patterns (n=254, 44.4%), consistently falling patterns (n=224, 39.2%), or falling patterns after an initial rise (n=94, 16.4%). Final diagnoses included 66 (11.5%) patients with UAP, 141 (24.7%) patients with non-STEMI, and 365 (63.8%) patients with hs-cTnT elevations not due to ACS. Rising values were found more frequently in patients with non-STEMI, as compared to non-ACS (OR 3.69, 95% CI 2.46–5.53; p<0.0001), and falling patterns were observed more frequently in patients with non-ACS conditions (OR 3.56, 95% CI 2.24–5.63; p<0.001). Addition of rising but not falling changes increased diagnostic performance of hs-cTnT concentrations at presentation: positive: AUC 0.680 (95% CI 0.618–0.742) vs. 0.861 (95% CI 0.822–0.900; p<0.0001), negative: AUC 0.678 (95% CI 0.545–0.812) vs. 0.741 (95% CI 0.635–0.847). A 20% criterion as proposed by ESC guidelines performed equally for positive and negative changes only when admission hs-cTnT values were considered: AUC 0.785 (95% CI 0.726–0.845) vs. AUC 0.763 (95% CI 0.681–0.845); p=ns.
Conclusions:
Detection of rising but not falling hs-cTnT values improves discrimination of non-STEMI from non-ACS in an unselected emergency department population.
Keywords: Diagnostic performance, high-sensitivity troponin T, negative changes, non-STEMI, positive changes
Introduction
Cardiac biomarkers play a key role in the management of patients with acute coronary syndromes (ACS) and thus are an essential part of the universal definition of acute myocardial infarction according to current ESC guidelines.1 As recommended by the ESC/ACCF/AHA/WHF Task Force for the redefinition of AMI, a rise and/or fall of cardiac biomarkers with at least one value exceeding the 99th percentile of the upper reference limit together with evidence of myocardial ischaemia is needed to make the diagnosis of acute myocardial infarction.2 Serial measurements of cardiac troponin (cTn) are required to distinguish acute elevations from those that may be associated with stable coronary or structural heart disease.3–8
At present, falling patterns which suggest a subacute event rank similar to rising patterns in their diagnostic value. Whether this is appropriate has, to the best of our knowledge, not been investigated in detail so far.
Accordingly, the scope of this analysis was to evaluate if kinetic changes add diagnostic information to baseline high-sensitivity cardiac troponin T (hs-cTnT concentrations and if positive or negative changes are equally effective in discriminating non-ST-elevation myocardial infarction (non-STEMI) and non-ACS. Furthermore, we evaluated whether rising and falling changes of 20%, as proposed by ESC guidelines, perform equally in discriminating non-STEMI and non-STE-ACS in unselected patients seen in an emergency department.
Methods
During a 6-month period, we screened 3327 consecutive patients presenting with acute symptoms to the internal medicine emergency department, including chest pain, dyspnoea, or unspecific symptoms including abdominal pain, nausea, fainting, or diaphoresis. To evaluate the relative importance of rising versus falling hs-cTnT concentrations, we included all consecutive patients with suspected non-STEMI ACS and all patients presenting with acute symptoms who had at least one hs-cTnT value above the 99th percentile of the upper reference range (URL).
Patients were triaged and treated at the discretion of the physician on duty considering not only admission hs-cTnT values and kinetic changes but also the GRACE risk score and the clinical presentation. Amongst patients with elevated hs-cTnT, the final diagnoses were subsequently readjudicated by two cardiologists into non-STEMI, unstable angina pectoris (UAP; if a cardiac cause of chest pain was most likely and hs-cTnT changes did not fulfil non-STEMI criteria according to the universal definition), or non-ACS conditions taking into account all available clinical data including chest pain characteristics, previous cardiac history, 12-lead ECG, coronary angiography, predischarge stress testing, or imaging modalities including cardiac magnetic resonance imaging. These physicians had access to the hs-cTnT data and diagnosis of non-STEMI was made according to the criteria of the third universal definition of myocardial infarction considering both rising or falling patterns.2 We excluded patients with ST-segment elevation or new left bundle branch block on presentation. We also excluded patients with non-STEMI post percutaneous coronary intervention (PCI) in whom a definite diagnosis of unstable angina or non-STEMI could not be established before PCI. However, we did not exclude patients with declining hs-cTnT values after PCI.
In the absence of clinical evidence suggestive of myocardial ischaemia, elevated hs-cTnT was interpreted as unrelated to AMI and the underlying reason of myocardial damage was sought actively including imaging, clinical and biochemical investigations. AMI without further differentiation into type I or type II MI was diagnosed according to the criteria of the Joint ESC/AACF/AHA/WHF Task Force definition.2
Diagnosis of AMI required the detection of a rising and/or falling pattern of the hs-cTnT assay with at least one of the following: symptoms of ischaemia, development of Q waves on ECG, or imaging evidence of new loss of viable myocardium. All patients with exclusively rising or falling patterns and those with falling patterns following an initial rise qualified for analysis. As the optimal magnitude of the rise and/or fall for the diagnosis of AMI is still not established, we analysed the effect of a broad range of relative-change cut offs from a minimum of 20% to 250% and tested its impact on diagnostic performance within 6 hours.4,5,8 In addition, we analysed the effect of incremental cut-offs for absolute concentration changes from 5 ng/l to 100 ng/l.3,7,9 ROC-optimized cut-offs for rising and falling kinetic changes were considered as diagnostic criterion for AMI. A minimal absolute concentration change of ≥5 ng/l between baseline and the highest consecutive value was used to adjust for high δ-changes at low absolute cTn concentrations, as suggested by Mueller et al.9
The relative change of hs-cTnT within 3 or 6 hours was calculated as Cmax (3h or 6h) – Cbaseline) divided by Cbaseline multiplied by 100, and is reported as percentage rise or fall. Negative percentage changes indicated a fall whereas positive changes marked a rise.
All medical decisions including the need and timing of coronary angiography, coronary intervention, or further diagnostic work up were left at the discretion of the interventional cardiologist on duty.
The study was performed according to the principles of the Declaration of Helsinki and approved by the local ethics committee. Written informed consent was obtained from all participating patients. Follow up was accomplished via telephone contact or questionnaire at least 6 months after discharge.
Laboratory measurements
Cardiac troponin was measured on COBAS E411 using the novel hs-cTnT assay (Roche Diagnostics, Rotkreuz, Switzerland) which is commercially available in Germany (not yet available in the USA). The limit of blank (3 ng/l) and limit of detection (5 ng/l) were determined in accordance with CLSI guideline EP17-A. The inter-assay coefficient of variation (CV) was 8% at 10 ng/l and 2.5% at 100 ng/l. The intra-assay CV was 5% at 10 ng/l and 1% at 100 ng/l.10 Normal reference values were established from a multicentre reference study and the 99th percentile value was determined at 14 ng/l.11 CVs were not reassessed for our central laboratory. A recent issue regarding lot-to-lot variation of the hs-cTnT assay was considered.12 None of the affected lots was used in this study. Furthermore, this problem affected particularly low concentration measurements (<8–20 ng/l) but only patients with admission hs-cTnT values exceeding 14 ng/l were enrolled in our study.
Statistical analysis
Continuous variables were tested for normal distribution using the D’Agostino-Pearson test and were presented either as means±standard deviation, or as medians (interquartile range). Groups were compared using the chi-squared test for categorical variables and analysis of variance for continuous variables. Independent samples were compared using the Mann–Whitney test. Alternatively, we used ANOVA after logarithmic transformation of the data. If the ANOVA test was found positive (p<0.05), then Student–Newman–Keuls test for pairwise comparison of subgroups was applied. We determined diagnostic performance for absolute and relative changes and rising and falling patterns within 6 hours from receiver-operating characteristic (ROC) curves on the basis of the continuously measured biomarker levels and compared areas-under-the-curve (AUC) using the test of DeLong et al.13 ROC-optimized cut-off-values were calculated using the point closest to the upper left corner according to the method proposed by Zweig and Campbell.14 In addition, we calculated sensitivities, specificities, negative predictive, and positive predictive values for rising and falling patterns of absolute and relative changes after 3 and 6 hours for classification of final diagnosis of non-STEMI. Diagnostic performance of admission hs-cTnT values alone and combined with ROC-optimized and 20% rising or falling kinetic changes was compared using integrated discrimination improvement index (IDI) and net reclassification index (NRI) calculation.15
A sample size calculation for the comparison of rising and falling kinetic changes could not be performed due to the lack of previous studies on this topic. However, to support the added value of kinetic changes over baseline values with a very conservative increase of AUC from 0.7 to 0.8 (with a type 1 error of 0.05 and a type II error of 0.2), a sample size of 203 cases per group would be needed.
SPSS 20.0 (IBM, Armonk, New York, USA) and MedCalc 11.1 (MedCalc, Mariakerke, Belgium) statistical software packages were used. All tests were two -tailed and a p-value <0.05 was considered statistically significant.
Results
Baseline
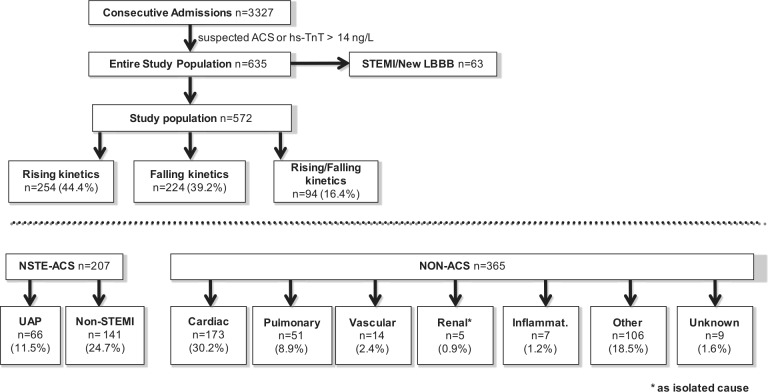
During 6 months, 635 of 3327 consecutive patients presenting to the emergency department were identified to have either unstable angina, or non-STEMI, or acute symptoms together with an hs-cTnT value >99th percentile URL. A total of 63 patients (9.9%) presenting with ST-segment elevation or presumably new left bundle branch block were excluded. Per inclusion criteria, a baseline hs-cTnT result and at least one additional hs-cTnT measurement within 6 hours was available in all patients. The final study population comprised 572 patients including patients with exclusively rising patterns (n=254, 44.4%), exclusively falling patterns (n=224, 39.2%), and falling patterns following an initial rise (n=94, 16.4%; Figure 1). No patient with an unchanged value was observed.
Figure 1.
Flow chart of study enrolment.
By diagnostic categories, the study group included 66 (11.5%) patients with an adjudicated diagnosis of UAP, 141 (24.7%) patients with non-STEMI, and 365 (63.8%) patients with hs-cTnT elevations not due to ACS. Categories of non-ACS-related conditions and corresponding numbers of patients in each category are listed in Figure 1. In patients with rising and falling kinetic changes, more hs-cTnT samples were taken than in patients with exclusively rising or falling patterns: rise/fall: 4.1±1.1 vs. 3.8±1.4 (rising) and 3.8±1.2 (falling); p<0.05. In addition, more hs-cTnT samples were taken in patients that received a final diagnosis of ACS: 4.4±1.2 (ACS) vs. 3.2±1.2 (non-ACS); p<0.0001.
The baseline characteristics of the entire study population subdivided by pattern characteristics are displayed in Table 1.
Table 1.
Baseline demographic, laboratory, and angiographic characteristics subdivided by non-STEMI, unstable angina, and non-ACS-related hs-cTnT elevation.
| Rising kinetic changes (n=254) | Falling kinetic changes (n=224) | Rising/falling kinetic changes (n=94) | |
|---|---|---|---|
| Age (years) | 71.1±13.0 | 73.6 (65.7–80.6) | 74.9 (68.9–80.7) |
| Age >75 (years) | 105 (41.3) | 97 (43.3) | 46 (48.9) |
| No. of hs cTnT samples/patient | 3.8±1.4 | 3.8±1.2 | 4.1±1.1* |
| Male gender | 171 (67.3) | 134 (59.8) | 61 (64.9) |
| NT-proBNP | 2359 (805.5–7593.8) | 2438.0 (732.0–7029.0) | 4129.0 (618.0–7301.0) |
| eGFR (ml/min/1.73m2) | 67.3±30.2 | 63.9±29.5 | 60.3±29.8 |
| eGFR<60 ml/min/1.73m2 | 110 (43.3) | 105 (46.9) | 50 (53.2) |
| eGFR<60 as isolated cause for hs-cTnT elevation | 1 (0.4) | 3 (1.3) | 1 (1.1) |
| Diagnosis | |||
| Non-STEMI | 96 (37.8) | 27 (12.1)*** | 18 (19.1)** |
| UAP | 26 (10.2) | 27 (12.1) | 13 (13.8) |
| Non-ACS | 132 (52.0) | 170 (75.9)*** | 63 (67.0)** |
| Leading symptom | |||
| Chest pain | 94 (37.0) | 45 (20.1)** | 26 (27.7) |
| Dyspnoea | 51 (20.1) | 65 (29.0)* | 26 (27.7) |
| Syncope | 11 (4.3) | 12 (5.4) | 6 (6.4) |
| Other | 92 (36.2) | 98 (43.8) | 36 (38.3) |
| History | |||
| CHF | 51 (20.1) | 45 (20.1) | 18 (19.1) |
| CAD | 193 (76.0) | 146 (65.2)* | 68 (72.3) |
| PCI | 82 (32.3) | 76 (33.9) | 34 (36.2) |
| CABG | 24 (9.4) | 22 (9.8) | 15 (16.0) |
| PAD | 27 (10.6) | 22 (9.8) | 12 (12.8) |
| Stroke | 16 (6.3) | 18 (8.0) | 11 (11.7) |
| COPD | 35 (13.8) | 44 (19.6) | 15 (16.0) |
| Risk factors | |||
| Diabetes mellitus | 89 (35.0) | 72 (32.1) | 37 (39.4) |
| Cholesterolaemia | 156 (61.4) | 111 (49.6)* | 60 (63.8) |
| Hypertension | 212 (83.5) | 181 (80.8) | 78 (83.0) |
| Active smoking | 31 (12.2) | 23 (10.3) | 10 (10.6) |
| Ex-smoker | 85 (33.5) | 80 (35.7) | 25 (26.6) |
| Family history | 30 (11.8) | 31 (13.8) | 12 (12.8) |
| GRACE score | 136.9±32.1 | 139.5±29.4 | 142.9±31.1 |
| Coronary angiography | |||
| CA performed | 143 (56.3) | 75 (33.5)*** | 42 (44.7) |
| 0 VD | 14 (5.5) | 22 (9.8) | 4 (4.3) |
| 1 VD | 22 (8.7) | 9 (4.0) | 5 (5.3) |
| 2 VD | 20 (7.9) | 12 (5.4) | 6 (6.4) |
| 3 VD | 86 (33.9) | 40 (17.9)** | 29 (30.9) |
| Left main trunk | 4 (1.6) | 1 (0.4) | 0 (0.0) |
| PCI | 84 (33.1) | 30 (13.4)*** | 21 (22.3) |
Values are mean±SD, n (%), or median (interquartile range).
Versus rising kinetic changes: *p<0.05; **p<0.01; ***p<0.0001.
eGFR, estimated glomerular filtration rate; CABG, coronary artery bypass graft; CAD, coronary artery disease; CHF, chronic heart failure; COPD, chronic obstructive pulmonary disease; NT-proBNP, N-terminal brain natriuretic peptide; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; UAP, unstable angina pectoris; VD, vessel disease.
Median hs-cTnT concentrations on presentation were significantly higher in patients with the final diagnosis of non-STEMI than in patients with non-ACS (95.3 ng/l, 95% CI 33.4–342.6 vs. 34.0 ng/l, 95% CI 21.2–68.6; p<0.0001).
Kinetic changes and final diagnoses
Rising values were found more frequently among patients with non-STEMI as compared to non-ACS (OR 3.69, 95% CI 2.46–5.53; p<0.0001). Conversely, falling values were noted more frequently among patients with non-ACS conditions (OR 3.56, 95% CI 2.24–5.63; p<0.001) and among non-STEMI patients presenting later than 6 hours after onset of symptoms (OR 1.74, 95% CI 0.81–3.77; p for trend=0.16). With respect to presenting symptoms, rising hs-cTnT concentrations were observed more often in patients with chest pain than other acute symptoms (OR 2.04, 95% CI 1.42–2.95; p=0.0001).
The magnitudes of rising hs-cTnT concentrations were significantly higher in patients with non-STEMI than in patients with non-ACS (Table 2) both for relative (103.9 vs. 16.2%; p=0.005) and absolute hs-cTnT changes (94.4 vs. 6.9 ng/l; p<0.001). The same relates to falling hs-cTnT concentration changes (relative 22.9 vs. 14.7%; absolute 21.0 vs. 6.1 ng/l; p<0.001; p=0.001).
Table 2.
Median relative and absolute kinetic changes of positive and negative kinetic patterns subdivided by diagnostic categories.
| Kinetic changes | Relative kinetic changes (%) |
Absolute kinetic changes (ng/l) |
||
|---|---|---|---|---|
| Rising | Falling | Rising | Falling | |
| Non-STEMI | 103.9 (31.5–492.6) | 22.9 (14.6–39.5) | 94.4 (35.9–354.6) | 21.0 (9.8–62.3)* |
| UAP | 17.0 (8.9–21.2) | 14.2 (7.8–20.1) | 6.0 (2.8–19.5) | 5.2 (3.4–10.7) |
| Non-ACS | 16.2 (7.0–52.9) | 14.7 (6.8–24.6)** | 6.9 (2.5–25.6) | 6.1 (3.2–13.3)* |
| Myocardial | 16.6 (6.8–53.2) | 10.5 (4.8–22.9)* | 7.3 (2.5–27.9) | 4.6 (2.1–10.3) |
| Pulmonary | 14.7 (4.7–32.4) | 14.4 (1.5–28.6) | 5.1 (1.2–11.0) | 6.1 (0.3–15.0) |
| Vascular | 48.8±47.9 | 18.5±12.4 | 23.0±15.5 | 14.5 (7.3–109.1) |
| Renal | a | a | a | a |
| Inflammatory | a | a | a | a |
| Other | 25.8 (9.8–63.1) | 15.5 (9.7–26.6)* | 8.0 (3.1–25.2) | 6.1 (3.4–11.4) |
| Unknown | a | a | a | a |
Values are median (interquartile range) or mean ±SD. an<10.
p<0.05; **p<0.01.
Discrimination between non-STEMI and non-ACS by direction and magnitude of kinetic changes
C-statistics on the performance of baseline and serial changes to discriminate between non-STEMI and non-ACS are displayed in Table 3. Absolute concentration changes outperformed relative changes. The performance of hs-cTnT elevations on presentation as index for the diagnosis of non-STEMI was moderate (AUC 0.705, 95% CI 0.650–0.759). Addition of serial changes of any direction improved the AUC from 0.680 (95% CI 0.618–0.742) to 0.861 (95% CI 0.822–0.900; p<0.0001; Figure 2). Split by the direction of concentration change, the ROC-optimized rising pattern increased AUC from 0.696 (95% CI 0.624–0.769) to 0.852 (95% CI 0.798–0.906) (p<0.01), whereas the ROC-optimized falling pattern did not improve performance significantly (AUC 0.678 (95% CI 0.545–0.812) vs. AUC 0.741 (95% CI 0.635–0.847); p=ns).
Table 3.
Diagnostic performance of positive and negative kinetic changes for the diagnosis non-STEMI.
| Rising | Falling | |
|---|---|---|
| Absolute changes | ||
| 5 ng/l | ||
| Sensitivity | 96.7 (90.6–99.3) | 3.5 (0.6–17.8) |
| Specificity | 40.7 (31.9–49.9) | 56.2 (46.9–65.2) |
| PPV | 54.4 (46.3–62.3) | 1.9 (0.3–9.9) |
| NPV | 94.3 (84.3–98.8) | 70.8 (60.7–79.7) |
| ROC-optimized* | ||
| Sensitivity | 95.6 (89.0–98.8) | 17.2 (5.9–35.8) |
| Specificity | 57.4 (48.1–66.3) | 31.4 (23.3–40.5) |
| PPV | 62.3 (53.7–70.4) | 5.7 (1.9–12.8) |
| NPV | 94.6 (86.7–98.5) | 61.3 (48.1–73.4) |
| 20 ng/l | ||
| Sensitivity | 88.4 (75.3–91.2) | 51.7 (32.5–70.5) |
| Specificity | 74.6 (66.5–81.7) | 85.1 (77.5–90.9) |
| PPV | 68.5 (59.0–77.0) | 45.5 (28.1–63.6) |
| NPV | 88.0 (80.7–93.3) | 88.0 (80.7–93.3) |
| 100 ng/l | ||
| Sensitivity | 47.8 (37.1–58.6) | 13.8 (4.0–31.7) |
| Specificity | 91.1 (84.6–95.4) | 96.7 (91.7–99.1) |
| PPV | 79.6 (66.5–89.4) | 50.0 (16.0–84.0) |
| NPV | 70.4 (62.7–77.4) | 82.4 (75.1–88.3) |
| Relative changes | ||
| 20% | ||
| Sensitivity | 82.2 (72.4–89.5) | 55.2 (35.7–73.5) |
| Specificity | 56.8 (47.6–65.6) | 67.2 (57.9–75.7) |
| PPV | 57.8 (48.8–66.5) | 29.6 (18.0–43.6) |
| NPV | 81.6 (71.9–89.1) | 85.7 (76.8–92.2) |
| ROC-optimized* | ||
| Sensitivity | 68.5 (57.8–78.0) | 89.7 (72.6–97.7) |
| Specificity | 78.2 (69.9–85.1) | 41.7 (32.6–51.3) |
| PPV | 69.3 (58.6–78.7) | 28.0 (19.2–38.2) |
| NPV | 77.6 (69.3–84.6) | 94.1 (83.7–98.7) |
| 100% | ||
| Sensitivity | 52.2 (41.4–62.9) | No patients |
| Specificity | 89.5 (82.7–94.3) | |
| PPV | 78.3 (65.8–87.9) | |
| NPV | 72.1 (64.3–79.0) | |
| 250% | ||
| Sensitivity | 35.6 (25.8–46.4) | No patients |
| Specificity | 96.8 (91.9–99.1) | |
| PPV | 88.9 (73.9–96.8) | |
| NPV | 67.4 (60.0–74.2) |
Values are % (95% CI).
ROC-optimized: absolute change: positive, 8.8 ng/l; negative, 9.3 ng/l; relative change: positive, 53.4%; negative, 10.7%.
Figure 2.
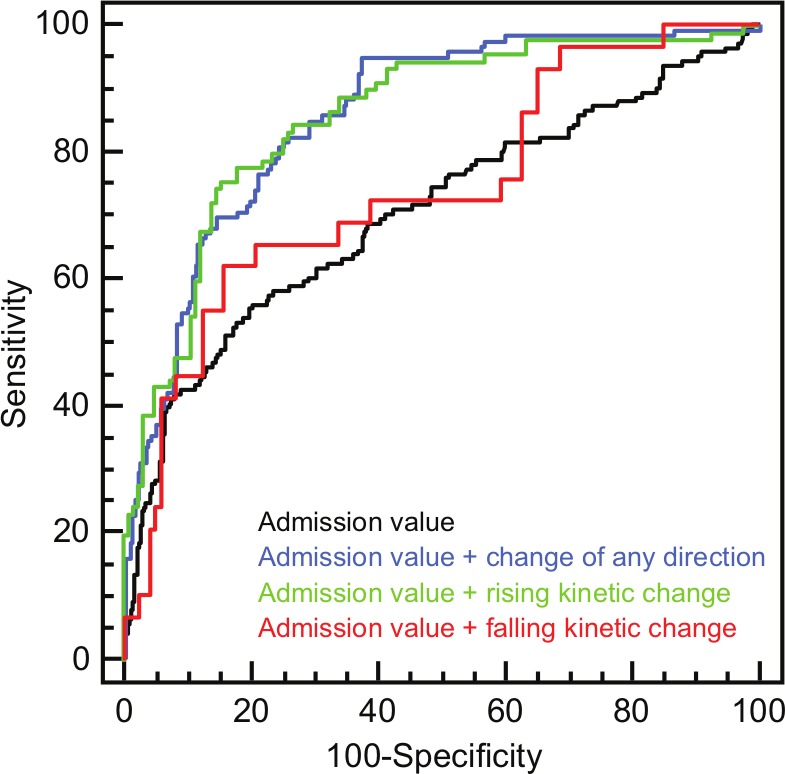
Diagnostic performance of admission hs-cTnT values, admission values combined with kinetic changes of any direction, admission values combined with rising kinetic changes, and admission values combined with falling kinetic changes within 6 hours.
Diagnostic performance of a 20% criterion
The performance of a concentration change of 20% as recently proposed by an ESC working group revealed a better performance of rising kinetic changes (AUC rise 0.757 (95% CI 0.674–0.840) vs. AUC fall 0.354 (95% CI 0.192–0.515); p=not applicable). However, no significant difference of positive changes as compared to negative changes was observed when admission hs-cTnT values were considered (AUC rise 0.785 (95% CI 0.726–0.845) vs. AUC fall 0.763 (95% CI 0.681–0.845); p=ns; Figure 3). ROC-optimized cut-offs for positive relative kinetic changes were higher (53.4%) than the proposed 20% criterion of ESC guidelines, whereas ROC-optimized falling kinetic changes were lower (10.7%). The comparison of ROC-optimized relative changes and the 20% criterion to admission hs-cTnT values indicated a higher net reclassification of positive kinetic changes (Table 4). Furthermore, the NRI analysis revealed a better performance of ROC-optimized changes as compared to the 20% criterion.
Figure 3.
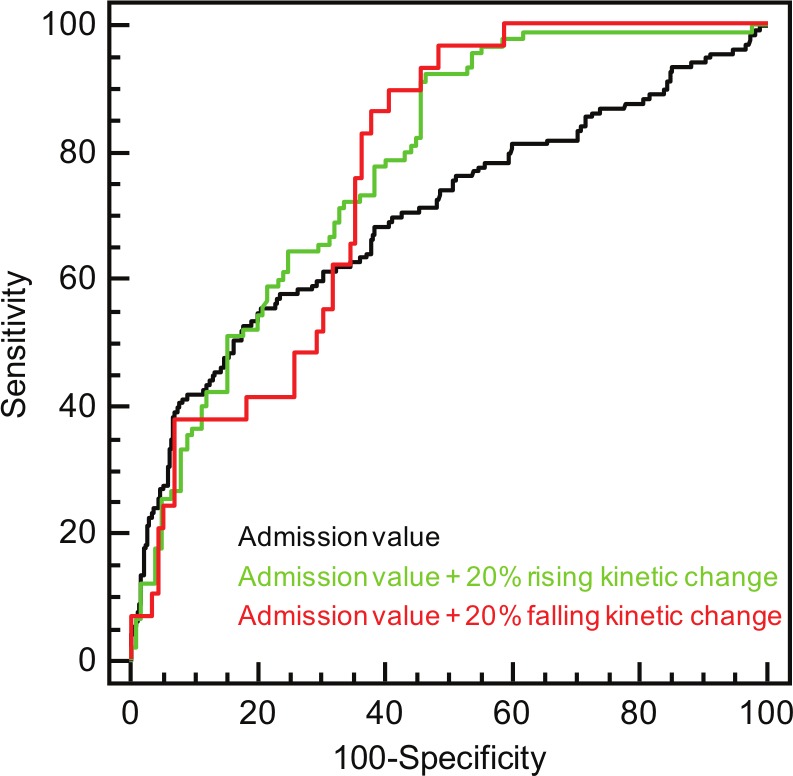
Diagnostic performance of admission hs-cTnT values, admission values combined with rising kinetic changes of 20%, and admission values combined with falling kinetic changes of 20% within 6 hours.
Table 4.
Net reclassification improvement by ROC-optimized and 20% positive and negative relative kinetic changes on top of admission hs-cTnT values.
| n | NSTEMI | AUC | p-value | IDI | NRI>0 | p-value | |
|---|---|---|---|---|---|---|---|
| hs-cTnT | 213 | 90 (42) | 0.704 (0.633–0.776) | ||||
| + ROC positive | 0.844 (0.791–0.897) | <0.0001 | 0.230 (0.171–0.289) | 0.917 (0.674–1.159) | <0.0001 | ||
| + 20% positive | 0.811 (0.754–0.868) | 0.0004 | 0.154 (0.106–0.203) | 0.783 (0.547–1.018) | <0.0001 | ||
| hs-cTnT | 144 | 29 (20) | 0.671 (0.533–0.808) | ||||
| + ROC negative | 0.751 (0.650–0.853) | 0.0936 | 0.090 (0.036–0.145) | 0.628 (0.342–0.914) | 0.0025 | ||
| + 20% negative | 0.772 (0.688–0.856) | 0.0416 | 0.032 (-0.011–0.078) | 0.443 (0.042–0.843) | 0.0332 |
Discussion
The major findings of our study are that both the magnitude of baseline values as well as the slope and direction of concentration changes carry important information for discrimination of non-STEMI versus non-ACS. Non-STEMI is more often found in the presence of higher baseline hs-cTnT concentrations and in the presence of rising hs-cTnT concentrations.
The ESC guidelines acknowledge the role of hs-cTnT as a major primary risk factor indicating macro-necrosis due to vulnerable plaque associated with an acute intracoronary thrombus formation in type I MI. Based on many multicentre trials, it is recommended to proceed to an early invasive treatment within 24 hours among patients with a relevant rise and/or fall of cTn concentrations.1 Unfortunately, the magnitude of qualifying cTn changes has not been defined. Furthermore, it is unresolved whether falling levels suggesting eventually a subacute event provide similar diagnostic information as rising values.
Previously, we reported on a moderate performance (AUC 0.731) of the absolute hs-cTnT value on presentation to discriminate between non-STEMI and non-ACS in patients with elevated hs-cTnT.9 The addition of serial changes within 6 hours increased AUC from 0.731 to 0.898 (p<0.0001). More recently, Reichlin et al.16 reported on a simple algorithm in patients presenting with acute chest pain to an emergency department. Taking into account hs-cTnT concentrations at presentation, changes within 1 hour, and ST-segment elevation on 12-lead ECG were reported to be extremely effective for discrimination of AMI from acute cardiac non-coronary events. Both reports consistently confirm the usefulness of baseline and serial cTn measurements for diagnosis of MI. The superior performance in the report of Reichlin et al.16 is likely explained by the inclusion of STEMI patients who are characterized by higher baseline values and steeper upslopes than the heterogeneous group of non-STEMI patients. On the other hand, exclusion of patients presenting with less specific symptoms such as acute dyspnoea is likely to reduce the fraction of patients with non-coronary cardiac diseases and will result in higher baseline values and steeper slopes. Our careful analysis reveals that only rising kinetic changes increase the diagnostic performance of baseline hs-cTnT values whereas falling kinetic changes do not add diagnostic information. The relative value of increasing or decreasing hs-cTnT concentrations has not been addressed appropriately, so far. In particular, relative and absolute concentration changes have only been reported for rising values,7,9,17 or without consideration of the direction of change.4,9 In larger MI, a distinct troponin release has been reported, which is characterized by a steep increase to an early peak on day 1 particularly in reperfused MI followed by a prolonged smaller second peak (biphasic release due to degradation of contractile proteins).18 It is tempting to speculate that patients with a MI will present earlier due to severe and typical symptoms whereas symptoms may vary widely among the numerous non-ACS-related differential diagnoses of elevated cTn. The results of our study are in accordance with this hypothesis. Patients presenting with rising hs-cTnT values had more often acute chest pain, presented earlier, qualified more often for a diagnosis of non-STEMI, and underwent more frequently and earlier an invasive procedure than patients with non-ACS. By contrast, patients with non-STEMI and falling hs-cTnT levels presented to the emergency department frequently later than 6 hours after onset of symptoms.
Our analysis revealed a better performance of absolute kinetic changes for discrimination of non-STEMI and non-ACS. As previously reported by our group, this is most likely explained by a better sensitivity of absolute changes, especially in the plateau phase of kinetic changes.9
ESC guidelines recommend a 20% rise and/or fall together with at least one value exceeding the 99th percentile of the URL to make the diagnosis of acute myocardial infarction.1 In our analysis, rising kinetic changes of 20% outperformed falling changes in diagnosing non-STEMI when admission hs-cTnT values were not considered (all patients had admission hs-cTnT values exceeding 14 ng/l). Interestingly, this superiority was not observed when admission hs-cTnT values were considered. This finding again supports the diagnostic value of admission hs-cTn values which was previously described by Keller et al.17 In our study, ROC-optimized cut-offs for positive relative kinetic changes were higher (53.4%) than recommended by the guidelines, whereas ROC-optimized falling kinetic changes were lower (10.7%). This suggests that a single cut-off for rising and falling kinetic changes as currently proposed might be problematic. According to our data, definition of distinct cut-offs for positive and negative kinetic changes would better reflect the value of these diagnostic classifiers.
In conclusion, our recommendation is to consider rising rather than falling, and absolute rather than relative, kinetic changes of high-sensitivity cTnT to diagnose non-STEMI. Although rising kinetic changes were more frequently associated with non-STEMI and falling kinetic changes were more often associated with non-ACS conditions, it is questionable if a standalone biomarker strategy is able to clearly differentiate between these groups. Therefore, the final diagnosis should always be made under consideration of the clinical context and biomarker testing.
Limitations
In study cohorts with high prevalence of baseline hs-cTnT elevations, the correct diagnosis of non-STEMI is paramount. Therefore, adjudication of ACS and non-ACS was accomplished by two independent cardiologists, who applied the prespecified universal MI definition criteria using a change of 20% or more to define a relevant change. Final diagnosis was based on all available clinical, biochemical, and imaging information including coronary angiography, multislice coronary computed tomography, cardiac magnetic resonance imaging, 2-D transthoracic, or transoesophageal echocardiography. Nevertheless, identification of the correct cause of cTn elevation in patients with suspected non-STEMI is challenging. Therefore, we cannot fully exclude that there was perhaps a certain proportion of falsely classified or excluded MIs as myocardial ischaemia may be involved, at least in part, in acute non-ACS conditions (type II MI) and since it may be particularly difficult to discriminate type II MI from non-ACS-related myocardial necrosis. For that reason, final diagnoses should ideally been adjudicated by an independent expert committee in forthcoming clinical trials before our results can eventually enter routine clinical practice.
Given the sample size of this study, our results are useful to generate a hypothesis which requires further confirmation in an independent validation cohort.
We included patients with a broad spectrum of symptoms and comorbidities and can therefore not exclude that our findings cannot be applied in patients presenting with chest pain as the leading symptom. However, cut-offs in our population were very similar to hs-cTnT cut-offs reported by Reichlin et al.3 in an independent study population including patients with chest pain.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest: EG has received financial support for clinical trials from Roche Diagnostics, Switzerland, Mitsubishi Chemicals, Germany, Siemens Healthcare, BRAHMS Biomarkers, Clinical Diagnostics Division, Thermo Fisher Scientific, Germany. He is consultant to Roche Diagnostics and BRAHMS Biomarkers and has received speaker’s honoraria from Roche Diagnostics, Siemens Healthcare, BRAHMS Biomarkers, and Mitsubishi Chemicals. HAK has developed the cTnT assay and holds a patent jointly with Roche Diagnostics. He has received grants and research support from several companies, and has received honoraria for lectures from Roche Diagnostics. MV has been reimbursed by several companies for travel expenses and fees associated with attending seminars and conferences.
The other authors declare no conflicts of interest.
References
- 1. Hamm CW, Bassand JP, Agewall S, et al. ;ESC Committee for Practice Guidelines. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011; 32: 2999–3054 [DOI] [PubMed] [Google Scholar]
- 2. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J 2012; 33:2551–255622922414 [Google Scholar]
- 3. Reichlin T, Irfan A, Twerenbold R, et al. Utility of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Circulation 2011; 124: 136–145 [DOI] [PubMed] [Google Scholar]
- 4. Giannitsis E, Becker M, Kurz K, et al. High-sensitivity cardiac troponin T for early prediction of evolving non-ST-segment elevation myocardial infarction in patients with suspected acute coronary syndrome and negative troponin results on admission. Clin Chem 2010; 56: 642–650 [DOI] [PubMed] [Google Scholar]
- 5. Apple FS, Pearce LA, Smith SW, et al. Role of monitoring changes in sensitive cardiac troponin I assay results for early diagnosis of myocardial infarction and prediction of risk of adverse events. Clin Chem 2009; 55: 930–937 [DOI] [PubMed] [Google Scholar]
- 6. Eggers KM, Jaffe AS, Venge P, et al. Clinical implications of the change of cardiac troponin I levels in patients with acute chest pain – an evaluation with respect to the Universal Definition of Myocardial Infarction. Clin Chim Acta 2011; 412: 91–97 [DOI] [PubMed] [Google Scholar]
- 7. Kavsak PA, Ko DT, Wang X, et al. Increasing cardiac troponin changes measured by a research high-sensitivity troponin I assay: absolute vs percentage changes and long-term outcomes in a chest pain cohort. Clin Chem 2010; 56: 1902–1904 [DOI] [PubMed] [Google Scholar]
- 8. Keller T, Zeller T, Ojeda F, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA 2011; 306: 2684–2693 [DOI] [PubMed] [Google Scholar]
- 9. Mueller M, Biener M, Vafaie M, et al. Absolute and relative kinetic changes of high-sensitivity cardiac troponin T in acute coronary syndrome and in patients with elevated troponin in the absence of acute coronary syndrome. Clin Chem 2012; 58: 8–10 [DOI] [PubMed] [Google Scholar]
- 10. Giannitsis E, Kurz K, Hallermayer K, et al. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem 2010; 56: 254–261 [DOI] [PubMed] [Google Scholar]
- 11. Saenger AK, Beyrau R, Braun S, et al. Multicenter analytical evaluation of a high-sensitivity troponin T assay. Clin Chim Acta 2011; 412: 748–754 [DOI] [PubMed] [Google Scholar]
- 12. Apple FS, Jaffe AS. Clinical implications of a recent adjustment to the high-sensitivity cardiac troponin T assay: user beware. Clin Chem 2012; 58 (11): 1599–600 [DOI] [PubMed] [Google Scholar]
- 13. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–845 [PubMed] [Google Scholar]
- 14. Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 1993; 39: 561–577 [PubMed] [Google Scholar]
- 15. Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 2011; 30:11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009; 361: 858–867 [DOI] [PubMed] [Google Scholar]
- 17. Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med 2009; 361: 868–877 [DOI] [PubMed] [Google Scholar]
- 18. Remppis A, Scheffold T, Greten J, et al. Intracellular compartmentation of troponin T: release kinetics after global ischemia and calcium paradox in the isolated perfused rat heart. J Mol Cell Cardiol 1995; 27: 793–803 [DOI] [PubMed] [Google Scholar]