Abstract
Background:
Intravenous sedation and analgesia are important therapies during mechanical ventilation (MV). However, daily interruption of these medications is associated with improved outcomes in mechanically ventilated patients. We tested a clinical pathway for the use of sedation and analgesia during MV in a cardiac intensive care unit (CICU).
Methods and results:
We evaluated all mechanically ventilated patients in a CICU during two phases: phase 1 prior to pathway implementation (PRE) and phase 2 post-pathway implementation (POST). A total of 198 patients (98 PRE and 100 POST) and 1012 days of intubation (574 PRE and 434 POST) were included in this analysis. We found an increase in the frequency of daily interruptions of sedation post-implementation (49.3% PRE and 58.4% POST, p=0.0041). There was a significant decrease in the mean duration of MV in the POST vs PRE periods (5.0±2.3 vs 6.1±2.8 days, p=0.015). There was also a significant decrease in total neuroimaging studies (9 vs 49, p=0.001) and a trend toward a decrease in tracheostomies (3.0% vs 6.1%, p=0.33). Mean CICU length of stay (LOS) and hospital LOS respectively were 10.4 days and 16.8 days PRE and 10.4 days and 17.9 days POST (p=0.99 and p=0.55). Mortality did not differ (PRE 36.7% vs POST 32.0% p=0.55).
Conclusions:
Implementation of a pragmatic pathway for sedation and analgesia in a CICU was associated with an increase in the daily interruption of sedation and a corresponding decrease in the duration of MV days and the need for neuroimaging.
Keywords: Mechanical ventilation, intensive care unit, sedation, analgesia, clinical pathway
Introduction
As the population ages with a rising burden of chronic disease, critical care has assumed an increasingly important role in modern medicine. As an example, from 2000–2005, there was a 6.5% increase in the number of intensive care unit (ICU) beds in the United States despite a decrease in the overall number of hospital beds.1 Cardiac intensive care units (CICUs) were initially developed in the 1960s as an environment to rapidly identify and treat arrhythmias after myocardial infarction.2 However, modern CICUs, in addition to treating patients with complex cardiovascular disease, commonly manage patients with multiorgan dysfunction, including respiratory failure, acute kidney injury, and sepsis.3,4 This changing landscape has necessitated advances in the organization and structure of the contemporary CICU, as well as implementation of interventions to improve care that have traditionally been focused on general medical and surgical ICUs.5,6
One such area of focus is the duration of mechanical ventilation (MV) and its associated complications.7 Compelling data from general ICUs have linked the use of continuous intravenous (IV) sedation during MV with a longer duration of MV, ICU length of stay, and total duration of hospitalization8 and demonstrated that daily interruption of IV sedation leads to shorter periods of MV.9,10 However, there are few data examining such interventions in the CICU environment, and prior experience, albeit important, has been very limited.11 Therefore, we designed and implemented a structured clinical pathway to guide selection and dosing of sedatives and analgesics in patients requiring MV in a CICU, and measured key indices of clinical process and quality prior to and after implementation of this pathway.
Methods
Sedation pathways
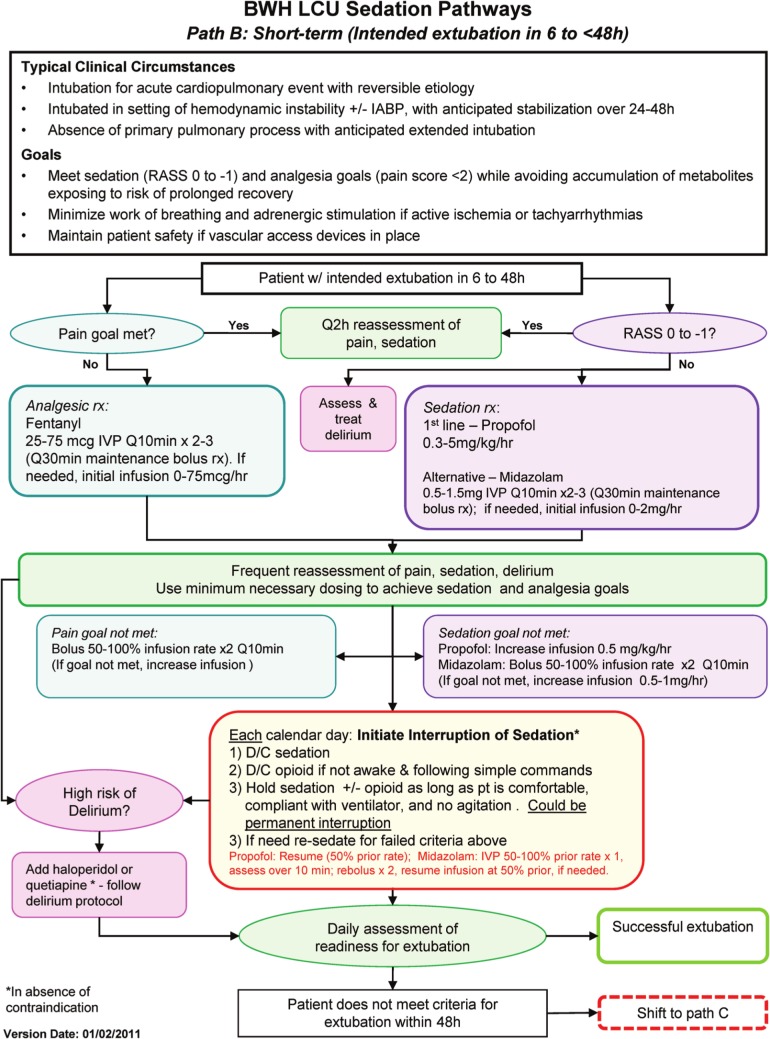
In January 2011, we implemented a multidisciplinary-designed set of structured clinical pathways for sedation and analgesia in the Samuel A Levine CICU (LCU) at Brigham and Women’s Hospital, Harvard Medical School (Boston, Massachusetts, USA). With the participation of pharmacy, respiratory therapy, nursing, and physician leadership, we developed three practical ‘sedation pathways’ based on the expected duration of MV. Pathway A was intended for patients with expected duration of MV of less than 6 h. Pathway B was designed for MV of 6–48 hours. Finally, Pathway C was used for an expected duration of MV greater than 48 h. These pathways specified approaches for pharmacologic analgesia and sedation, frequency of reassessment, strategies for modifying medication dosages, and guidelines for trial interruptions of sedation. Each of the pathways is shown in the Online Supplement with an example (Pathway B) shown in Figure 1. Selection between the available pathways was based on the expected duration of MV rather than being dictated by specific diagnoses.
Figure 1.
Brigham and Women’s Hospital (BWH) Levine Cardiac Intensive Care Unit (CICU) Sedation Pathways. Pathway B is shown as an example. The full set of sedation pathways are provided in the Online Supplement. Benzo: benzodiazepine; D/C: discontinue; IABP: intra-aortic balloon pump; IVP: intravenous push; min: minutes; LCU: Samuel A Levine CICU; PVAD: percutaneous ventricular assist device; Q10 min: every 10 min; RASS: Richmond Agitation Sedation Scale; rx: therapy.
The pathways were developed to be consistent with external and internal hospital guidelines for the treatment of pain and agitation in the mechanically ventilated adult patient. Prior to implementation of the sedation pathways, care was guided by these same guidelines but without a specific practical clinical pathway in place. After creation by the multidisciplinary working group, the pathways were distributed to the entire CICU nursing, respiratory therapy, and physician staff for a six-week period of open feedback before finalization and implementation. All practitioners received training on each of the pathways.
Consideration of daily interruption of sedation was incorporated into daily multidisciplinary rounds for every patient who was mechanically ventilated. Every member of the team, including nursing, pharmacy, respiratory therapy, physician trainees, and the attending physician, was empowered to advocate for daily interruption. If the team agreed that daily interruption was not contraindicated, the nurse was responsible for implementing the interruption according to the sedation pathway for daily interruption (Figure 1 and Online Supplement).
Data collection
In order to assess the impact of implementation of the sedation pathways on processes of care, we designed a quality improvement assessment that captured data from the same period in the year prior to and after implementation of the clinical pathways. We collected data from all patients receiving MV from February 2010–September 2010 in the CICU by retrospective chart review. Data were collected from the time of intubation to the time of extubation, tracheostomy, transfer out of the CICU, or death. The second period for data collection was prospectively performed between February 2011–September 2011, after initiation of the sedation and analgesia pathway. All patients undergoing MV in the CICU from both periods were included without exclusion. Ventilator acquired complications (pneumonia) were collected prospectively in both periods as part of hospital-wide monitoring of these events. Approval for data collection was obtained from the hospital’s institutional review board.
Statistical analysis
Categorical variables are presented as numbers and percentages. Continuous variables are summarized as the mean and standard deviation. Average infusion doses of medications were calculated by determining the total daily dose of each medication and then averaged for an hourly rate (over the infusion duration). The average hourly rate across ventilation days was then determined. Comparison of baseline characteristics, medication usage, and metrics of care were compared using the t-test for continuous variables, and either the chi-square test or Fisher’s exact test for categorical variables. Two-tailed p-value <0.05 were considered significant. Analyses were performed using GraphPad Software (GraphPad Software, Inc., La Jolla, California, USA).
Results
Baseline characteristics
Data were collected for 98 patients (574 observation days) in the period prior to pathway implementation (designated PRE) and 100 patients (434 observation days) in the period post-pathway implementation (designated POST). The majority of patients were male, and nearly all patients were admitted for medical management rather than post-operative care (Table 1). The most common reasons for admission in both the PRE and POST groups were cardiogenic shock, cardiac arrest, and acute coronary syndrome. There were no significant differences between the PRE and POST groups with respect to patient age, gender, weight, or reason for admission (Table 1).
Table 1.
Patient demographics.
| Variable | PRE (n=98) | POST (n=100) | p-value |
|---|---|---|---|
| Age; years | 62.4±16.4 | 62.5±15.8 | 0.96 |
| Male; n (%) | 66 (67.3) | 62 (62) | 0.43 |
| Actual weight, kg | 88.3±24.1 | 82.3±27.1 | 0.10 |
| CICU patient type, n (%) | |||
| Cardiac medical | 96 (98) | 94 (96) | 0.28 |
| Cardiac surgical | 2 (2) | 6 (6) | 0.28 |
| Reason for admission, n (%) | |||
| Cardiogenic shock | 23 (23.5) | 18 (18) | 0.44 |
| Cardiac arrest | 12 (12.2) | 18 (18) | 0.35 |
| Acute coronary syndrome | 17 (17.3) | 18 (18) | 0.91 |
| Arrhythmia | 10 (10.2) | 7 (7) | 0.46 |
| Heart failure (without shock) | 11 (11.2) | 5 (5) | 0.12 |
| Valvular disease | 6 (6.1) | 9 (9) | 0.59 |
| Primary pulmonary process | 10 (10.2) | 13 (13) | 0.67 |
| Sepsis/septic shock | 7 (7.1) | 4 (4) | 0.37 |
| Cardiac other | 2 (2) | 8 (8) | 0.10 |
CICU: cardiac intensive care unit; POST: post-pathway implementation; PRE: prior to pathway implementation.
Use of sedating medications
Compared with use in patients observed during the PRE period, the proportion of patients in the POST period receiving propofol vs midazolam did not change (1:1 ratio in both periods) with a numerically higher number of patients receiving fentanyl alone (5 PRE vs 10 POST). In addition, the average infusion rate of the intravenous analgesic fentanyl was significantly reduced (52.9 mcg/h PRE vs 37.6 mcg/h POST, p<0.01, Figure 2) as were the average infusion rates of the sedatives propofol (1.4 mg/h PRE vs 1.1 mg/h POST, p<0.01) and midazolam (2.1 mg/h PRE vs 1.1 mg/h POST, p<0.01).
Figure 2.
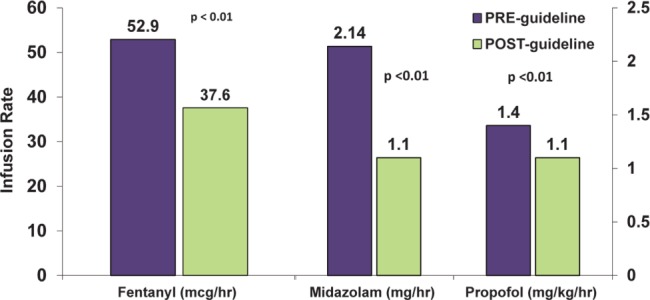
Average infusion rates of fentanyl, midazolam, and propofol in the prior to pathway implementation (PRE) and post-pathway implementation (POST) periods.
As intended, utilization of the sedation pathway was associated with an increased proportion of patient-days undergoing daily interruption of pharmacologic sedation (49.3% PRE vs 58.4% POST, p<0.01, Table 2). Prior to implementing the sedation pathways, the most common reason for deferring daily interruption of sedation was recorded as hemodynamic instability (24.7% PRE vs 15% POST, p=0.02). In contrast, during the POST period, the most common reason for deferring interruption was a planned surgery or procedure (11.9% PRE vs 20.5% POST, p=0.02). Similarly, a RASS of greater than −1 or patient agitation was less commonly cited as a reason for deferring sedation interruption after implementation of our pathway (15.3% PRE vs 6.1% POST, p<0.01). Capture of the reason for deferring daily interruptions was improved in the POST period with fewer unrecorded indications for deferring daily pharmacologic sedation (Table 2).
Table 2.
Interruption of intravenous sedating medications.
| Variable | PRE (n=98) | POST (n=100) | p-value |
|---|---|---|---|
| DI completed, n (%) | 283 (49.3) | 254 (58.4) | <0.01 |
| Opportunities for DI that were not completeda | 235 | 179 | |
| Reasons for no DI, n (%) | |||
| Hemodynamic instability | 58 (24.7) | 27 (15) | 0.02 |
| Planned surgery/procedure | 28 (11.9) | 37 (20.5) | 0.02 |
| Ventilatory dyssynchrony | 8 (3.4) | 22 (12.2) | <0.01 |
| RASS goal <–2 | 27 (11.5) | 27 (13.3) | 0.35 |
| Comfort measures/deceased | 15 (6.4) | 12 (6.7) | 0.89 |
| Sedation initiated <12 h | 53 (22.6) | 34 (18.9) | 0.45 |
| Active ischemia | 7 (3) | 1 (0.6) | 0.15 |
| Alcohol withdrawal | 3 (1.3) | 7 (3.9) | 0.11 |
| RASS currently >–1/agitation | 36 (15.3) | 11 (6.1) | <0.01 |
| Active seizures | 0 (0) | 1 (0.6) | 0.81 |
DI: drug interruption; POST: post-pathway implementation; PRE: prior to pathway implementation; RASS: Richmond Agitation Sedation Scale.
Restricted to analysis of instances where the reason for not completing sedating DI was recorded. The % for DI completed is among the total opportunities for DI. The reasons (%) are among opportunities for DI that were not completed and the reason was known. Reasons for deferring DI were unknown in 59 ‘opportunities’ PRE and 0 POST.
Patient outcomes
The outcomes in the PRE and POST groups with respect to duration of MV, CICU length of stay, and hospital length of stay are displayed in Table 3. Patients in the POST group had a significantly shorter duration of MV (5.2 days vs 6.1 days, p=0.015). The total number of neuroimaging studies (including repeat imaging) to investigate causes of altered mental status in the study population was also significantly lower during the POST period (9 over 434 observation days POST vs 49 studies over 574 observation days PRE, p<0.001). A trend toward a reduction in ventilator acquired pneumonia was observed in the POST vs PRE periods (2.6, 95% confidence interval (CI) 0.5–7.5, per 1000 vent-days vs 4.6, 95% CI 1.7–10, per 1000 vent-days). There was no significant difference with respect to CICU length of stay or hospital length of stay (Table 3). Although numerically lower in the POST period, there was no difference in the frequency of tracheostomy, or in-hospital mortality (36% PRE vs 32% POST, p=0.48, Table 3).
Table 3.
Patient outcomes.
| Variable | PRE (n=98) | POST (n=100) | p-value |
|---|---|---|---|
| Mechanical ventilation (days) | 6.1±2.8 | 5.0±2.3 | 0.015 |
| CICU length of stay (days) | 10.4±8.9 | 10.4±7.3 | 0.99 |
| Hospital length of stay (days) | 16.8±13.4 | 17.9±12.7 | 0.55 |
| Tracheostomy (%) | 6.1 | 3.0 | 0.29 |
| In-hospital mortality (%) | 36.7 | 32.0 | 0.48 |
CICU: cardiac intensive care unit; POST: post-pathway implementation; PRE: prior to pathway implementation.
Discussion
In this assessment of a set of practical clinical pathways for the use of analgesic and sedating medications in the CICU compared to our routine practice, we found that introduction of the sedation pathways was associated with a significant decrease in the duration of MV. After implementation of the clinical pathway, we observed the intended increase in the proportion of patients on MV who underwent daily interruption of sedating medications and an overall reduction in the average dose of intravenous sedating medications. These changes in practice are plausibly responsible for the decrease in the duration of MV that we observed. Of note, we did not find a measurable change in the overall ICU length of stay, duration of hospitalization, or mortality, which was in excess of 30% in these high-risk patients. These findings highlight the potential value of introducing a pragmatic clinical pathway to improve practice in the use of sedatives and analgesics in MV patients in the CICU environment, as well as the need for continued research to maximize the impact of this and other interventions to reduce CICU length of stay and mortality in complex CICU patients undergoing MV.
These findings are similar to those from the growing body of literature in general medical and surgical ICUs suggesting that the use of pharmacologic sedation to assist in MV is not benign.8–10,12 Surveys indicate 33–71% of ICU patients require ventilatory support at any given time.13 In medical ICUs, the survival rate of patients requiring MV is only 69%, and survival decreases with prolonged duration of support. Early efforts to decrease the time of MV emphasized the importance of respiratory mechanics, ventilatory mode, and daily spontaneous breathing trial (SBT) in the process of weaning.14,15 More recent studies have demonstrated that daily interruption of IV sedation leads to shorter periods of MV and ICU stay as well as decreased symptoms of post-traumatic stress.9,10 MV without any form of IV sedation similarly decreases the duration of MV and ICU admission but leads to increased delirium.16 Interestingly, some data suggest a nurse-controlled sedation algorithm is superior to daily discontinuation of sedation.17 The specifics of the weaning protocol are particularly important because weaning over more than seven days is associated with increased mortality.12
Although the specific mechanism by which the sedation pathway led to a decreased duration of MV cannot be definitively determined in an observational study, our findings reemphasize the importance of goal-directed analgesia and sedation as well as regular reassessments of sedation needs. In our study, the sedation pathway led to decreased infusion rates of fentanyl, midazolam, and propofol and also led to an ~10% absolute increase in daily interruptions of sedation. Interestingly, implementation of the sedation pathway led to fewer instances of continued sedation for elevated RASS scores or agitation, suggesting that regularly assessing and adjusting sedation may lead to less distress for patients. Although not directly measured in our study, there is a potential for reduction in overall costs through a decrease in the duration of MV and number of neuroimaging tests in our post-intervention group. By significantly decreasing neuroimaging, there are potential benefits from decreased follow-up studies or workup of incidental findings. Perhaps most importantly, despite the fact that nearly all patients in both populations were admitted with primary cardiac rather than non-cardiac issues, our study found no evidence to support the notion that this specialized population should be managed differently from non-cardiac populations with respect to analgesia and sedation.5,11 Further research is necessary to probe these potential benefits and identify sub-populations that may require further specialized sedation strategies, such as patients requiring percutaneously placed mechanical circulatory support.
Limitations
There are several limitations of this study. It is possible that a larger sample size could reveal additional effects of implementation of structured sedation pathways in the CICU environment, such as an impact on ventilator-acquired pneumonia, length of stay, and survival. Given the significant risks of MV, including immobility-associated complications such as deconditioning, pressure ulcers, and deep vein thrombosis, it is possible that our intervention led to improvements in morbidity that were not captured by this study. Additionally, we were only able to assess in-hospital mortality, and long-term follow-up may have provided additional insights into the effects of decreased MV time. Our study was further limited in that implementation of the sedation pathways was not randomized and could not be blinded. General educational efforts that were part of the sedation pathways program also could have contributed to the improved adherence to best practice goals for management of sedating and analgesic medications, including daily interruptions of sedation. Finally, prior to implementing our standardized sedation pathway, documentation of the reason for deferring daily sedation interruption was more commonly missing.
Conclusions
Implementation of a pragmatic pathway for sedation and analgesia in a CICU was associated with increased daily interruption of sedation, decreased use of pharmacologic sedation, decreased utilization of neuroimaging studies, and decreased duration of MV. Our findings support the relevance of structured quality improvement initiatives with respect to analgesia and sedation to the specialized environment of the CICU. The absence of a significant impact of this pathway on length of ICU stay or survival highlights the need for continued investigation of these and other practice changes in the CICU.
Footnotes
Funding: There was no external funding for this hospital-based quality improvement project.
Conflict of interest: The authors declare that they have no related conflicts of interest.
References
- 1. Halpern NA, Pastores SM. Critical care medicine in the United States 2000–2005: An analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med 2010; 38: 65–71 [DOI] [PubMed] [Google Scholar]
- 2. Julian DG. The history of coronary care units. Br Heart J 1987; 57: 497–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Katz JN, Turer AT, Becker RC. Cardiology and the critical care crisis: A perspective. J Am Coll Cardiol 2007; 49: 1279–1282 [DOI] [PubMed] [Google Scholar]
- 4. Katz JN, Shah BR, Volz EM, et al. Evolution of the coronary care unit: Clinical characteristics and temporal trends in healthcare delivery and outcomes. Crit Care Med 2010; 38: 375–381 [DOI] [PubMed] [Google Scholar]
- 5. Morrow DA, Fang JC, Fintel DJ, et al. Evolution of critical care cardiology: Transformation of the cardiovascular intensive care unit and the emerging need for new medical staffing and training models: A scientific statement from the American Heart Association. Circulation 2012; 126: 1408–1428 [DOI] [PubMed] [Google Scholar]
- 6. O'Malley RG, Olenchock B, Bohula-May E, et al. Organization and staffing practices in US cardiac intensive care units: A survey on behalf of the American Heart Association Writing Group on the Evolution of Critical Care Cardiology. Europ Heart J Acute Cardiovasc Care 2012; 2: 3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Esteban A, Anzueto A, Frutos F, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: A 28-day international study. JAMA 2002; 287: 345–355 [DOI] [PubMed] [Google Scholar]
- 8. Kollef MH, Levy NT, Ahrens TS, et al. The use of continuous i.v. sedation is associated with prolongation of mechanical ventilation. Chest 1998; 114: 541–548 [DOI] [PubMed] [Google Scholar]
- 9. Kress JP, Pohlman AS, O'Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 2000; 342: 1471–1477 [DOI] [PubMed] [Google Scholar]
- 10. Kress JP, Gehlbach B, Lacy M, et al. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med 2003; 168: 1457–1461 [DOI] [PubMed] [Google Scholar]
- 11. Piotto RF, Maia LN, Machado MN, et al. Effects of the use of mechanical ventilation weaning protocol in the Coronary Care Unit: Randomized study. Rev Bras Cir Cardiovasc 2011; 26: 213–221 [DOI] [PubMed] [Google Scholar]
- 12. Penuelas O, Frutos-Vivar F, Fernandez C, et al. Characteristics and outcomes of ventilated patients according to time to liberation from mechanical ventilation. Am J Respir Crit Care Med 2011; 184: 430–437 [DOI] [PubMed] [Google Scholar]
- 13. Esteban A, Anzueto A, Alia I, et al. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med 2000; 161: 1450–1458 [DOI] [PubMed] [Google Scholar]
- 14. Esteban A, Frutos F, Tobin MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med 1995; 332: 345–350 [DOI] [PubMed] [Google Scholar]
- 15. Sahn SA, Lakshminarayan S. Bedside criteria for discontinuation of mechanical ventilation. Chest 1973; 63: 1002–1005 [DOI] [PubMed] [Google Scholar]
- 16. Strøm T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: A randomised trial. The Lancet 2010; 375: 475–480 [DOI] [PubMed] [Google Scholar]
- 17. De Wit M, Gennings C, Jenvey WI, et al. Randomized trial comparing daily interruption of sedation and nursing-implemented sedation algorithm in medical intensive care unit patients. Crit Care 2008; 12: R70. [DOI] [PMC free article] [PubMed] [Google Scholar]