Abstract
Background:
Persistent oxidative stress may play a key role in microvascular obstruction (MVO). We aimed at assessing the role of platelet gp91phox (NOX2), the catalytic subunit of NADPH oxidase in MVO.
Methods:
We enrolled 40 patients with ST-elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention within 12 h from symptoms onset, either with angiographic MVO (n=20) or good angiographic myocardial reperfusion (MR) (n=20). Angiographic MVO was defined as a final thrombolysis in myocardial infarction (TIMI) flow ≤2 or TIMI flow of 3 with myocardial blush grade <2. NOX2 and isoprostanes (8-iso-PGF2α) levels, as assessed by enzyme-linked immunoadsorbent assay (ELISA) or by an enzyme immunoassays, respectively, were measured on admission, at 24 h and pre-discharge.
Results:
NOX2 levels increased from baseline to pre-discharge in patients with angiographic MVO (20.25 (15–24.75) pg/ml vs 25.50 (17–29.25) pg/ml, p=0.02), but not in MR patients (p=0.45), with a significant interaction between baseline and pre-discharge levels among the two groups (p=0.04). The levels of 8-iso-PGF2α showed a trend to increase from baseline to pre-discharge in angiographic MVO patients (295 (183.50–389.25) pmol/l vs 322 (206–370) pmol/l, p=0.06), but not in patients with MR (p=0.56), with a trend for interaction between baseline and pre-discharge levels among the two groups (p=0.09).
Conclusion:
Patients with MVO, but not those with myocardial reperfusion, have a sustained increase of NOX2 and 8-iso-PGF2α. Therapies targeting NOX2 or high dosage antioxidants should be tested for MVO prevention and treatment.
Keywords: ST-elevation myocardial infarction, primary percutaneous coronary intervention, microvascular obstruction, oxidative stress
Introduction
Microvascular obstruction (MVO) is an important complication of reperfusion therapy in patients with ST-elevation myocardial infarction (STEMI).1 Importantly, it occurs in up to 50% of cases despite successful reopening of the occluded epicardial coronary artery,1 and is associated with a high rate of major adverse cardiac events.2,3 The pathogenesis of MVO is complex.4 Distal embolization and ischemic time are important factors and many efforts have been recently made in order to prevent embolization of plaque-thrombus material 5 or to reduce time from vessel occlusion to reopening. However, another crucial pathogenetic mechanism is reperfusion injury,6 as shown in several experimental studies.7,8 Yet, attempts to prevent reperfusion injury in humans have been disappointing, possibly due to the selection of inappropriate therapeutic targets.9 Of note, reperfusion of a previously ischemic myocardium leads to plugging of microcirculation by platelet and neutrophil aggregates and importantly to release of oxygen radicals that in turn may injure myocardial and endothelial cells.10 Furthermore, oxygen radicals may lead to lipid peroxidation that stimulates platelet activation due to the production of an isoprostane (8-iso-PGF2α), that is not sensitive to aspirin.11 Interestingly, a progressive impairment of myocardial reperfusion has been observed after reperfusion, a process that may be due to persistent reperfusion injury.10
Platelets may release reactive oxygen species (ROS), with nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) oxidase being the main source.12 Indeed, platelets have all the subunits of NADPH oxidase including gp91phox (NOX2), its catalytic sub-unit.13 ROS in turn may oxidate arachidonic acid leading to the production of 8-iso-PGF2α.14 Of note, 8-iso-PGF2α plays an important role in platelet recruitment leading to platelet aggregate stabilization and thrombus growth.15 Platelet-mediated low density lipoprotein oxidation further contributes to phospholipase A2 activation and thromboxane A2 production that sustains platelet activation in a vicious circle.16
In this study we aimed at evaluating the time course of serum NOX2 and 8-iso-PGF2α in patients with STEMI treated by primary percutaneous coronary intervention (PPCI) according to MVO occurrence.
Materials and methods
Patient population, study design, and PPCI
From April 2011–January 2012, all patients admitted to our hospital with a first episode of STEMI undergoing successful PPCI were screened. Inclusion criteria were: prolonged chest pain (>30 min) with pain onset <12 h, ST segment elevation >0.2 mV in at least two contiguous leads in the initial ECG, and elevated serum troponin T levels, successful PPCI (residual coronary stenosis <20%) performed within 12 h of the onset of chest pain and blood sampling prior to PPCI. Exclusion criteria were: age less than 18 years (n=0), cardiogenic shock (n=2), pregnancy (n=0), history of renal failure (serum creatinine >3 mg/dl) (n=2), contraindications to contrast agents (n=0), or other study medications (n=1), culprit lesion non-identified and left main disease (n=1).
This study is a case-control study in its design. Indeed, we decided to enroll patients with angiographic MVO vs those without angiographic MVO. We enrolled 40 patients: the first 20 with angiographic MVO and the first 20 with angiographic myocardial reperfusion. We did not enroll any other patients after reaching the prespecified number. A total of 23 potentially eligible patients were excluded because of rescue PCI (n=5), stent thrombosis (n=2), residual stenosis after PPCI > 20% (n=2), missing blood sampling (n=11), and non-eligibility for IIb/IIIa inhibitor administration (n=3). Finally, 10 patients were excluded despite having good reperfusion as the prespecified number of patients with good myocardial reperfusion was reached before that in the angiographic MVO group.
All patients were treated with aspirin (300 mg) and clopidogrel (600 mg) on admission in the emergency room. All PPCI were performed through a radial or femoral access according to operator preference, using a 6 French catheter. A bolus of 5000 IU of heparin was administrated. Glycoprotein IIb/IIIa inhibitors were administrated after diagnostic angiogram at the start of PPCI in all patients, as well as manual thrombus aspiration.
Cardiovascular risk factors were carefully examined, including family history of early coronary artery disease (first degree relative with a history of myocardial infarction <60 years), diabetes (fasting blood glucose >126 mg/dl or treated diabetes), dyslipidemia (low density lipoprotein>130 mg/dl, high density lipoprotein<45 mg/dl, or triglycerides >150 mg/dl, or total cholesterol >200 mg/dl), smoking and hypertension (systolic blood pressure >140 mm Hg and/or diastolic blood pressure > 90 mm Hg or treated hypertension). Time to PPCI was also recorded, as well as medications on admission along with body mass index. Moreover, echocardiographic left ventricular ejection fraction was assessed in all patients soon after PPCI (Simpson method). The study was approved by the University Ethics Committee, and all patients gave their consent to use part of their blood for scientific purposes.
The study was approved by the local Ethics Committee and after a complete explanation of the aims and details of the study, all patients gave their informed consent before entering the study.
Laboratory assays
Blood samples were drawn from a brachial vein in all patients and collected in ethylenediaminetetraacetic acid (EDTA) tubes or tubes without any anticoagulant, and centrifuged. Plasma and serum aliquots were stored at −80°C in appropriate cuvettes until assayed. Routine laboratory data including complete hemochrome, serum glucose, low density lipoprotein (LDL), fibrinogen were measured on admission using standardized methods. Peak creatine kinase, creatine kinase-MB, troponin T were also evaluated. Creatinine clearance was estimated by using the Cockcroft-Gault equation. Serum samples were immediately stored at −80°C until use with the antioxidant butylated hydroxytoluene at final concentration of 20 μmol/l.
NOX2 and 8-iso-PGF2α levels were measured on admission, at 24 h after PPCI and at pre-discharge (mean time four days). Variation (Δ) in biomarker levels between pre-discharge and basal values was calculated and related to MVO.
Serum levels of soluble NOX2-derived peptide (sNOX2-dp), which maximally reflect the activity of NOX2 by blood cells including platelets, leucocytes and monocytes, were detected by the enzyme-linked immunoadsorbent assay (ELISA) method.17 The peptide was recognized by the specific monoclonal antibody against the amino acidic sequence (224–268) of the extra membrane portion of NOX2. Values were expressed as pg/ml, intra-assay and inter-assay coefficients of variation were 5.2% and 6%, respectively, for serum and platelets.
Serum concentration of 8-iso-PGF2α was measured by an enzyme immunoassay method (Tema Ricerca, Italy) and expressed as pmol/l as previously described.18 Intra-assay and inter-assay coefficients of variation were 4.4% and 8.8%, respectively.
Coronary angiographic analysis and procedural data
Angiographic assessment was performed by two independent angiographers (GN and NC) who were unaware of patients’ characteristics and laboratory data; final agreement was of the order of 95% (discordances were resolved by consensus). Thrombolysis in myocardial infarction (TIMI) flow and myocardial blush grade (MBG) assessment was performed within 24 h from the index procedure in order to allocate patients in either group (angiographic myocardial reperfusion vs angiographic MVO). TIMI flow and MBG were assessed according to previous studies.19,20 As previously reported, we defined angiographic MVO as a final TIMI flow ≤2 or final TIMI flow of 3 with an MBG <2 (modified from Gibson et al.21). Angiographic data including culprit vessel, collateral evaluation as assessed by Rentrop classification,22 and thrombus grade according to the Gibson score23 (high thrombus grade (4–5) vs low-thrombus grade (0–3)) were also recorded at the time of PPCI. Patients with at least two-vessel disease were defined to have a multi-vessel disease. Stent type (drug-eluting stent or bare-metal stent) was also recorded, along with predilation or postdilation when performed. Finally, the number of patients undergoing complete revascularization during the in-hospital staying was reported.
Electrocardiogram (ECG) assessment of ST-resolution
A 12-lead ECG was recorded before PPCI, at 90 min after PPCI and at pre-discharge. ST-segment elevation was measured 20 ms after the end of QRS complex, with TP-segment as isoelectric line. According to validated algorithms, summed ST elevation was calculated from leads V1–V6, I, and AVL for anterior acute myocardial infarction and from leads II, III, AVF, V5, and V6 for inferior acute myocardial infarction. A reduction in ST elevation value less than 70%, compared with baseline ECG, was considered as MVO.24
Study endpoints
Endpoints of the study were: (a) comparison of baseline NOX2 and 8-iso-PGF2α levels in patients showing angiographic reperfusion as compared to those showing angiographic MVO; (b) the correlation between biomarker changes over time and reperfusion status (angiographic myocardial reperfusion vs angiographic MVO); (c) comparison of baseline NOX2 and 8-iso-PGF2α levels in patients showing ECG reperfusion as compared to those showing ECG MVO; (d) the correlation between biomarker changes over time and reperfusion status (ECG myocardial reperfusion vs ECG MVO).
Statistical analysis
Continuous variables were expressed as mean±standard deviation or median (interquartile range), if they followed a normal or non-normal distribution, respectively, while dichotomous variables were expressed as numbers and percentages. The unpaired T-test or Mann Whitney U-test were used for the comparison of continuous variables between the two groups (angiographic reperfusion vs angiographic MVO; ECG reperfusion vs ECG MVO); categorical variables were compared using the chi square test or Fisher’s exact test, as appropriate. Continuous variables were assessed at three time points (baseline, 24 h and at pre-discharge) in all patiens. Continuous variables assessed at these time points were then compared with ANOVA or Kruskal Wallis Test, as appropriate, between patients with angiographic reperfusion vs angiographic MVO or between patients with ECG reperfusion vs ECG MVO. Bonferroni’s correction for multiple comparisons was applied and a Bonferroni-adjusted (Ba)-p value was reported in the text for variables showing an overall p value<0.05. Finally, two-way analysis of variance for repeated measures was used to compare biomarker values at different time points (basal, at 24 h after PPCI, and at pre-discharge), in the two groups of patients (angiographic reperfusion vs angiographic MVO or ECG reperfusion vs ECG MVO). A two-tailed p value <0.05 was the level of statistical significance. All analysis were performed using the SPSS 17.0 software (Florence, Italy).
Results
Patient population
Baseline clinical and laboratory features of the overall study population are shown in Table 1, while angiographic and procedural characteristics are shown in Table 2. We enrolled 40 patients (age 63±11, 72.5% males) at first onset of ischemic heart disease for STEMI. Of note, 12 patients (30%) were diabetics, while mean time to PPCI was 211±110 min. The left anterior descending coronary artery was the most involved culprit lesion (n=21 patients, 52.5%), while 19 patients (47.5%) had multi-vessel disease. Of note, 33 potentially eligible patients were excluded, as previously reported, and they did not differ for baseline main clinical, laboratory and angiographic characteristics as compared to those included in the study (data not shown).
Table 1.
Baseline clinical characteristics, therapy on admission, laboratory and echocardiographic data of the study population and according to angiographic or electrocardiogram (ECG) microvascular obstruction (MVO).
| Variables | Overall population 40 (100%) | Angiographic reperfusion n=20 | Angiographic MVO n=20 | p | ECG reperfusion n=16 | ECG MVO n=24 | p |
|---|---|---|---|---|---|---|---|
| Clinical characteristics | |||||||
| Age (years) | 63±11 | 65±10 | 62±12 | 0.34 | 62±10 | 64±12 | 0.48 |
| Sex, men (%) | 29 (72) | 16 (80) | 13 (65) | 0.48 | 11 (69) | 18 (75) | 0.73 |
| Hypertension, n (%) | 21 (52) | 10 (50) | 11 (52) | 0.99 | 8 (50) | 13 (54) | 0.95 |
| Current smoker, n (%) | 25 (62) | 13 (65) | 12 (60) | 0.97 | 10 (62) | 15 (62) | 0.99 |
| Dyslipidemia, n (%) | 20 (50) | 9 (45) | 11 (55) | 0.75 | 8 (50) | 12 (50) | 0.99 |
| Diabetes mellitus, n (%) | 12 (30) | 5 (25) | 7 (35) | 0.73 | 4 (25) | 8 (33) | 0.73 |
| Family history of CAD, n (%) | 14 (35) | 6 (43) | 8 (57) | 0.74 | 5 (31) | 9 (37) | 0.74 |
| Body mass index, kg/m2 | 26.9±3.0 | 26.9±1.9 | 26.9±1.8 | 0.90 | 26.3±2.8 | 27.3±3.1 | 0.31 |
| Time pre-PPCI, min | 211±110 | 158±72 | 223±99 | 0.04 | 166±96 | 213±97 | 0.05 |
| Therapy on admission | |||||||
| Beta-blockers, n (%) | 7 (17) | 4 (20) | 3 (15) | 0.95 | 3 (18.8) | 4 (16.7) | 0.95 |
| ACE inhibitors, n (%) | 27 (67) | 8 (61) | 5 (25) | 0.50 | 7 (44) | 6 (25) | 0.31 |
| Statins, n (%) | 11 (27) | 5 (25) | 6 (30) | 0.95 | 2 (12) | 9 (37) | 0.15 |
| Aspirin, n (%) | 18 (45) | 8 (40) | 10 (50) | 0.75 | 5 (31) | 13 (54) | 0.21 |
| Laboratory data | |||||||
| Blood glucose, mg/dl | 146 (105–212) | 137 (108–187) | 148 (112–205) | 0.49 | 139 (111–182) | 147 (111–197) | 0.53 |
| White blood cells, 109/l | 11.2±3.7 | 9.4±3.1 | 1.1±3.5 | 0.18 | 10.6±0.3 | 11.5±0.3 | 0.43 |
| Platelets, 109/l | 262±58 | 233±47 | 262±58 | 0.57 | 239±61 | 253±51 | 0.76 |
| Creatinine clearance, ml/min | 78±20 | 81±18 | 78±19 | 0.30 | 76±23 | 79±17 | 0.74 |
| LDL, mg/dl | 113±30 | 103±20 | 110±27 | 0.40 | 99±24 | 111±23 | 0.13 |
| C-reactive protein (mg/dl | 2.54 (0.99–3.47) | 2.14 (0.96–2.43) | 2.88 (1.59–3.33) | 0.12 | 2.79 (1.57–3.18) | 2.29 (1.19–2.84) | 0.12 |
| Fibrinogen, mg/dl | 265±83 | 247±77 | 281±81 | 0.44 | 298±94 | 352±91 | 0.06 |
| Peak creatine kinase, IU/l | 1571 (487–2874) | 814 (273–1669) | 1752 (508–2748) | 0.01 | 780 (302–1541) | 1602 (511–2417) | 0.01 |
| Peak creatine kinase MB, ng/ml | 159 (76–314) | 113 (44–278) | 179 (54–301) | 0.03 | 121 (54–289) | 181 (57–314) | 0.04 |
| Peak troponin-T, ng/ml | 2.99 (1.78–6.42) | 2.48 (1.40–5.32) | 4.01 (3.11–7.57) | 0.01 | 2.32 (1.19–5.42) | 3.80 (2.51–6.99) | 0.06 |
| Echocardiographic data | |||||||
| Ejection fraction | 0.47±0.9 | 0.51±0.6 | 0.44±10 | 0.08 | 0.44±9 | 0.49±0.7 | 0.37 |
ACE inhibitor: angiotensin-converting-enzyme inhibitor; CAD: coronary artery disease; LDL: low density lipoprotein; PPCI: primary percutaneous coronary intervention. Data are expressed as mean±standard deviation or median and interquartile range unless otherwise stated.
Table 2.
Procedural and angiographic characteristics of the study population and according to angiographic or electrocardiogram (ECG) microvascular obstruction (MVO).
| Variables | Overall population 40 (100%) | Angiographic reperfusion n=20 | Angiographic MVO n=20 | p | ECG reperfusion n=16 | ECG MVO n=24 | p |
|---|---|---|---|---|---|---|---|
| Angiographic data | |||||||
| Culprit vessel | |||||||
| Left anterior descending artery | 21 (52) | 11 (55) | 10 (50) | 7 (44) | 14 (58) | ||
| Circumflex artery | 4 (10) | 2 (10) | 2 (10) | 0.73 | 2 (12) | 2 (8) | 0.41 |
| Right coronary artery | 15 (37) | 7 (35) | 8 (40) | 7 (44) | 8 (33) | ||
| Multivessel disease | 19 (47) | 8 (40) | 11 (55) | 0.53 | 5 (31) | 14 (58) | 0.12 |
| Pre-TIMI 0 | 28 (70) | 15 (75) | 13 (65) | 0.73 | 10 (62) | 18 (75) | 0.49 |
| Pre-TIMI 1 | 12 (30) | 5 (25) | 7 (35) | 6 (37) | 6 (25) | ||
| Pre- MBG 0–1 | 36 (90) | 18 (90) | 18 (90) | 0.95 | 15 (94) | 21 (87) | 0.90 |
| Post-TIMI 0–1 | 4 (10) | 0 | 4 (20) | 0.05 | 1 (6) | 2 (8) | 0.001 |
| Post-MBG 0–1 | 15 (37) | 0 | 15 (75) | 0.001 | 2 (13) | 13 (54) | 0.004 |
| Rentrop 0–1 | 4 (10) | 2 (10) | 2 (10) | 0.95 | 2 (12) | 2 (8) | 0.95 |
| High thrombus score (4–5) | 27 (67) | 13 (65) | 14 (70) | 0.95 | 10 (63) | 17 (71) | 0.74 |
| Procedural data | |||||||
| Predilation | 18 (45) | 10 (50) | 8 (40) | 0.74 | 7 (43.7) | 11 (45.8) | 0.95 |
| Drug-eluting stent | 27 (67) | 15 (75) | 12 (60) | 0.50 | 14 (87) | 13 (54) | 0.04 |
| Postdilation | 24 (60) | 13 (65) | 11 (55) | 0.74 | 10 (62) | 14 (58) | 0.95 |
| Complete revascularization | 31 (77) | 16 (80) | 15 (75) | 0.90 | 13 (81) | 18 (75) | 0.72 |
MBG: myocardial blush grade; TIMI: thrombolysis in myocardial infarction. Data are n (%) unless otherwise stated.
Correlates of angiographic or ECG MVO among baseline clinical data
Patients showing angiographic MVO were similar for demographics and therapy on admission as compared to patients with angiographic reperfusion, but exhibited significantly longer time to PPCI (158±72 min vs 223±99 min, p=0.04). Twenty-four patients (60%) had ECG MVO. Patients showing ECG MVO were similar for demographics and therapy on admission as compared to patients with ECG reperfusion. Patients with ECG MVO had significantly longer time to PPCI as compared to those presenting with ECG myocardial reperfusion (166±96 min vs 213±97 min, p=0.05). Patients with ECG MVO tended to have higher serum levels of fibrinogen as compared to those showing ECG reperfusion (352±91 mg/dl vs 298±94 mg/dl, p=0.06).
NOX2 and 8-iso-PGF2α levels in patients with angiographic or ECG MVO
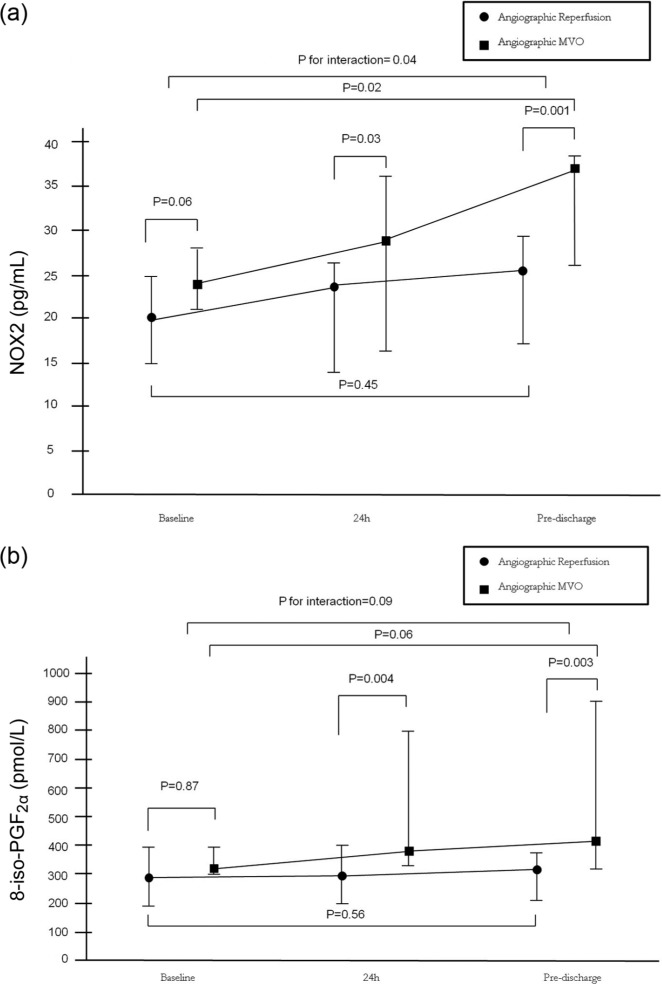
Time profiles of NOX2 and 8-iso-PGF2α levels according to angiographic or ECG patterns of myocardial reperfusion before PPCI, 24 h after PPCI and at pre-discharge are reported in Table 3 and Figures 1 and 2. Baseline NOX2 levels tended to be higher in patients presenting angiographic MVO (24 (21–27.75) pg/mL) as compared with those with myocardial reperfusion (20.25 (15–24.75) pg/mL), p=0.06, while 8-iso-PGF2α levels were similar between the two groups (p=0.87). NOX2 levels increased over time in patients with angiographic MVO (p=0.02), while they did not change over time in patients with angiographic myocardial reperfusion (p=0.45), with a significant interaction between baseline and pre-discharge levels in the two groups (p=0.04). The levels of 8-iso-PGF2α tended to increase over time in patients with angiographic MVO (p=0.06), while they did not modify over time in patients with angiographic myocardial reperfusion (p=0.56), with a trend of interaction between baseline and pre-discharge levels in the two groups (p=0.09).
Table 3.
Temporal evolution of gp91phox (NOX2) and 8-iso-PGF2α (ISO) levels in the study population and according to angiographic or electrocardiogram (ECG) microvascular obstruction (MVO).
| Variables | Overall population 40 (100%) | Angiographic reperfusion n=20 | Angiographic MVO n=20 | p | ECG reperfusion n=16 | ECG MVO n=24 | p |
|---|---|---|---|---|---|---|---|
| NOX2 T0 | 21.5 (18–25.35) | 20.25 (15–24.75) | 24 (21–27.75) | 0.06 | 21.25 (15.25–22.75) | 25 (21.25–27.75) | 0.13 |
| NOX2 T1 | 22 (12.35–31.25) | 23.75 (14–26.25) | 29 (16.25–36.00) | 0.03 | 20.50 (14–30.75) | 22 (13.25–29.25) | 0.77 |
| NOX2 T2 | 22.25 (13.25–27.25) | 25.50 (17–29.25) | 37.25 (26.25–38) | 0.001 | 23 (12–29.25) | 24.50 (14.25–35) | 0.38 |
| ISO T0 | 333.50 (272–457.30) | 295 (183.50–389.25) | 305 (292.50–392.50) | 0.87 | 320 (292.50–400) | 300 (203–378) | 0.73 |
| ISO T1 | 334 (231–478) | 300 (197–400) | 385 (326.25–797.50) | 0.004 | 347.50 (197–400.75) | 359.50 (300–512.50) | 0.13 |
| ISO T2 | 378 (289–480) | 322 (206–370) | 375 (320–900) | 0.003 | 322 (185–370) | 370.50 (308–472.75) | 0.04 |
T0: baseline; T1: 24 h; T2: pre-discharge. Data are expressed as median and interquartile range unless otherwise stated.
Figure 1.
(a) Time profiles of gp91phox (NOX2) levels according to angiographic patterns of myocardial reperfusion (angiographic myocardial reperfusion vs angiographic microvascular obstruction (MVO)) before primary percutaneous coronary intervention (PPCI), 24 hours after PPCI and at pre-discharge. (b)Time profiles of 8-iso-PGF2α levels according to angiographic patterns of myocardial reperfusion (angiographic myocardial reperfusion vs angiographic MVO) before PPCI, 24 h after PPCI and at pre-discharge. Data are given as median and ranges, • indicates angiographic myocardial reperfusion, ■ indicates angiographic MVO.
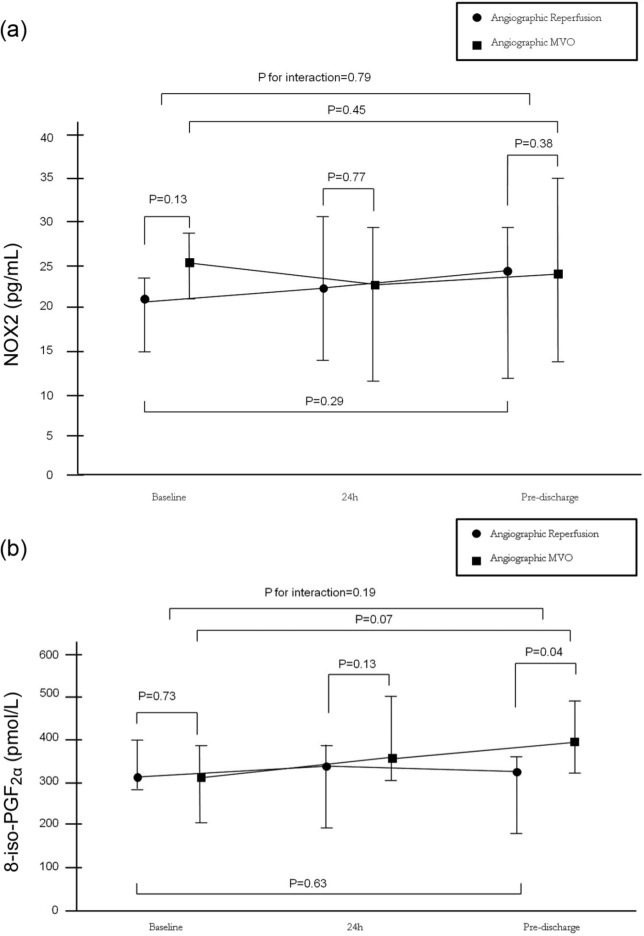
Figure 2.
(a)Time profiles of gp91phox (NOX2) levels according to angiographic patterns of myocardial reperfusion (electrocardiogram (ECG) myocardial reperfusion vs ECG microvascular obstruction (MVO)) before primary percutaneous coronary intervention (PPCI), 24 h after PPCI and at pre-discharge. (b)Time profiles of 8-iso-PGF2α levels according to angiographic patterns of myocardial reperfusion (ECG myocardial reperfusion vs ECG MVO) before PPCI, 24 h after PPCI and at pre-discharge. Data are given as median and ranges, • indicates electrocardiographic myocardial reperfusion, ■ indicates electrocardiographic MVO.
Baseline NOX2 and 8-iso-PGF2α levels were similar between patients with ECG MVO and ECG reperfusion (p=0.13 and p=0.73, respectively). NOX2 levels did not change over time in patients with ECG myocardial reperfusion or ECG MVO. Levels of 8-iso-PGF2α tended to increase over time in patients with ECG MVO (p=0.07), while they did not change over time in patients with ECG myocardial reperfusion (p=0.63), with no significant interaction between baseline and pre-discharge levels in the two groups (p=0.19).
Correlates of NOX2 and 8-iso-PGF2α levels
Correlates of baseline NOX2 and 8-iso-PGF2α levels and of changes of levels over time with main dichotomous and continuous variables in the overall population are shown in the Online Appendix (Online Appendix, Table 1). Patients with arterial hypertension, dyslipidemia and diabetes had significant higher levels of NOX2 levels as compared to those without (25 (17.5–29.35) pg/mL vs 21.25 (16.25–24.25) pg/mL, p=0.04; 25.75 (14.50–30.25) pg/mL vs 22.50 (14.25–25.75) pg/mL, p=0.03, 26.50 (15.25–32.50) pg/mL vs 22.25 (13.25–26.25) pg/mL, p=0.02, respectively). Moreover, current smokers tended to have higher levels of NOX2 levels as compared to those not smoker (p=0.06).
Concordantly, 8-iso-PGF2α levels were, or tended to be, significantly higher in patients with these cardiovascular risk factors as compared to those patients without (Online Appendix, Table 1). Interestingly, patients on statin treatment tended to have lower NOX2 and 8-iso-PGF2α levels as compared to those not taking statins on admission (20.50 (14.75–27.50) pg/mL vs 24.75 (17.25–31.50) pg/mL, p=0.08; 295.25 (201–354.50) pmol/L vs 334 (222.25–400.25) pmol/L, p=0.07). Finally, NOX2 levels tended to positively correlate with admission glycemia (r=0.34, p=0.09), and C-reactive protein (r=0.021, p=0.07) and peak creatine kinase-MB (r=0.37, p=0.08) and peak troponin T (r=0.41 and p=0.06). Similarly findings were observed for 8-iso-PGF2α levels (Online Appendix, Table 1).
Discussion
This study shows that patients with MVO after PPCI, but not those with myocardial reperfusion, have a sustained increase of both NOX2 and 8-iso-PGF2α levels, reliable markers of oxidative stress that persist at pre-discharge, suggesting that platelet-mediated ROS generation may be involved in MVO.
The primary therapeutic goal in patients with STEMI is the timely reopening of the infarct-related artery.25 However, despite successful reopening of the epicardial vessel, the obstruction of the microcirculation may hamper myocardial reperfusion.4 Such a phenomenon, firstly described in the animal model,7 has been defined no-reflow and more recently MVO as the obstruction of coronary microcirculation due to cell plugging, distal embolization, vasoconstriction and extramural compression is the main pathogenetic mechanism.26 Accordingly, manual thrombus-aspiration is recommended by the guidelines.25 In the last few years, many efforts have been made in order to counteract further reperfusion injury but with conflicting results.9 Pharmacological targets of reperfusion injury may be platelets, neutrophils or other mediators with ROS playing a crucial role.6
Among the cellular sources of ROS, NADPH oxidase plays a major role; detection of its catalytic sub-unit NOX2 in serum maximally (90%) reflects NOX2 activity by platelets, leucocytes and monocytes.13 Our study shows for the first time that patients with MVO have a sustained release of serum NOX2 that may unfavorably affect the microcirculation through different mechanisms.
It is worth noting, however, that soluble NOX2 reflects maximally NOX2 generated by blood cells, and therefore we cannot provide insight on NOX2 potentially generated by endothelial cells.11–17 Even if this is a limitation of the study, we believe that our analysis may be relevant for oxidant-mediated MVO because both platelets and white cells may interact with damaged endothelium, further determining vasoconstriction. Additionally, our biochemical data derive from systemic blood, not from the coronary circulation. However, our samples were obtained immediately after myocardial revascularization, thus suggesting that NOX2 increases mainly reflect the ischemia-reperfusion process.
NOX2 may cause damage in a number of ways. Firstly, NOX2 per se has been shown to impair arterial dilatation in isolated aorta;27 accordingly patients with hereditary deficiency of the NOX2 have significantly lower flow-mediated dilation as compared to healthy subjects.28 Secondly, NOX2 leads to the production of ROS that may directly damage mitochondria leading to irreversible reperfusion injury.29 Thirdly, NOX2 is involved in the generation of isoprostanes that in turn trigger platelet activation, with a mechanism insensitive to aspirin.11 Fourthly, NOX2, by oxidating phospholipase A2, leads to the production of thromboxane A2 that is a potent vasoconstrictor and platelet activator.11 Finally, a pro-inflammatory role of NOX2 has been recently suggested, that may enhance MVO by causing edema and extramural compression.30
The sustained increased production of 8-iso-PGF2α found in our study in patients with MVO may also play a contributory role in its pathogenesis and persistence. A previous study showed increased urinary excretion of 8-iso-PGF2α in patients with STEMI treated by thrombolytic therapy with a peak after 6 h and return to baseline levels after 24 h.31 Our findings expand this observation and suggest that patients with MVO are prone to sustained production of 8-iso-PGF2α as compared to those having good reperfusion. Again, mechanisms by which isoprostanes may favor MVO are multiple. 8-iso-PGF2α is a vasoconstrictor and stimulates platelet activation and aggregation.32 Interestingly, platelet incubation with aspirin fully prevents anoxia-reoxygenation induced thromboxane A2 production but it does not affect 8-iso-PGF2α production.11 Accordingly, the increase of coronary sinus 8-iso-PGF2α levels during PCI33 is not prevented by aspirin.11 8-iso-PGF2α may also favor MVO by inducing endothelin-1 release, another mediator of MVO,34 and by inducing beta2-integrin mediated adhesion of neutrophils35 that, along with platelets, plays a major role in reperfusion injury. Furthermore, a recent study found that isoprostanes inhibit angiogenesis that in turn negatively modulates the reversibility of MVO over time.36
Our results suggest that baseline levels of NOX2 and 8-iso-PGF2α levels may be affected by some risk factors and therapy on admission. However, neither risk factors nor admission therapies were related to NOX2 and 8-iso-PGF2α level changes over time, while patients with or without MVO had no differences in risk factors or baseline therapies. Interestingly, in our study, 8-iso-PGF2α level changes over time showed a statistically borderline association with ECG MVO, while this was not observed for NOX2.
Our study may have some therapeutic implications. Indeed, NOX2-mediated release of ROS may be antagonized by drugs. In particular, a recent study suggests that in patients with hypercholesterolemia, a dose of 40 mg of atorvastatin can reduce NOX2 and 8-iso-PGF2α levels starting two hours after administration.37 This finding may be useful for patients with acute STEMI where rapid inhibition of ROS production may be of importance. Furthermore, the emerging anti-thrombotic effects of statins are in keeping with meta-analysis data showing that peri-procedural myocardial infarction in urgent or elective PCI is reduced by pre-procedural statin administration.38 Of note in the study by Pignatelli et al., a dose of 40 mg of atorvastatin was also able to reduce thromboxane A2 levels after 24 h, thus suggesting that multiple mechanisms leading to platelet activation may be afforded by such drugs.37
Another potential approach able to antagonize ROS detrimental effects is the administration of intravenous infusion of high dose of vitamin C. Indeed, a study by Basili et al. showed that blood thromboxane A2, 8-iso-PGF2α and NOX2-derived peptide levels did not change after PCI after two hours in patients treated with 1 g of vitamin C while they increased in placebo-treated patients.11 The same group also showed that the rate of TIMI myocardial perfusion grade <2, a measure of microvascular perfusion after PCI, was higher in placebo-treated patients (32%) as compared to vitamin C-treated patients (4%).39
Finally, accordingly to previous data,1 we found that patients with a long ischemic time had a higher incidence of MVO. A trend to a higher rate of MVO occurrence was observed in our study in diabetic patients and in patients with a raised inflammatory milieu, as assessed by C-reactive protein levels. Indeed, we acknowledge the small sample size may have underpowered these statistical associations.
In conclusion, patients with MVO are prone to sustained NOX2-mediated ROS production leading also to sustained 8-iso-PGF2α release. Therapies targeting NOX2 or high dosage antioxidant shown to be effective in lowering NOX2 activity should be tested in randomized trials for the prevention or the treatment of MVO.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Niccoli G, Burzotta F, Galiuto L, et al. Myocardial no-reflow in humans. J Am Coll Cardiol 2009; 54: 281–292 [DOI] [PubMed] [Google Scholar]
- 2. Kammler J, Kypta A, Hofmann R, et al. TIMI 3 flow after primary angioplasty is an important predictor for outcome in patients with acute myocardial infarction. Clin Res Cardiol 2009; 98: 165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu KC, Zerhouni EA, Judd RM, et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 1998; 97: 765–772 [DOI] [PubMed] [Google Scholar]
- 4. Ito H. No-reflow phenomenon in patients with acute myocardial infarction: Its pathophysiology and clinical implications. Acta Med Okayama 2009; 63: 161–168 [DOI] [PubMed] [Google Scholar]
- 5. Svilaas T, Vlaar PJ, van der Horst IC, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med 2008; 358: 557–567 [DOI] [PubMed] [Google Scholar]
- 6. Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007; 357: 1121–1135 [DOI] [PubMed] [Google Scholar]
- 7. Kloner RA, Ganote CE, Jennings RB. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest 1974; 54: 1496–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rochitte CE, Lima JA, Bluemke DA, et al. Magnitude and time course of microvascular obstruction and tissue injury after acute myocardial infarction. Circulation 1998; 98: 1006–1014 [DOI] [PubMed] [Google Scholar]
- 9. Niccoli G, Kharbanda RK, Crea F, et al. No-reflow: Again prevention is better than treatment. Eur Heart J 2010; 31: 2449–2455 [DOI] [PubMed] [Google Scholar]
- 10. Ambrosio G, Weisman HF, Mannisi JA, et al. Progressive impairment of regional myocardial perfusion after initial restoration of postischemic blood flow. Circulation 1989; 80: 1846–1861 [DOI] [PubMed] [Google Scholar]
- 11. Basili S, Pignatelli P, Tanzilli G, et al. Anoxia-reoxygenation enhances platelet thromboxane A2 production via reactive oxygen species-generated NOX2: Effect in patients undergoing elective percutaneous coronary intervention. Arterioscler Thromb Vasc Biol 2011; 31: 1766–1771 [DOI] [PubMed] [Google Scholar]
- 12. Seno T, Inoue N, Gao D, et al. Involvement of NADH/NADPH oxidase in human platelet ROS production. Thromb Res 2001; 103: 399–409 [DOI] [PubMed] [Google Scholar]
- 13. Pignatelli P, Sanguigni V, Lenti L, et al. Gp91phox-dependent expression of platelet CD40 ligand. Circulation 2004; 110: 1326–1329 [DOI] [PubMed] [Google Scholar]
- 14. Morrow JD, Roberts LJ., 2nd The isoprostanes. Current knowledge and directions for future research. Biochem Pharmacol 1996; 51: 1–9 [DOI] [PubMed] [Google Scholar]
- 15. Pignatelli P, Carnevale R, Di Santo S, et al. Inherited human gp91phox deficiency is associated with impaired isoprostane formation and platelet dysfunction. Arterioscler Thromb Vasc Biol 2011; 31: 423–434 [DOI] [PubMed] [Google Scholar]
- 16. Carnevale R, Pignatelli P, Lenti L, et al. LDL are oxidatively modified by platelets via gp91(phox) and accumulate in human monocytes. FASEB J 2007; 21: 927–934 [DOI] [PubMed] [Google Scholar]
- 17. Pignatelli P, Carnevale R, Cangemi R, et al. Atorvastatin inhibits gp91phox circulating levels in patients with hypercholesterolemia. Arterioscler Thromb Vasc Biol 2010; 30: 360–367 [DOI] [PubMed] [Google Scholar]
- 18. Hoffman SW, Roof RL, Stein DG. A reliable and sensitive enzyme immunoassay method for measuring 8-isoprostaglandin F2 alpha: A marker for lipid peroxidation after experimental brain injury. J Neurosci Methods 1996; 68: 133–136 [DOI] [PubMed] [Google Scholar]
- 19. TIMI Study Group The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. N Engl J Med 1985; 312: 932–936 [DOI] [PubMed] [Google Scholar]
- 20. Van ’t Hof AW, Liem A, Suryapranata H, et al. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: Myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation 1998; 97: 2302–2306 [DOI] [PubMed] [Google Scholar]
- 21. Gibson CM, Murphy SA, Morrow DA, et al. Angiographic perfusion score: An angiographic variable that integrates both epicardial and tissue level perfusion before and after facilitated percutaneous coronary intervention in acute myocardial infarction. Am Heart J 2004; 148: 336–340 [DOI] [PubMed] [Google Scholar]
- 22. Rentrop KP, Cohen M, Blanke H, et al. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol 1985; 5: 587–592 [DOI] [PubMed] [Google Scholar]
- 23. Gibson CM, de Lemos JA, Murphy SA, et al. Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction: A TIMI 14 substudy. Circulation 2001; 103: 2550–2554 [DOI] [PubMed] [Google Scholar]
- 24. Sorajja P, Gersh BJ, Costantini C, et al. Combined prognostic utility of ST-segment recovery and myocardial blush after primary percutaneous coronary intervention in acute myocardial infarction. Eur Heart J 2005; 26: 667–674 [DOI] [PubMed] [Google Scholar]
- 25. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127: 529–555 [DOI] [PubMed] [Google Scholar]
- 26. Bekkers SC, Yazdani SK, Virmani R, et al. Microvascular obstruction: Underlying pathophysiology and clinical diagnosis. J Am Coll Cardiol 2010; 55: 1649–1660 [DOI] [PubMed] [Google Scholar]
- 27. Takenouchi Y, Kobayashi T, Matsumoto T, et al. Gender differences in age-related endothelial function in the murine aorta. Atherosclerosis 2009; 206: 397–404 [DOI] [PubMed] [Google Scholar]
- 28. Violi F, Sanguigni V, Carnevale R, et al. Hereditary deficiency of gp91(phox) is associated with enhanced arterial dilatation: Results of a multicenter study. Circulation 2009; 120: 1616–1622 [DOI] [PubMed] [Google Scholar]
- 29. Sorescu D, Weiss D, Lassegue B, et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 2002; 105: 1429–1435 [DOI] [PubMed] [Google Scholar]
- 30. Lassegue B, Griendling KK. NADPH oxidases: Functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol 2010; 30: 653–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reilly MP, Delanty N, Roy L, et al. Increased formation of the isoprostanes IPF2alpha-I and 8-epi-prostaglandin F2alpha in acute coronary angioplasty: Evidence for oxidant stress during coronary reperfusion in humans. Circulation 1997; 96: 3314–3320 [DOI] [PubMed] [Google Scholar]
- 32. Patrono C, FitzGerald GA. Isoprostanes: Potential markers of oxidant stress in atherothrombotic disease. Arterioscler Thromb Vasc Biol 1997; 17: 2309–2315 [DOI] [PubMed] [Google Scholar]
- 33. Iuliano L, Pratico D, Greco C, et al. Angioplasty increases coronary sinus F2-isoprostane formation: Evidence for in vivo oxidative stress during PTCA. J Am Coll Cardiol 2001; 37: 76–80 [DOI] [PubMed] [Google Scholar]
- 34. Xia Z, Kuo KH, Godin DV, et al. 15-F(2t)-isoprostane exacerbates myocardial ischemia-reperfusion injury of isolated rat hearts. Am J Physiol Heart Circ Physiol 2005; 289: H1366–H1372 [DOI] [PubMed] [Google Scholar]
- 35. Fontana L, Giagulli C, Cominacini L, et al. Beta2 integrin-dependent neutrophil adhesion induced by minimally modified low-density lipoproteins is mainly mediated by F2-isoprostanes. Circulation 2002; 106: 2434–2441 [DOI] [PubMed] [Google Scholar]
- 36. Benndorf RA, Schwedhelm E, Gnann A, et al. Isoprostanes inhibit vascular endothelial growth factor-induced endothelial cell migration, tube formation, and cardiac vessel sprouting in vitro, as well as angiogenesis in vivo via activation of the thromboxane A(2) receptor: A potential link between oxidative stress and impaired angiogenesis. Circ Res 2008; 103: 1037–1046 [DOI] [PubMed] [Google Scholar]
- 37. Pignatelli P, Carnevale R, Pastori D, et al. Immediate antioxidant and antiplatelet effect of atorvastatin via inhibition of Nox2. Circulation 2012; 126: 92–103 [DOI] [PubMed] [Google Scholar]
- 38. Patti G, Cannon CP, Murphy SA, et al. Clinical benefit of statin pretreatment in patients undergoing percutaneous coronary intervention: A collaborative patient-level meta-analysis of 13 randomized studies. Circulation 2011; 123: 1622–1632 [DOI] [PubMed] [Google Scholar]
- 39. Basili S, Tanzilli G, Mangieri E, et al. Intravenous ascorbic acid infusion improves myocardial perfusion grade during elective percutaneous coronary intervention: Relationship with oxidative stress markers. JACC Cardiovasc Interv 2010; 3: 221–229 [DOI] [PubMed] [Google Scholar]