Abstract
Rationale
Several studies suggest that repeated nicotine administration causes alterations in glutaminergic transmission that may play an important role in developing and maintaining nicotine addiction. Chronic nicotine administration in rats decreases the expression of the glutamate transporter-1 (GLT-1) and cysteine–glutamate exchanger (system xC−) in the nucleus accumbens. We hypothesized that ceftriaxone, a GLT-1 and system xC− activator, would decrease murine behavioral aspects of nicotine dependence.
Objective
This study aimed to investigate the effect of repeated ceftriaxone administration on the behavioral effects of nicotine using mouse models of conditioned reward and withdrawal.
Method
Using male ICR mice, the ability of repeated ceftriaxone injections to modulate the development and reinstatement of a nicotine-conditioned place preference (CPP) was evaluated. Additionally, nicotine withdrawal-associated signs were assessed. These included both physical (somatic signs and hyperalgesia) and affective (anxiety-related behaviors) withdrawal signs in mice. Finally, the effects of ceftriaxone on nicotine-induced antinociception and hypothermia after acute nicotine injection were measured.
Result
Ceftriaxone had no effect on the development of nicotine preference but significantly attenuated nicotine-induced reinstatement of CPP. Furthermore, ceftriaxone reversed all nicotine withdrawal signs measured in mice.
Conclusion
Altogether, these findings show that a β-lactam antibiotic reduces nicotine withdrawal and nicotine-seeking behavior. Our results suggest that the documented efficacy of ceftriaxone against cocaine and morphine dependence-related behaviors effects extends to nicotine.
Keywords: Ceftriaxone, GLT-1, Conditioned place preference, Nicotine, Reward, Withdrawal, Reinstatement
Introduction
Tobacco use is the leading cause of premature death in the USA and around the world. Nicotine is the principal reinforcing component in tobacco smoke thought to be responsible for the addictive properties of cigarettes. The reinforcing and withdrawal effects of nicotine are widely accepted to be responsible for the initiation and maintenance of the tobacco smoking habit (Castane et al. 2005; Stolerman and Jarvis 1995). Despite the proven efficacy of some current pharmacotherapies for tobacco addiction, relapse rates remain high, indicating that novel or more efficacious medications and/or novel approaches are needed.
While ample evidence supports a crucial role of the dopamine system in nicotine addiction, increasing data suggest that nicotine-induced adaptations in glutamatergic neurotransmission play an important role in the development of nicotine dependence (Liechti and Markou 2008; Watkins et al. 2000). For example, nicotine administration increases glutamatergic transmission in brain areas involved in reward processes, including the nucleus accumbens and the ventral tegmental area (Reid et al. 2000; Saellstroem Baum et al. 2006). In addition, several studies by Markou and colleagues showed that repeated administration of nicotine leads to a decrease in glutamate signaling in several brain regions (for reviews, see Kenny and Markou 2004; Markou 2008). More specifically, during early withdrawal, chronic nicotine self-administration resulted in the downregulation of predominantly presynaptic mGlu2/3 receptor function in areas such as the nucleus accumbens and the ventral tegmental area (Liechti et al. 2007). Furthermore, antagonism of the rat mGlu5 receptor by MPEP selectively decreased nicotine, but not food, self-administration and appears to worsen the somatic signs and reward deficits of nicotine withdrawal (Liechti and Markou 2007; but see Stoker et al. 2012). Collectively, these reports suggest that a “hypoglutamatergic” tone may play an important role in maintaining nicotine dependence. Thus, ameliorating the nicotine-induced alterations in glutamatergic transmission may help promote smoking cessation by reversing a state of withdrawal and by preventing the reinstatement of nicotine seeking.
The glial glutamate transporter-1 (GLT-1), a sodium-dependent transporter found on astrocytes, is responsible for the removal of at least 90 % of extrasynaptic glutamate (Anderson and Swanson 2000; Rothstein et al. 1994). Both cocaine and nicotine self-administration in rodents downregulate the expression of GLT-1 in the accumbens core (Kau et al. 2008; Knackstedt et al. 2009, 2010). Ceftriaxone, a β-lactam antibiotic, increases the synthesis and membrane insertion of GLT-1 (Miller et al. 2008; Rothstein et al. 2005). Interestingly, chronically treating animals trained to self-administer cocaine with ceftriaxone restored GLT-1 and prevented reinstatement of rat cocaine-seeking behavior (Knackstedt et al. 2010; Sari et al. 2009). Moreover, ceftriaxone also inhibits the development of physical dependence on morphine in rats (Rawls et al. 2010). However, the effects of ceftriaxone on nicotine dependence are currently unknown. Recently, ceftriaxone was reported to enhance nicotine-induced antinociception in the tail flick test and to attenuate the development of chronic nicotine tolerance in rats (Schroeder et al. 2011).
Based on these earlier studies, we hypothesize here that ceftriaxone administration will also influence the behavioral effects of nicotine. For that, we assessed the effects of repeated ceftriaxone administration on nicotine reward using the conditioned place preference (CPP) paradigm and physical (somatic signs and hyperalgesia) and affective (anxiety-related behaviors) nicotine withdrawal signs in mice. In addition, the effects of repeated administration on drug-induced reinstatement of nicotine in the CPP test were measured. Finally, we measured the effects of ceftriaxone on nicotine-induced antinociception and hypothermia after a single injection of nicotine.
Methods and materials
Drugs and chemicals
(−)-Nicotine hydrogen tartrate salt, ceftriaxone, and mecamylamine hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO, USA). All drugs were dissolved in physiological saline (0.9 % sodium chloride). All injections were administered in a volume of 10 ml/kg. Nicotine and mecamylamine were administered via subcutaneous injection (s.c.), while ceftriaxone was given intraperitoneally (i.p.). All doses are expressed as the free base of the drug.
Animals
Naive male 8- to 10-week-old ICR mice (Harlan Laboratories, Indianapolis, IN, USA) served as subjects. Mice were housed five per cage in a 21 °C humidity-controlled facility with ad libitum access to food and water on a normal light cycle (lights on 0700 h). The animal facility is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All experiments were performed during the animal’s light cycle by the same investigator at the same time of day and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Acute nicotine assessment
Naive mice were injected with either i.p. saline or ceftriaxone (200 mg/kg, i.p., twice per day at 9:00 am and 4:00 pm) for four consecutive days. On day 5, mice were treated with either saline or nicotine (0.5 or 2.5 mg/kg, s.c.). Antinociception using the tail flick and hot plate tests was measured 5 min after nicotine injection and changes in body temperature were measured 30 min after injection. These doses were based on previous studies of nicotine potency and time-course of these responses in the ICR mouse strain (Damaj et al. 1999).
Tail flick test
Antinociception was assessed by the tail flick method of D’Amour and Smith (1941). Mice were lightly restrained while a radiant heat source was directed onto the upper portion of the tail. A control response (average of 2–4 s) was determined for each mouse before treatment, and test latency was determined after drug administration. The apparatus has an automatic cutoff of 10 s to minimize tissue damage. Antinociceptive response for the tail flick test was expressed as the mean ± standard error of the mean (SEM) of the latency after drug treatment.
Hot plate test
Antinociception was also assessed using the hot plate test. Mice were placed into a 10-cm wide glass cylinder on a hot plate (Thermojust Apparatus, Columbus, OH, USA). The hot plate is a rectangular heated surface maintained at 55 °C and surrounded by a plexiglass enclosure. The device is connected to a manually operated timer that records the amount of time that the mouse spends on the heated surface before showing signs of nociception (e.g., jumping or paw licks). A control response (8–12 s) was determined for each mouse before treatment, and test latency was determined after drug administration. The timer has an automatic cutoff of 40 s to avoid tissue damage. Antinociceptive response for the hot plate test is expressed as the mean ± SEM of the latency after drug treatment.
Body temperature
Rectal temperature was measured by a thermistor probe (inserted 24 mm) and digital thermometer (YSI Inc., Yellow Springs, OH, USA). Readings were taken just before and 30 min after nicotine injection. The difference in rectal temperature before and after treatment was calculated for each mouse. The ambient temperature of the laboratory varied from 21 to 24 °C from day to day.
Nicotine CPP studies
An unbiased mouse CPP paradigm was utilized in all studies as described by Kota et al. (2007). Briefly, place conditioning chambers consisted of two distinct compartments separated by a smaller intermediate compartment with openings that allowed access to either side of the chamber. On day 1, animals were confined to the intermediate compartment for a 5-min habituation period and then allowed to move freely between compartments for 15 min. Time spent in each compartment was recorded. These data were used to separate the animals into groups of approximately equal bias. Days 2–4 were the conditioning days during which the saline group received saline in both compartments and drug groups received nicotine (0.5 mg/kg, s.c.) in one compartment and saline in the opposite compartment. This dose of nicotine has been established to produce significant CPP after s.c. injection in the ICR mouse (Kota et al. 2007). Separate groups of animals were pretreated with either saline or ceftriaxone (100 and 200 mg/kg, i.p.) 10 min before nicotine. Nicotine-paired compartments were randomized among all groups. Day 5 was the drug-free test day and the procedure was the same as day 1. Time spent on each side was measured and the preference score was expressed as time spent on the nicotine-paired side postconditioning minus time spent on the nicotine-paired side preconditioning. A positive number indicated a preference for the nicotine-paired side, whereas a negative number indicated an aversion to the nicotine-paired side. A number at or near zero indicated no side preference. Locomotor activity counts expressed as the number of cell interrupts on each side were recorded via photobeam breaks (Med Associates, St. Albans, VT, USA).
Nicotine-primed reinstatement of nicotine CPP
A nicotine CPP reinstatement paradigm was utilized, as described by Jackson et al. (2012). Briefly, two individual cohorts of nine mice were tested in the CPP paradigm and classified into group A and group B. On day 6, 1 day after test day (described previously), the test day procedure was repeated in both groups for three additional days to measure extinction of nicotine CPP. All mice received saline injections (s.c.) prior to being placed in the chambers on these days. During the 3 days of extinction, animals received either vehicle or ceftriaxone pretreatment (200 mg/kg, i.p.) 10 min before the saline injection. On the reinstatement test session on day 9, mice received an injection of nicotine (0.1 mg/kg, s.c.) and were immediately placed into the middle chamber for the evaluation of nicotine CPP reinstatement.
Chronic nicotine administration protocol
Mice were anesthetized with sodium pentobarbital (45 mg/kg, i.p.) and implanted with Alzet osmotic mini pumps [model 1007D (7 days) Durect Corporation, Cupertino, CA, USA] filled with (−)-nicotine or saline solution as described by Jackson et al. (2008). The concentration of nicotine was adjusted according to animal weight and mini pump flow rate.
Nicotine withdrawal assessment
Withdrawal studies were conducted as previously described by Jackson et al. (2008). Mice received nicotine (36 mg/kg/day) for 7 days. Beginning on day 4, mice were injected i.p. with either saline or ceftriaxone (20 or 200 mg/kg, twice per day at 9:00 am and 4:00 pm) for 4 days. On the morning of day 8, mice were injected with the nonselective nicotinic antagonist, mecamylamine (2 mg/kg, s.c.) and testing was initiated 15 min later. The mice were first evaluated for 5 min in the plus maze test for anxiety-related behavior, followed by a 20-min observation of somatic signs measured as paw and body tremors, head shakes, backing, jumps, curls, and ptosis. Hyperalgesia was evaluated in the hot plate test (on a 52 °C plate) immediately following the somatic sign observation period. The testing sequence was chosen based on our prior studies showing that this order of testing reduced within-group variability and produced the most consistent results (Jackson et al. 2008). An observer blinded to the experimental treatments scored behavior and analyzed the data.
Statistical analysis
A statistical analysis of all behavioral studies was performed with analysis of variance (ANOVA) with the post hoc Newman–Keuls test when appropriate. p values of <0.05 were considered to be statistically significant.
Results
Ceftriaxone did not disrupt the acquisition of nicotine CPP
In Fig. 1, as previously reported by our laboratory (Walters et al. 2006), mice conditioned with nicotine (0.5 mg/kg, s.c.) produced a robust and significant CPP in mice [two-way ANOVA pretreatment (F2, 46=0.4685, p =0.6289) × CPP treatment (F1, 46=80.99, p<0.0001)]. Repeated pretreatment with ceftriaxone (100 or 200 mg/kg, i.p.) did not significantly alter the development of nicotine CPP in mice conditioned with 0.5 mg/kg nicotine (p=0.63). In saline-conditioned mice, ceftriaxone did not produce a significant response.
Fig. 1.

Effects of ceftriaxone on the development of nicotine-induced CPP in the mouse. Nicotine (0.5 mg/kg, s.c.) induced a significant CPP in mice. Ceftriaxone had no effect on the development of nicotine CPP in mice conditioned with 0.5 mg/kg nicotine. Each point represents the mean ± SEM of eight mice per group. *p<0.05 vs. the saline and ceftriaxone control groups. CEF ceftriaxone, NIC nicotine, SAL saline
Ceftriaxone blocked nicotine-primed reinstatement of nicotine CPP
A significant main effect revealed a robust CPP established in mice in response to nicotine (0.5 mg/kg, s.c.) in both cohorts [F3, 12 =6.001, p =0.0097; Fig. 2]. After 3 days extinction from nicotine side pairing, no nicotine-paired side preference was observed, as supported by a significant two-way ANOVA treatment [F9, 111 = 3.157, p = 0.0020]. Repeated ceftriaxone treatment (200 mg/kg, i.p., 3 days) blocked nicotine-primed (0.1 vmg/kg, s.c.) CPP reinstatement (Fig. 2). Activity counts (number of interrupts) on the test day did not significantly differ between postconditioning reinstatement groups (vehicle/nicotine = 523.1 ±42.7; ceftriaxone/vehicle=573.3±71.1; ceftriaxone/nicotine= 577.5±73.4).
Fig. 2.

Effects of ceftriaxone on nicotine-primed reinstatement of nicotine CPP in the mouse. Mice conditioned with 0.5 mg/kg nicotine (s.c.) expressed a significant CPP on test day. Nicotine CPP was extinguished by extinction day 3. On day 8, nicotine CPP was reinstated by nicotine (0.1 mg/kg, s.c.). Ceftriaxone pretreatment blocked the reinstatement of CPP. Each point represents the mean ± SEM of five to seven mice per group. *p<0.05 vs. the corresponding saline group; ^p<0.05 vs. SAL–NIC(0.1) group. CEF ceftriaxone, NIC nicotine, SAL saline
Physical and affective precipitated nicotine withdrawal signs are attenuated by ceftriaxone
The nonselective nicotinic antagonist mecamylamine was used to precipitate affective and physical nicotine withdrawal signs in nicotine-infused mice. Anxiety-related behavior (affective), somatic signs, and hyperalgesia (physical) were measured in these mice. Following mecamylamine injection, animals exhibited a significant anxiety-related response [F4, 25 = 6.129, p < 0.01], an increase in total somatic signs [F4, 25=19.83, p <0.0001], and a decrease in hot plate latency following nicotine withdrawal [F4, 25= 7.128, p<0.0001] (Fig. 3). Repeated pretreatment with ceftriaxone (20 or 200 mg/kg, i.p.) significantly blocked somatic and hyperalgesia signs of nicotine withdrawal in a dose-related manner. In addition, mice exhibited a total loss of anxiety-related behavior [F4, 25=6.129, p<0.0014], attenuation of somatic signs [F4, 25=19.83, p<0.05], and an increased latency on the hot plate [F4, 25=7.128, p<0.05] after pretreatment with ceftriaxone at 200 mg/kg, i.p. (Fig. 3). The ceftriaxone doses used in this assessment did not significantly affect behavioral responses in any withdrawal test and did not precipitate nicotine withdrawal signs in nicotine-dependent mice (data not shown).
Fig. 3.
Effects of ceftriaxone pretreatment on nicotine withdrawal in the mouse. Mice chronically infused with nicotine for 7 days (36 mg/kg/day) were withdrawn on day 8 after injection of mecamylamine (2 mg/kg, s.c.). Significant a anxiety-related response, b increase in somatic withdrawal signs, and c hyperalgesia response were observed in nicotine-withdrawn mice. Ceftriaxone (200 mg/kg, i.p.) significantly attenuated the expression of both the physical and affective nicotine withdrawal responses in mice. Each point represents the mean ±SEM of six to eight mice per group. *p<0.05 vs. the SAL–SAL–MEC and SAL–CEF(200)–MEC control groups; ^p<0.05 vs. NIC–SAL–MEC group. CEF ceftriaxone, NIC nicotine, SAL saline
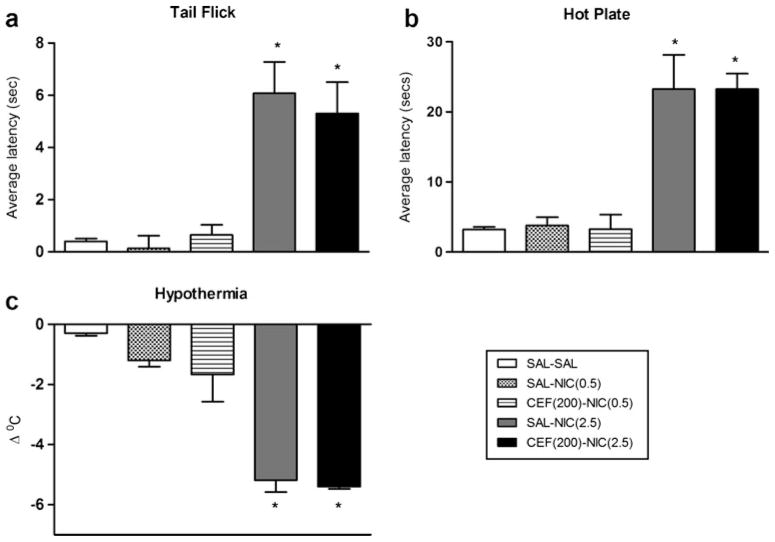
Effect of ceftriaxone on nicotine-induced hypothermia and antinociception
Mice were injected with nicotine (0.5 or 2.5 mg/kg, s.c.) and assessed for changes in body temperature and pain threshold. Antinociception was measured 5 min after injection using the tail flick and hot plate tests. Next, body temperature was assessed 30 min after injection. Acute nicotine produced a significant increase in response latency in the tail flick [F4, 23=15.08, p<0.0001] and hot plate tests [F4, 23= 19.54, p<0.0001] in a dose-related manner (Fig. 4a, b). A significant decrease in body temperature was also observed after acute injection of nicotine [F4, 23=19.45, p<0.0001] (Fig. 4c). Repeated ceftriaxone failed to enhance the low dose of nicotine (0.5 mg/kg) in the tail flick [F(4, 23)=15.08, p> 0.05] or the hot plate assays [F(4, 23)=19.54, p>0.05] or body temperature assessments [F(4, 23)=19.45, p>0.05] (Fig. 4). Similarly, ceftriaxone failed (p>0.05) to block the effects of an active dose of nicotine (2.5 mg/kg) in these tests (Fig. 4). When tested in nicotine-naive mice, ceftriaxone did not produce significant effects in the responses measured here (data not shown).
Fig. 4.
Effects of ceftriaxone on nicotine-induced hypothermia and antinociception. Mice received single injection of either saline or nicotine (0.5 or 2.5 mg/kg, s.c.) and were tested 5 min later for antinociception in the a tail flick and b hot plate tests; c changes in body temperature were measured 30 min later. A 4-day pretreatment with ceftriaxone failed to enhance or block the responses of the low and high doses of nicotine, respectively. Each point represents the mean ± SEM of six mice per group. *p<0.05 vs. the SAL–SAL, SAL–NIC(0.5), and CEF(200)–NIC(0.5) groups. CEF ceftriaxone, NIC nicotine, SAL saline
Discussion
The present study investigated the effect of ceftriaxone, a β-lactam antibiotic, on the behavioral effects of nicotine in mice thought to model critical aspects of nicotine dependence in humans. We report here that ceftriaxone attenuates the reinstatement of nicotine preference in mice. In contrast, the establishment of nicotine CPP was unaltered by ceftriaxone. Furthermore, ceftriaxone reversed the expression of both somatic and affective withdrawal signs in nicotine-dependent mice. Ceftriaxone has failed to block nicotine-induced hypothermia and antinociception after acute administration.
We report here for the first time that ceftriaxone given to mice in the days immediately prior to reinstatement testing attenuated nicotine-primed reinstatement in the CPP test. In contrast, a similar repeated administration of ceftriaxone failed to attenuate the acquisition of a nicotine preference. This finding is consistent with previous studies showing that ceftriaxone did not affect the acquisition of cocaine self-administration, but was able to attenuate reinstatement in rats (Knackstedt et al. 2010; Sondheimer and Knackstedt 2011). This attenuation of cocaine reinstatement was accompanied by a restoration of GLT-1 expression in the nucleus accumbens of rats (Knackstedt et al. 2010; Sari et al. 2009; Sondheimer and Knackstedt 2011). In addition to reinstatement results, here, we present the novel finding that physical (somatic signs and hyperalgesia) and affective (anxiety-related behavior) nicotine withdrawal signs in mice were attenuated by pretreatment with ceftriaxone. As this was a preliminary animal study, the current results do not provide a clear mechanism by which ceftriaxone blocked dependence-like behaviors of nicotine in the mouse. However, a body of literature suggests that cysteine–glutamate exchanger (system xC−) and/or GLT-1 in the brain are likely candidates. Of important relevance, withdrawal after chronic nicotine exposure is characterized by decreased glutamate transmission and compensatory changes in glutamate receptors (Mansvelder et al. 2002). Furthermore, Knackstedt et al. (2009) recently reported that rats in early withdrawal after chronic nicotine self-administration exhibited the downregulation of GLT-1 expression in the nucleus accumbens and the ventral tegmental area (Knackstedt et al. 2009). Another potential mechanism by which ceftriaxone could modify the effects of nicotine is through the upregulation of system xC−. System xC− exchanges extracellular cysteine for intracellular glutamate in a sodium-independent manner. In the nucleus accumbens (Baker et al. 2002) and hippocampus (De Bundel et al. 2011), system xC− has been found to control basal extracellular glutamate levels. System xC−′ is composed of a 4FH2 heavy chain that is generic to amino acid transporters and the catalytic subunit xCT. Lewerenz et al. (2009) showed that ceftriaxone induces a known transcriptional regulator of xCT, Nrf2, and also increases system xC− activity. Knackstedt et al. (2010) showed that, indeed, ceftriaxone restored xCT expression following cocaine self-administration. Furthermore, ceftriaxone treatment increases basal glutamate following cocaine self-administration, likely by increasing export via system xC−(Trantham-Davidson et al. 2012). Despite this earlier work, additional experiments are needed to evaluate the involvement of these ceftriaxone targets under the experimental conditions used in our study.
Our present data with nicotine is further supported by evidence that morphine physical dependence in rats is reduced by ceftriaxone (Rawls et al. 2010). It should be noted that the ceftriaxone dose of 20 mg/kg, which disrupted nicotine somatic signs of withdrawal in mice (this study), is much lower than the doses (150–200 mg/kg) required to decrease morphine physical dependence (Rawls et al. 2010) or hyperthermia in rats (Rawls et al. 2007).
It was somewhat surprising that ceftriaxone blocked the expression of all somatic and affective withdrawal signs measured here, since these signs implicate various neuronal pathways and nicotinic acetylcholine receptor subtypes (Jackson et al. 2008). Our data do not certainly argue for a common glutamatergic pathway or mechanism for nicotine withdrawal signs, but certainly the dopamine–glutamate interactions in key brain regions involved in dependence may help explain the generalized effects of ceftriaxone observed here.
While repeated treatment with ceftriaxone reduced nicotine withdrawal signs and nicotine-primed reinstatement of a CPP, ceftriaxone failed to alter acute pharmacological responses of nicotine. In as much as ceftriaxone positively modulates GLT-1 and xCT, these results suggest no similar role of these proteins in nicotinic antinociceptive and hypothermic effects after acute administration in mice. In contrast to our data, a recent study done in rats showed that pretreatment for 5 days with ceftriaxone (200vmg/kg, i.p) enhanced antinociceptive response to a high dose (2.5 mg/kg) but not low dose (1 mg/kg) of nicotine in the tail flick assay (Schroeder et al. 2011). However, the increase was modest (~20 %) and limited to one time point (2 min) after nicotine injection. Interestingly, unlike saline-injected controls, rats treated with ceftriaxone did not develop tolerance to nicotine’s antinociceptive effects after repeated injection of the drug in days 4 and 7 (Schroeder et al. 2011).
While the current dataset is promising, a number of limitations should be noted concerning the potential translation of our findings. First, the study was not designed to evaluate the effects of ceftriaxone after chronic administration to determine the maintenance of its effects with long-term treatment. In addition, the effects of ceftriaxone on nicotine withdrawal were only evaluated after maintaining mice on nicotine for 7 days. While this period of nicotine exposure is relatively short for patients seeking to quit smoking, preclinical models, with apparent predictive validity, frequently report the activity of drugs after a 7- or 14-day treatment regimen. Thus, while a longer period of nicotine exposure will be required to more fully evaluate ceftriaxone clinical utility, the translational potential of these data are supported by a large preclinical withdrawal literature using relatively brief nicotine exposure protocols. Lastly, further structure–activity development around ceftriaxone, as a promising lead compound, will likely be required given the antibacterial activity and intramuscular route of administration of the parent compound.
In summary, the results of the present study showed for the first time that ceftriaxone administration reversed nicotine withdrawal signs and attenuated nicotine-primed reinstatement of nicotine CPP without affecting acquisition. Further studies are needed to investigate the potential mechanisms by which ceftriaxone reduces nicotine dependence-related behaviors in the mouse.
Acknowledgments
This work was supported by the National Institute of Drug Abuse grant DA-12610 to MID. The authors wish to thank Cindy Evans and Tie Han for their technical assistance with this study.
Abbreviations
- CPP
Conditioned place preference
- s.c
Subcutaneous injection
- i.p
Intraperitoneal injection
- GLT-1
Glial glutamate transporter-1
- System xC
Cysteine-glutamate exchanger
Footnotes
There are no conflicts of interest to disclose for this research.
Contributor Information
M. Alajaji, Department of Pharmacology and Toxicology, Virginia Commonwealth University, Richmond, VA 23219, USA
M. S. Bowers, Virginia Institute for Psychiatric and Behavioral Genetics, Department of Psychiatry, Virginia Commonwealth University, Richmond, VA 23298, USA
L. Knackstedt, Department of Psychology, University of Florida, Gainesville, FL 32611, USA
M. I. Damaj, Email: mdamaj@vcu.edu, Department of Pharmacology and Toxicology, Virginia Commonwealth University, Richmond, VA 23219, USA
References
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. GLIA. 2000;32:1–14. doi: 10.1002/1098-1136(200010)32:1<1:AID-GLIA10>3.0.CO2-W. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–41. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castane A, Berrendero F, Maldonado R. The role of the cannabinoid system in nicotine addiction. Pharmaco Biochem Behav. 2005;81:381–386. doi: 10.1016/j.pbb.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Glassco W, Aceto MD, Martin BR. Antinociceptive and pharmacological effects of metanicotine, a selective nicotinic agonist. J Pharmacol Exp Ther. 1999;291:390–398. [PubMed] [Google Scholar]
- D’Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- De Bundel D, Schallier A, Loyens E, Fernando R, Miyashita H, Van Liefferinge J, Vermoesen K, Bannai S, Sato H, Michotte Y, Smolders I, Massie A. Loss of system x(c)− does not induce oxidative stress but decreases extracellular glutamate in hippocampus and influences spatial working memory and limbic seizure susceptibility. J Neurosci. 2011;31(15):5792–5803. doi: 10.1523/JNEUROSCI.5465-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 2008;325:302–312. doi: 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, McLaughlin JP, Carroll FI, Damaj MI. Effects of the kappa opioid receptor antagonist, norbinaltorphimine, on stress and drug-induced reinstatement of nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2716-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau KS, Madayag A, Mantsch JR, Grier MD, Abdulhameed O, Baker DA. Blunted cystine-glutamate antiporter function in the nucleus accumbens promotes cocaine-induced drug seeking. Neuroscience. 2008;155:530–537. doi: 10.1016/j.neuroscience.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny P, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Knackstedt L, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas P. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt L, Melendez R, Kalivas P. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Lewerenz J, Albrecht P, Tien ML, Henke N, Karumbayaram S, Kornblum HI, et al. Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitro. J Neurochem. 2009;111(2):332–343. doi: 10.1111/j.1471-4159.2009.06347.x. [DOI] [PubMed] [Google Scholar]
- Liechti M, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti M, Markou A. Interactive effects of the mGlu5 receptor antagonist MPEP and the mGlu2/3 receptor antagonist LY341495 on nicotine self-administration and reward deficits associated with nicotine withdrawal in rats. Eur J Pharmacol. 2007;554:164–174. doi: 10.1016/j.ejphar.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti M, Markou A. Role of the glutamatergic system in nicotine dependence: implications for the discovery and development of new pharmacological smoking cessation therapies. CNS Drugs. 2008;22:705–724. doi: 10.2165/00023210-200822090-00001. [DOI] [PubMed] [Google Scholar]
- Mansvelder H, Keath JR, McGehee D. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Markou A. Review. Neurobiology of nicotine dependence. Phil Trans R Soc Biol Sci. 2008;363:3159–3168. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, Kennedy RT, Rebec GV. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington’s disease phenotype in the R6/2 mouse. Neuroscience. 2008;153:329–337. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Tallarida R, Robinson W, Amin M. The beta-lactam antibiotic, ceftriaxone, attenuates morphine-evoked hyperthermia in rats. Br J Pharmacol. 2007;151:1095–1102. doi: 10.1038/sj.bjp.0707309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls S, Zielinski M, Patel H, Sacavage S, Baron D, Patel D. Beta-lactam antibiotic reduces morphine analgesic tolerance in rats through GLT-1 transporter activation. Drug Alcohol Depend. 2010;107:261–263. doi: 10.1016/j.drugalcdep.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Fox L, Ho LB, Berger SP. Nicotine stimulation of extracellular glutamate levels in the nucleus accumbens: neuropharmacological characterization. Synapse. 2000;35:129–136. 2-D. doi: 10.1002/(SICI)1098-2396(200002)35:2<129::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Rothstein J, Patel S, Regan M, Haenggeli C, Huang Y, Bergles D, Jin L, Dykes Hoberg M, Vidensky S, Chung D, Toan S, Bruijn L, Su Z, Gupta P, Fisher P. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Saellstroem Baum S, Huebner A, Krimphove M, Morgenstern R, Badawy AA, Spies C. Nicotine stimulation on extracellular glutamate levels in the nucleus accumbens of ethanol-withdrawn rats in vivo. Alcohol Clin Exp Res. 2006;30:1414–1421. doi: 10.1111/j.1530-0277.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- Sari Y, Smith K, Ali P, Rebec G. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J, Quick K, Landry P, Rawls S. Glutamate transporter activation enhances nicotine antinociception and attenuates nicotine analgesic tolerance. Neuro Report. 2011;22:970–973. doi: 10.1097/WNR.0b013e32834d87eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondheimer I, Knackstedt L. Ceftriaxone prevents the induction of cocaine sensitization and produces enduring attenuation of cue-and cocaine-primed reinstatement of cocaine-seeking. Behav Brain Res. 2011;225:252–258. doi: 10.1016/j.bbr.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker A, Olivier B, Markou A. Involvement of metabotropic glutamate receptor 5 in brain reward deficits associated with cocaine and nicotine withdrawal and somatic signs of nicotine withdrawal. Psychopharmacology (Berl) 2012;221:317–327. doi: 10.1007/s00213-011-2578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacol (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Lalumiere RT, Reissner KJ, Kalivas PW, Knackstedt LA. Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport, and export following cocaine self-administration and extinction training. J Neurosci. 2012;32(36):12406–12410. doi: 10.1523/JNEUROSCI.1976-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters C, Brown S, Changeux J, Martin B, Damaj MI. The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacol (Berl) 2006;184:339–344. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nico Tob Res. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]