Abstract
Affective nicotine withdrawal symptoms are of major motivational significance in contributing to relapse and continued tobacco use; thus, it is important to understand the molecular and receptor-mediated mechanisms that mediate affective withdrawal behaviors. Previous work using the conditioned place aversion (CPA) model has shown that nicotine withdrawal is associated with a negative affective state, and place aversion to previously neutral environmental stimuli represents a motivational component in the maintenance of drug use. Thus, the purpose of this study was to evaluate the role of genotype, sex, and age and to extend previous studies examining the role of various nicotinic receptor subtypes in the development of nicotine withdrawal aversion using the CPA model. Mice were chronically treated with nicotine and conditioned for two days with various nicotinic receptor antagonists. The major findings showed that mecamylamine and dihydro-β-erythroidine (DHβE), but not hexamethonium or methyl-lycaconitine citrate (MLA), precipitated significant aversion in the CPA model. This pharmacological data support our previous knockout mouse data suggesting that nicotine CPA is mediated by central β2-containing nicotinic receptors, but not α7 nicotinic receptors. Further, we show that sex and age are contributing factors to the development of nicotine CPA. Overall, the results of our study provide some insight into pharmacological and behavioral factors involved in the development of an aversive motivational component associated with nicotine withdrawal.
Keywords: Nicotinic receptors, Conditioned place aversion, Nicotine withdrawal
1. Introduction
It has been widely accepted that chronic nicotine use in humans leads to tolerance and a withdrawal syndrome consisting of both somatic and affective symptoms. While avoidance of somatic nicotine withdrawal symptoms contributes to the maintenance of smoking behavior, it is hypothesized that affective nicotine withdrawal symptoms are of greater motivational significance in contributing to relapse and continued tobacco use (Koob et al., 1993; Markou et al., 1998). Several groups have reported utilization of rodent models to assess the nicotine withdrawal syndrome by measuring physical and affective nicotine withdrawal signs. The relative lack of selective agonists and antagonists for the different nicotinic receptor subtypes has lead to the generation of several α-conotoxins, which have greater selectivity for specific nicotinic acetylcholine receptor (nAChR) subtypes, as well as genetically modified animals, such as knockout mice; thus, we have established nicotine withdrawal models in the mouse in order to use genetically modified mice to elucidate nicotinic receptor involvement in nicotine withdrawal. Previously, our laboratory characterized a nicotine withdrawal model in the mouse that allowed us to measure both physical and affective withdrawal signs in one setting (Damaj et al., 2003). More recently, we reported testing nicotinic receptor knockout mice in the conditioned place aversion (CPA) model, an affective measure of nicotine withdrawal (Jackson et al., 2008).
The CPA model measures the aversive state associated with nicotine withdrawal. It is a form of classic Pavlovian conditioning where the animal learns to avoid a compartment that was previously paired with an aversive stimulus. Previous work using this model in the rat has shown that nicotine withdrawal is associated with a negative affective state, and place aversion to previously neutral environmental stimuli represents a motivational component in the maintenance of drug use (Suzuki et al., 1996). Because the model also tests the animals in an antagonist-free state, it evaluates the important role of environmental stimuli in the maintenance of drug use. While the nicotine CPA model has been well defined in rats, this model has not been fully evaluated or characterized in mice. Indeed, the use of a mouse model would be advantageous in that it offers the possibility of exploring the underlying mechanisms of nicotine withdrawal through the use of genetically modified mice.
Previously, we tested β2, α7, and α5 knockout mice in the CPA model to identify the role of various nicotinic receptor subunits involved in affective nicotine withdrawal (Jackson et al., 2008); however, due to the issue of compensation in knockout animals, in the current study, we tested available antagonists for major nicotinic receptors to complement of our knockout data. Furthermore, we extended our pharmacological investigation by examining the role of various factors, such as genotype, sex, and age, on the development of nicotine CPA.
2. Methods
2.1. Animals
Male 129P3/J (129) and male and female C57Bl6/J mice were purchased from Jackson Laboratories. The mice were 8–10 weeks of age at the start of all studies. This is an adult age in the mouse, and published studies utilizing mice between 8 and 12 weeks of age do not report major behavior differences to nicotine in mice in this age range. Male adult and adolescent ICR mice were purchased from Harlan Laboratories. Adult mice were 8–10 weeks of age and weighed approximately 20–25 g at the start of the experiment. Adolescent mice were approximately 3 weeks of age [postnatal day (PND) 21] at the start of the experiment. Animals were housed in groups of five on a 12 h light/dark cycle with free access to food and water. Animals were maintained in an Association for Assessment and Accreditation of Laboratory Animal C are-approved animal care facility and the studies were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
2.2. Drugs
(−)-Nicotine hydrogen tartrate salt, mecamylamine hydrochloride, dihydro-β-erythroidine (DHβE) and methyllycaconitine citrate (MLA) were purchased from Sigma–Aldrich Inc. (St. Louis, MO, USA). Hexamethonium dichloride was purchased from Sigma/RBI (Natick, MA). All drugs were dissolved in physiological saline (0.9% sodium chloride) at a volume of 10 ml/kg body weight. Hexamethonium injections were administered intraperitoneally (i.p.). All other drugs were administered subcutaneously (s.c.). Doses are expressed as free base of the drug.
2.3. Chronic nicotine administration
Mice were implanted with Alzet osmotic mini pumps [model 2002 (14 days) or model 2004 (28 days) Durect Corporation, Cupertino, CA] filled with (−)-nicotine or saline solution. The concentration of nicotine was adjusted according to animal weight and the mini pump flow rate, resulting in 36 mg/kg/day for 14 or 28 days. Because the period of early adolescence lasts approximately from PND 21 to 36, adult and adolescent mice used for the age assessment were chronically infused with 48 mg/kg/day for 7 days to ensure a sufficient level of dependence. The mini pumps were surgically implanted subcutaneously under sterile conditions with sodium pentobarbital anesthesia (45 mg/kg, i.p.). An incision was made in the back of the animal, and a pump was inserted. The wound was closed with wound clips, and the animal was allowed to recover before being returned to its home cage.
2.4. Nicotine CPA
The mice were chronically exposed to nicotine for 7 or 14 days prior to initiation of testing to induce dependence. Infusion continued throughout the duration of testing. We used a biased and counterbalanced CPA protocol to measure aversion, as the biased nicotine CPA protocol is generally utilized for rat studies (Suzuki et al., 1996; Ise et al., 2002; Malin et al., 2006; O’Dell et al., 2007). The CPA apparatus consisted of a three-chambered box with a white compartment, a black compartment, and a center grey compartment separated by partitions. The black and white compartments also had different floor textures to help the mice further differentiate between the two environments. Day 1 of CPA testing (Day 8 or 15 of chronic nicotine administration) was the pre-conditioning day. The mice were placed in the grey center compartment for a 5 min habituation period, followed by a 15 min test period. During habituation, mice did not have access to the other compartments. During the test period, the partitions were raised and mice were allowed to roam freely between compartments. The CPA boxes were connected to a computer, which recorded the amount of time the mouse spent in each compartment. A pre-preference score was determined for each mouse and was used to pair the mouse with the antagonist to its initially preferred compartment. On days 2 and 3 of CPA testing, all mice received injections of saline in the morning and were immediately confined to their non-drug-paired compartment for 30 min. Four hours later, mice received an injection of antagonist and were immediately confined to their drug-paired compartment for 30 min. Day 4 was the antagonist-free test day. Mice moved freely between compartments as on day 1 and activity counts and time spent on each side were recorded via photosensors using Med Associates interface and software. Data were expressed as time spent on drug-paired side minus time spent on saline-paired side. A reduction in time spent in the initially preferred compartment was interpreted as aversion.
2.5. Statistical analysis
For all data, statistical analyses were performed using StatView® (SAS, Cary, NC, USA). All studies were analyzed with one-way ANOVAs [with treatment as the between subject factor] or two-way ANOVAs [with treatment and genotype, sex, or age as the between subject factors] using the Neuman–Keuls post hoc test. p values of less than 0.05 were considered significant.
3. Results
3.1. Acquisition of aversion in the CPA model
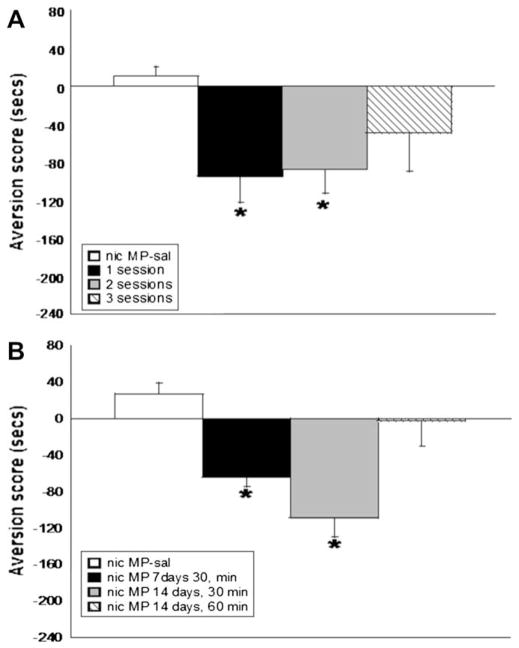
To determine the acquisition of aversion in the CPA model, C57BL/6 mice were subjected to one, two, or three conditioning sessions (day 2, days 2 and 3, or days 2, 3, and 4 respectively) with mecamylamine (3.5 mg/kg) before the antagonist-free test day. Test day was day 3, day 4, or day 5, based on the number of mecamylamine conditioning sessions. One mecamylamine conditioning session was sufficient to produce significant mecamylamine-precipitated aversion in chronic nicotine infused mice (F(3,31) = 6.098, p < 0.05). A significant aversive response was also noted after two conditioning sessions (F(3,31) = 6.098, p < 0.05); however, there was no significant effect after three days of mecamylamine conditioning (Fig. 1A). Mice were also placed in the chambers for 30 or 60 min after mecamylamine injection, and testing was initiated after 7 or 14 days of chronic nicotine infusion(day 8 or 15 of nicotine administration). Mice developed significant aversion after 7 (F(3,31) = 3.098, p < 0.05) and 14 days of prior nicotine infusion, but only after a 30 min mecamylamine conditioning session (F(3,35) = 9.530, p < 0.0001) (Fig. 1B). No significant aversion was noted after the 60 min conditioning session (Fig. 1B). Based on these results, for the remaining experiments, mice were chronically exposed to nicotine for 14 days prior to initiation of testing, and subjected to two 30 min mecamylamine conditioning sessions.
Fig. 1.
Acquisition of aversion in the CPA model. (A) Mice acquire aversion in the CPA model after one and two conditioning sessions; however, no significant effect was observed after three conditioning sessions. (B) Aversion is precipitated in mice chronically infused with nicotine for 7 and 14 days prior to test initiation. 30 min conditioning sessions are sufficient to develop aversion. The effect is lost after 60 min conditioning sessions. Each point represents ±S.E.M. of 10–12 mice per group. * denotes p < 0.05. MP = mini pump, nic = nicotine, sal = saline.
3.2. Assessment of various antagonists in the CPA model
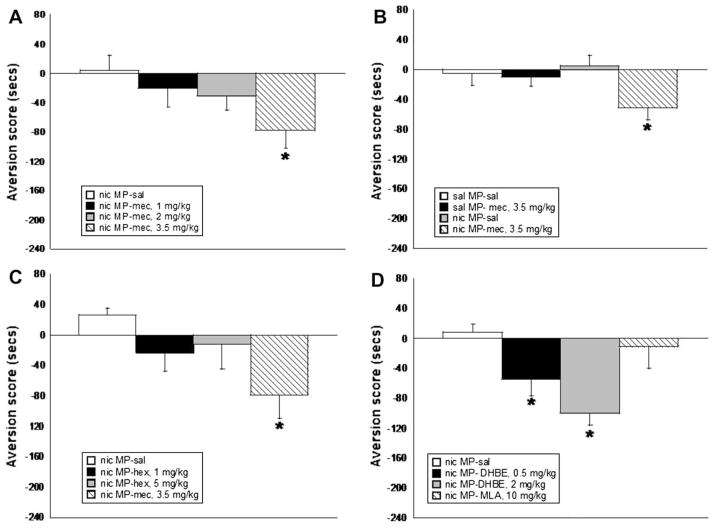
The role of various nicotinic receptors in the aversion associated with nicotine withdrawal was evaluated using nicotinic receptor antagonists. Mecamylamine, a non-selective receptor antagonist that blocks both central and peripheral nicotinic receptors, dose-dependently precipitated aversion in C57BL/6 mice. Significant aversion was observed with 3.5 mg/kg (s.c.) mecamylamine, but not with 1 or 2 mg/kg (F(3,32) = 4.022, p < 0.05) (Fig. 2A). Further, the highest dose of mecamylamine used for the studies did not precipitate aversion in chronic saline infused mice, suggesting that this dose used is not behaviorally active by itself (Fig. 2B). Hexamethonium (1 and 5 mg/kg, i.p.), a peripheral nicotinic receptor antagonist that does not cross the blood brain barrier, did not produce significant aversion as seen in mice treated with mecamylamine (Fig. 2C). DHβE, however, a β2-selective antagonist, dose-dependently precipitated aversion at 0.5 and 2 mg/kg (s.c.) (F(3,32) = 7.330, p < 0.05), while MLA, an α7 antagonist, did not precipitate aversion at 10 mg/kg (s.c.), a dose that effectively blocks α7 nicotinic receptors (Ward et al., 1990) (Fig. 2D).
Fig. 2.
Assessment of various antagonists in the CPA model. (A) Mecamylamine (mec) dose-dependently precipitates aversion in the CPA model. (B) The dose of mecamylamine used in the model, 3.5 mg/kg, does not precipitate aversion in saline infused mice, suggesting that the dose is not behaviorally active by itself. (C) Hexamethonium (hex), a peripheral nicotinic receptor antagonist, does not precipitate aversion in chronic nicotine infused mice. (D) The β2 nicotinic receptor antagonist, DHβE, but not the α7 antagonist, MLA, dose-dependently precipitates aversion in chronic nicotine infused mice. Each point represents ±S.E.M. of 10–12 mice per group. * denotes p < 0.05 vs. saline groups. MP = mini pump, nic = nicotine, sal = saline.
3.3. Sex and strain assessment in the CPA model
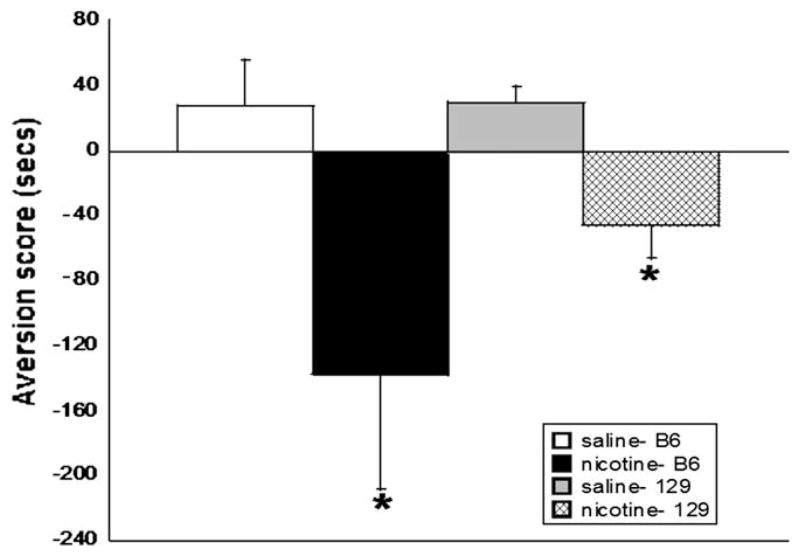
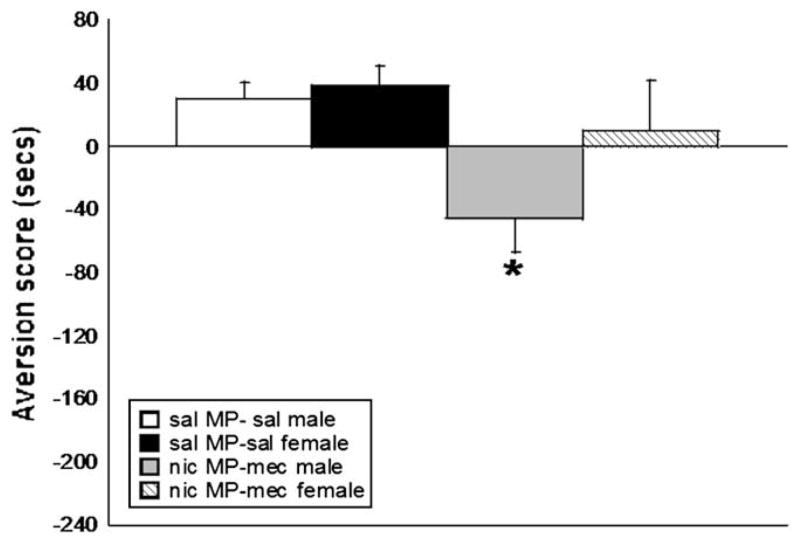
The knockout mice used in our studies are maintained on a C57BL/6 background and bred with 129P3 mice to generate B6129P3 hybrids. It was therefore necessary to evaluate the background strains in order to make the most efficient interpretation of our knockout results. Additionally, because of the limited number of knockout animals produced in each litter, it is sometimes necessary to use both male and female animals for our studies; thus, we also evaluated differences between male and female C57BL/6 mice in our CPA model. Results show that mecamylamine (3.5 mg/kg, s.c.) precipitated aversion in male C57BL/6 and 129 mice (F(3,32) = 3.105, p < 0.05 for treatment) (Fig. 3). Female C57BL/6 mice were also evaluated in the nicotine CPA model. As noted, mecamylamine (3.5 mg/kg, s.c.) precipitated significant aversion in male C57BL/6 mice, but CPA was absent in female C57BL/6 mice (F(3,34) = 6.017, p < 0.05 for treatment) (Fig. 4). Based on these results, only male mice were used for subsequent studies.
Fig. 3.
CPA assessment using 129 and C57BL/6 male mice. Mecamylamine precipitates aversion in the 129 and C57BL/6 inbred strains. Each point represents ±S.E.M. of 12 mice per group. * denotes p < 0.05 vs. the corresponding saline group.
Fig. 4.
Assessment of male and female C57BL/6 mice in the development of aversion in the CPA model. Chronic nicotine infused male C57BL/6 mice develop aversion in the CPA model; however, nicotine infused female C57BL/6 mice do not develop aversion in this testing scheme. Each point represents ±S.E.M. of 12 mice per group. * denotes p < 0.05 vs. the corresponding saline group. MP = mini pump, nic = nicotine, sal = saline.
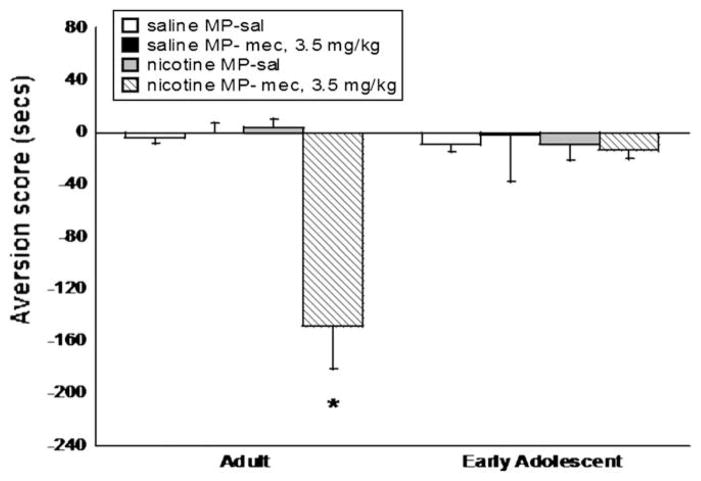
3.4. Evaluation of age differences in the CPA model
To complete characterization of a nicotine CPA model in mice, we wanted to evaluate the importance of age in the development of CPA. Early adolescent and adult ICR mice were tested in the CPA model to evaluate age effects. Mecamylamine (3.5 mg/kg, s.c.) precipitated significant aversion in adult mice (F(3,32) = 9.920, p < 0.0001), but no significant effect was observed in adolescent mice, suggesting that adolescents are less sensitive to the aversive effects of nicotine withdrawal (Fig. 5). The dose of mecamylamine used did not precipitate significant aversion in adult or adolescent saline controls, suggesting that the dose was not behaviorally active by itself.
Fig. 5.
Age differences in development of aversion in the CPA model. (A) Mecamylamine (mec, 3.5 mg/kg, s.c.) precipitates aversion in adult mice, but (B) not in early adolescent mice. The dose of mecamylamine used did not precipitate aversion in saline-treated adult or adolescent mice. Each point represents ±S.E.M. of 10–12 mice per group. * denotes p < 0.05 vs. control groups. MP = mini pump, sal = saline.
4. Discussion
The present research aimed to determine the role of genotype, sex, and age on the development of nicotine withdrawal-associated aversion. The adaptation of such a model for mouse studies allows us to define the underlying receptor mechanisms of affective nicotine withdrawal through the use of genetically modified mice. The major findings showed that mecamylamine and DHβE, but not hexamethonium or MLA, precipitated significant aversion in the CPA model, suggesting that the aversion associated with nicotine withdrawal is mediated by central populations of β2-containing nAChRs. Further, we show that sex and age are contributing factors to the development of nicotine CPA.
Acquisition of significant aversion was observed after one and two conditioning sessions, but surprisingly, a significant effect was lost after three conditioning sessions. It is possible that some adaptation to the aversive stimuli occurred during the three day consecutive conditioning, thus decreasing the degree of CPA expression on test day. In the current study, mice were conditioned to mecamylamine for three consecutive days. Prior nicotine CPA studies conducted in rats used one (Suzuki et al., 1996, 1999; Ise et al., 2002; Malin et al., 2006) or four mecamylamine conditioning sessions on alternating days (O’Dell et al., 2007). In another recent study by Guillem et al. (2007), rats were treated for five consecutive conditioning sessions with no adaptation to the aversive stimuli; however, this study was conducted in rats, where species differences could be a factor.
Results also showed that 7 or 14 days of prior nicotine exposure was sufficient to obtain mecamylamine-precipitated aversion after a 30 min, but not 60 min conditioning session. While the half-life of mecamylamine in the rat is approximately 1 h (Debruyne et al., 2003), it is not known if this duration is applicable to mice. In our study, a fairly high dose of mecamylamine (3.5 mg/kg) was necessary to precipitate a significant CPA. Significant aversion was not precipitated at 2 mg/kg mecamylamine, suggesting that the half-life dose of mecamylamine would not be sufficient to precipitate significant CPA in chronic nicotine infused mice. Although we cannot assume that the half-life of mecamylamine is the same in rats and mice, a possible explanation for the observed data may be attributed to the duration of action of mecamylamine in the mouse. Studies establishing the plasma mecamylamine levels in mice at various time points are necessary to further assess these effects.
Results using different antagonists suggest the involvement of central, but not peripheral nicotinic receptor populations. While mecamylamine, a non-selective nicotinic receptor antagonist that acts on both central and peripheral nicotinic receptor populations, precipitated significant aversion in chronic nicotine infused mice, hexamethonium, a peripheral nicotinic receptor antagonist that does not cross the blood brain barrier, did not precipitate aversion at any dose tested. These results are consistent with studies which indicate that affective nicotine withdrawal signs are mediated solely by central nicotinic receptor populations (Watkins et al., 2000). Additionally, results suggest that β2, but not α7 nicotinic receptors, are involved in development of nicotine CPA. DHβE, an antagonist with preferential selectivity for β2-containing receptors, dose-dependently precipitated significant aversion, while MLA failed to precipitate aversion at a dose that effectively blocks α7 nicotinic receptors (Ward et al., 1990). It is also noted that DHβE was more potent than mecamylamine in this assessment, as DHβE was able to precipitate aversion at lower doses than mecamylamine. The current results are also consistent with previous studies from our lab showing that DHβE precipitates anxiety-related behavior in the plus maze test in nicotine-dependent mice (Damaj et al., 2003). Further, these pharmacological studies complement our transgenic mouse data, which also indicate a role for β2-containing, but not α7 nicotinic receptors, in affective nicotine withdrawal (Jackson et al., 2008). Taken together, the results suggest that β2-containing nicotinic receptor subtypes are involved in affective nicotine withdrawal behaviors and that CPA is a centrally mediated effect.
In our strain assessment, significant aversion was precipitated in both male 129 and C57BL/6 mice and there was no significant difference in intensity of aversion between strains. Previous results, however, show that physical nicotine withdrawal and anxiety-related behavior is more intense in C57BL/6 mice than 129 mice (Damaj et al., 2003), suggesting that genotypic factors are involved in nicotine withdrawal behaviors. Studies testing additional mouse strains may be necessary to support a role for the involvement of genotypic factors in the development of nicotine CPA. Our evaluation using male and female C57BL/6 mice suggests that sex factors contribute to the development of nicotine withdrawal aversion. Mecamylamine precipitated a significant CPA in male, but not in female C57BL/6 mice. Prior evaluations of female mice in nicotine withdrawal revealed that female mice did not express an anxiety-related response, suggesting that female mice are less sensitive to affective nicotine withdrawal (Kota et al., 2008). Another explanation could be the influence of sex hormones. Indeed, sex hormones can modulate the effects of nicotine and may contribute to differences in nicotine’s responses between males and females (Damaj, 2001). Human studies also suggest that hormonal changes during different menstrual cycle phases impact severity of the nicotine withdrawal syndrome (Carpenter et al., 2006). In the current study, we did not control for the estrous cycle in female mice. Consequently, it is possible that the female mice were in different phases of the estrous cycle, thus impacting our results.
In our final assessment, results revealed a significant contribution of age to the development of a significant CPA. Mecamylamine (3.5 mg/kg, s.c.) precipitated aversion in chronic nicotine infused adult, but not adolescent mice. Our findings are consistent with previous findings from Kota et al. (2007), showing that male adolescent mice are less sensitive to the affective measures of nicotine withdrawal than male adult mice, as adolescents do not express an anxiety-related response on the plus maze. Further, O’Dell et al. (2007) showed that CPA was lower in chronic nicotine infused adolescent rats vs. adult rats using a biased experimental design. It is possible that adolescent rodents may be less able than adults to associate environmental cues with aversive nicotine withdrawal effects; however, the aforementioned study addressed this possibility using another aversive stimulus, lithium chloride (LiCl) injections. The effect was shown to be specific to nicotine withdrawal aversion, as there was no significant difference between adolescents and adults in learning place aversion to LiCl (O’Dell et al., 2007). Additionally, in the current study, two conditioning sessions were used to acquire a nicotine CPA. The possibility that adolescent mice require more conditioning sessions to acquire a significant CPA cannot be ruled out; however, such an assessment was beyond the scope of the current study. Furthermore, we did not measure nicotine plasma levels to establish that they were equivalent across sex or ages; therefore, we cannot rule out the possibility that the differences in either study are attributed to differences in metabolism between sex and age.
In summary, the results of our study demonstrate the importance of pharmacological, biochemical, and behavioral mechanisms associated with a motivational component of nicotine dependence.
Acknowledgments
This work was supported by the National Institute on Drug Abuse grants # DA-12610 and DA-05274.
References
- Carpenter MJ, Upadhyaya HP, LaRowe SD, Saladin ME, Brady KT. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: a review. Nicotine Tob Res. 2006;8:627–638. doi: 10.1080/14622200600910793. [DOI] [PubMed] [Google Scholar]
- Damaj MI. Influence of gender and sex hormones on nicotine acute and pharmacological effects in mice. J Pharmacol Exp Ther. 2001;296:132–140. [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Debruyne D, Sobrio F, Hinschberger A, Camsonne R, Coquerel A, Barré L. Short-term pharmacokinetics and brain distribution of mecamylamine as a preliminary to carbon-11 labeling for nicotinic receptor investigation. J Pharm Sci. 2003;92:1051–1057. doi: 10.1002/jps.10302. [DOI] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Koob GF, Cador M, Stinus L. Monoamine oxidase inhibition dramatically prolongs the duration of nicotine withdrawal-induced place aversion. Biol Psychiatry. 2007;63:158–163. doi: 10.1016/j.biopsych.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Ise Y, Narita M, Nagase H, Suzuki T. Modulation of κ-opiodergic systems on mecamylamine-precipitated nicotine-withdrawal aversion in rats. Neurosci Lett. 2002;323:164–166. doi: 10.1016/s0304-3940(02)00074-5. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 2008;325:302–312. doi: 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GP, Heinrichs SC, Pich EM, Menzaghi F, Baldwin H, Miczek K, Britton KT. The role of corticotropin-releasing factor in behavioral responses to stress. Ciba Found Symp. 1993;172:277–289. doi: 10.1002/9780470514368.ch14. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Damaj MI. Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology. 2008;198:201–210. doi: 10.1007/s00213-008-1117-8. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Smith TD, Khambati HN, Meyers-Paal RL, Montellano AL, Jennings RE, Erwin DS, Presley SE, Perales BA. Bupropion attenuates nicotine abstinence syndrome in the rat. Psychopharmacology. 2006;184:494–503. doi: 10.1007/s00213-005-0135-z. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Torres OV, Natividad LA, Tejeda HA. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicol Teratol. 2007;29:17–22. doi: 10.1016/j.ntt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Ise Y, Tsuda M, Maeda J, Misawa M. Mecamylamine-precipitated nicotine-withdrawal aversion in rats. Eur J Pharmacol. 1996;314:281–284. doi: 10.1016/s0014-2999(96)00723-6. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Ise Y, Maeda J, Misawa M. Mecamylamine-precipitated nicotine-withdrawal aversion in Lewis and Fischer 344 inbred rat strains. Eur J Pharmacol. 1999;369:159–162. doi: 10.1016/s0014-2999(99)00086-2. [DOI] [PubMed] [Google Scholar]
- Ward J, Cockcroft V, Lunt G, Smillie F, Wonnacott S. Methyllycaconitine: a selective probe for neuronal α-bungarotoxin binding sites. FEBS Lett. 1990;270:45–48. doi: 10.1016/0014-5793(90)81231-c. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther. 2000;292:1053–1064. [PubMed] [Google Scholar]