Abstract
Rationale
Several studies implicate stress as a risk factor for the development and maintenance of drug addictive behaviors and drug relapse. Kappa opioid receptor (KOR) antagonists have been shown to attenuate behavioral responses to stress and stress-induced reinstatement of cocaine and ethanol seeking and preference.
Objectives
In the current study, we determined whether the selective KOR antagonist, norbinaltorphimine (nor-BNI), would block stress-induced reinstatement of nicotine preference.
Methods
Adult Institute of Cancer Research mice were conditioned with 0.5 mg/kg nicotine, injected subcutaneously (s.c.) for 3 days and tested in the nicotine-conditioned place preference (CPP) model. After 3 days extinction, nor-BNI (10 mg/kg, s.c.) was administered 16 h prior to a priming dose of nicotine (0.1 mg/kg, s.c.), and mice were tested in the CPP model for nicotine-induced reinstatement of CPP. A separate group of mice was subjected to a 2-day modified forced swim test (FST) paradigm to induce stress after 3 days extinction from CPP. Mice were given vehicle or nor-BNI (10 mg/kg, s.c.) 16 h prior to each FST session.
Results
Nor-BNI pretreatment significantly attenuated stress-induced reinstatement of nicotine-CPP, but had no effect on nicotine-primed reinstatement.
Conclusions
Blockade of KORs by selective antagonists attenuates stress-induced reinstatement of nicotine-CPP. Overall, the kappa opioid system may serve as a therapeutic target for suppressing multiple signaling processes which contribute to maintenance of smoking, smoking relapse, and drug abuse in general.
Keywords: Nicotine, Kappa opioid receptors, Nor-BNI, Drug reinstatement, Forced swim test, Nicotine-CPP
Introduction
Stress is implicated as a risk factor in the development and maintenance of drug addictive behaviors and relapse to drug abuse. Indeed, exposure to stressors in humans has been shown to increase cigarette puff rate, volume of smoke inhaled (Rose et al. 1983), smoking and nicotine intake (Pomerleau and Pomerleau 1987; Todd 2004), and desire to smoke (Perkins and Grobe 1992; Todd 2004). Similarly, exposure to stressors is a predictor for relapse to cocaine (McMahon 2001), and the exaggerated stress response often observed in former opiate and cocaine abusers has been linked to drug relapse (Kreek 1987). Stressful situations also increase alcohol intake in humans (Pohorecky 1981). In support of these findings, animal studies report enhanced drug-induced reinstatement of cocaine self-administration (Beardsley et al. 2005), enhanced cocaine reward (McLaughlin et al. 2003), and enhanced ethanol reward and ethanol intake in the two-bottle choice paradigm (Sperling et al. 2010) after exposure to stressors.
One mechanism for this enhanced response to drugs of abuse following stressors involves kappa opioid receptor (KOR) pathways. Dynorphin, the endogenous KOR ligand, is released in response to stress, activating the kappa opioid system and mediating behavioral responses to stressors (see Bruchas et al. 2010 for review). A study by McLaughlin et al. (2003) reported that the selective KOR antagonist, nor-binaltorphimine (nor-BNI), blocks stressed-induced analgesia, reduces stress-induced immobility in the forced swim test (FST), and blocks stress-induced potentiation of cocaine-conditioned place preference (CPP) in mice. Similar blockade of stress-induced analgesia and potentiation of cocaine-CPP was also observed with the selective KOR antagonist arodyn, a novel peptide ligand based on the structure of dynorphin A (Carey et al. 2007). In addition to the FST, nor-BNI attenuated enhanced cocaine reinstatement of CPP from stress induced by footshock in mice (Redila and Chavkin 2008). Nor-BNI also significantly blocked stress-induced enhancement of ethanol consumption and reward in mice (Sperling et al. 2010). Consistent with these results, mice null for the prodynorphin gene, the precursor protein for dynorphin, did not show stress-induced enhancement of cocaine-CPP (McLaughlin et al. 2003; Redila and Chavkin 2008) or ethanol consumption (Sperling et al. 2010). Additionally, nor-BNI and (3R)-7-hydroxy-N-[(1 S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-di methyl-1-piperidinyl]methyl]-2-methylpropyl]-1,2,3,4-tet rahydro-3-isoquinoline-carboxamide (JDTic), a potent, selective KOR antagonist, decreased immobility in the FST and reduced the ability of a footshock stressor to reinstate cocaine self-administration in rats (Beardsley et al. 2005). Despite these studies indicating an apparent role for KORs in stress-induced relapse of cocaine and ethanol, to date, it is unclear if this blockade extends to nicotine. Nicotine is known to affect stress pathways, inducing alterations in the corticotrophin-releasing factor, hypothalamic-pituitary-adrenal axis, and autonomic nervous system pathways, and changes in heart rate and blood pressure (Wilkins et al. 1982; Cinciripini et al. 1989; Mendelson et al. 2005; Chen et al. 2008). Such nicotine-induced alterations to stress and dopamine pathways contribute to the negative affect, craving, and increased relapse susceptibility associated with nicotine withdrawal (Sinha 2008; Renthal and Nestler 2008). Indeed, a major issue in smoking cessation is the high rate of relapse, which, after 1 year of initial treatment, is approximately 75 % (Gonzales et al. 2006).
We therefore extended the studies to nicotine and tested whether the selective KOR antagonist, nor-BNI, would block stress-induced reinstatement of nicotine-CPP, using the modified FST to induce stress in mice. To test if the effect was specific to stress-induced reinstatement, we also assessed the ability of nor-BNI to block nicotine-primed reinstatement of nicotine-CPP.
Methods
Animals
Male Institute for Cancer Research mice, purchased from Jackson Laboratories (Bar Harbor, ME), were housed in a 21°C humidity-controlled Association for Assessment and Accreditation of Laboratory Animal Care-approved animal care facility with food and water available ad libitum. The rooms were on a 12-h light/dark cycle (lights on at 7:00 A.M.). Mice were 8–10 weeks of age and weighed approximately 20–25 g at the start of all the experiments. All experiments were performed during the light cycle (between 7:00 A.M. and 7:00 P.M.) and approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University and in accordance with the National Institutes of Health Guide for Animal Care and Use.
Drugs
(−)-Nicotine hydrogen tartrate salt [(−)-1-methyl-2-(3-pyr idyl)pyrrolidine (+)-bitartrate salt] was purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). Norbinaltorphimine dihydrochloride was a generous gift from the NIDA Drug Supply Program (Research Triangle Park, NC). All drugs were dissolved in a physiological saline solution (0.9 % sodium chloride) and injected subcutaneously (s.c.) at a volume of 10 ml/kg body weight. All doses are expressed as the free base of the drug.
Nicotine-CPP
An unbiased CPP paradigm was utilized in this study as described in Kota et al (2007). Briefly, place-conditioning chambers consisted of two distinct compartments separated by a smaller intermediate compartment with openings that allowed access to either side of the chamber. On day 1, animals were confined to the intermediate compartment for a 5-min habituation period and then allowed to move freely between compartments for 15 min. Time spent in each compartment was recorded. These data were used to separate the animals into groups of approximately equal bias. Days 2–4 were the conditioning days during which the saline group received saline in both compartments, and drug groups received nicotine (0.5 mg/kg, s.c.) in one compartment and saline in the opposite compartment. Drug-paired compartments were randomized among all groups. Day 5 was the drug-free test day, and the procedure was the same as day 1. Activity counts and time spent on each side were recorded via photosensors using Med Associates interface and software. Data were expressed as time spent on the drug-paired side postconditioning minus time spent on the drug-paired side preconditioning. A positive number indicated a preference for the drug-paired side, whereas a negative number indicated an aversion to the drug-paired side. A number at or near zero indicated no preference for either side.
Nicotine-primed reinstatement of nicotine-CPP
Two individual cohorts of seven to nine mice were tested in the CPP paradigm and classified into groups A and B. On day 6, 1 day after test day, the test day procedure was repeated in both groups for three additional days to measure extinction of nicotine-CPP. All mice received saline injections (s.c.) prior to entering the chambers on these days. After day 8 test session, group A mice received an injection of nor-BNI (10 mg/kg, s.c.), and group B mice received an injection of vehicle, 16 h prior to day 9 test session. On day 9, mice received an injection of nicotine (0.1 mg/kg, s.c.), and a subset of animals from group A, the nor-BNI pre-treated group (n=4 for saline and nicotine) received vehicle, and were immediately placed in the chamber for testing. A timeline for the experiment is shown in Fig. 1a.
Fig. 1.
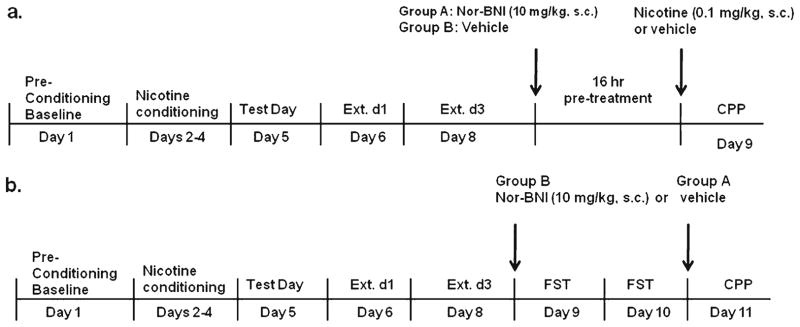
Timelines for nicotine-CPP reinstatement studies. a Timeline for the nicotine-primed nicotine-CPP reinstatement and b stress-induced nicotine-CPP reinstatement study
Stress-induced reinstatement of nicotine-CPP
As in the nicotine-primed reinstatement study, two individual cohorts of six to eight mice, groups A and B, were tested in the CPP paradigm. After three CPP extinction days (day 8), nicotine-conditioned mice from group B received injections of nor-BNI (10 mg/kg, s.c.), while nicotine-conditioned mice from group A received vehicle. Saline-conditioned mice served as a control group (unstressed) and were treated with vehicle (group A) or nor-BNI (group B) in the home cage, according to the same treatment schedule as mice subjected to the FST. On day 9, 16 h after nor-BNI pretreatment, to induce stress, mice were exposed to a 2-day modified forced swim test (Porsolt et al. 1977; McLaughlin et al. 2003). Briefly, mice swam in opaque 5-l beakers filled with 3.5 l of 30°C water. After each trial, the mice were dried with towels and returned to their home cages before further testing. On day 1 of the FST (experiment day 9), mice were placed in water to swim for a single trial of 15 min. At the end of FST day 1, mice were again pretreated with nor-BNI or vehicle 16 h prior to FST day 2. On day 2 of the FST (experiment day 10), mice were placed in water to swim through a series of four trials, each 6 min long. Trials were separated by a 10-min return to the home cage. Difficulties in swimming or staying afloat were the criteria for exclusion in this study; however, no mice met these criteria. On day 11, mice were reexposed to the CPP chambers for testing. A timeline for the experiment is shown in Fig. 1b.
Statistical analysis
Statistical analyses for both studies were conducted using repeated measures analysis of variance testing with between-subjects factors. All comparisons were between cohorts (groups A and B), test day, postconditioning days, and treatment (saline vs. nicotine). Values of p<0.05 were considered to be statistically significant. Significant results were further assessed using Neuman–Keuls post-hoc analysis.
Results
Effects of nor-BNI on nicotine-primed reinstatement of nicotine-CPP
A significant between-subjects main effect revealed a robust CPP established in mice in response to nicotine (0.5 mg/kg, s.c.) in both cohorts (F(1,80) =31.12, p <0.0001; Fig. 2). After 1 day extinction from CPP, nicotine preference was still expressed; however, this effect was extinguished after 3 days extinction from nicotine-CPP, as supported by a significant within-subject×between-subject interaction (F(5,80) =5.69, p<0.01). At a dose of 0.1 mg/kg nicotine, CPP was reinstated in vehicle pretreated mice (Fig. 2). Nicotine-primed reinstatement of CPP was not blocked by pretreatment with nor-BNI (10 mg/kg, s.c.), administered 16 h prior to test day, as these mice also exhibited a significant reinstatement of nicotine-CPP. There were no significant differences between cohorts on any days tested. Nor-BNI alone had no significant effect on reinstatement of nicotine-CPP at the dose tested. CPP locomotor activity counts did not significantly differ between postconditioning reinstatement groups (Veh/Nic=516.8±32.8; nor-BNI/Veh=473.3±61.4; nor-BNI/Nic=496.7±83.3).
Fig. 2.
Effect of nor-BNI on nicotine-primed reinstatement of nicotine-CPP. Mice conditioned with 0.5 mg/kg nicotine (s.c.) expressed a significant CPP on test day (TD). Significant CPP is present on extinction day 1 (ED1), but is extinguished by day 3 (ED3). On day 8, nicotine-CPP is reinstated by nicotine (RS V/N) (0.1 mg/kg, s.c.) and is not blocked by nor-BNI (RS NB/N) (10 mg/kg, s.c.). Nor-BNI pretreatment had no significant effect on reinstatement of CPP (RS NB/V). Each point represents the mean±SEM of seven to nine mice per group (n=5 per group for RS NB/N, n=4 per group for RS NB/V). *p <0.05 vs. the corresponding saline group
Effects of nor-BNI on stress-induced reinstatement of nicotine-CPP
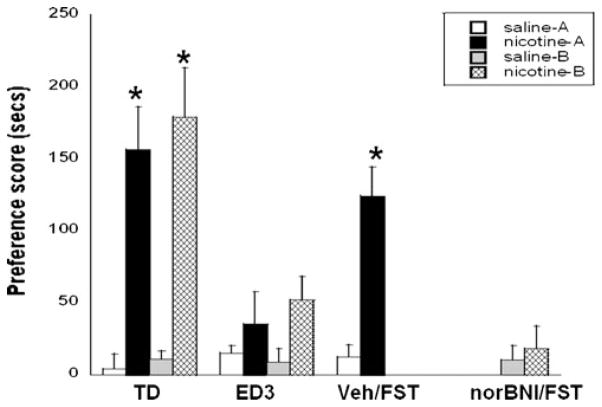
A robust nicotine-CPP was observed in both cohorts on test day (F(1,54) =78.61, p <0.0001; Fig. 3). Following three CPP extinction days, stress was induced in nicotine-conditioned mice using a 2-day modified FST. Mice were pretreated with vehicle or nor-BNI (10 mg/kg, s.c.) 16 h before each FST session. Saline-conditioned mice received the same treatment in the home cages, but were not subjected to the FST (unstressed). The FST reinstated nicotine-CPP in vehicle pretreated mice (F(3,54)=10.09, p <0.01; Fig. 3). Alternatively, stress-induced reinstatement of nicotine-CPP was significantly blocked in mice pretreated with nor-BNI (F(3,54)= 10.27, p<0.01). The dose of nor-BNI tested alone had no significant effect on CPP in unstressed mice. There were no significant differences between cohorts on any days tested. CPP locomotor activity counts did not significantly differ between postconditioning reinstatement groups (unstressed/Sal=483.3±71.6; unstressed/nor-BNI=524.7±107.3; Veh/FST=492.7±112.3; nor-BNI/FST=508.7±41.3).
Fig. 3.
Effect of nor-BNI on stress-induced reinstatement of CPP. Mice conditioned with 0.5 mg/kg nicotine (s.c.) expressed a robust CPP on test day (TD) and were exposed to the FST to induce stress after three extinction days (ED3). Stress induced a significant reinstatement of nicotine-CPP in vehicle (Veh/FST) pretreated mice, but not in nor-BNI pretreated mice (nor-BNI/FST). A significant CPP was not expressed in vehicle-treated unstressed mice. Nor-BNI had no significant effect on CPP in unstressed mice. Each point represents the mean±SEM of six to eight mice per group. *p<0.05 vs. the corresponding saline/unstressed and nor-BNI-treated groups
Discussion
The primary finding of this study was that the KOR antagonist, nor-BNI, significantly blocked FST stress-induced reinstatement of nicotine-CPP. Exposure to stress via the modified 2-day FST induced a significant CPP in vehicle-treated mice, an effect that was blocked by nor-BNI pre-treatment. Nor-BNI failed to block nicotine-primed reinstatement of nicotine-CPP, indicating an effect specific to stress-induced mechanisms. These results coincide with a recent study by Smith et al. (2012), where the FST reinstated nicotine-CPP in saline, but not nor-BNI pretreated mice. Our findings are also consistent with previous reports with cocaine (McLaughlin et al. 2003; Beardsley et al. 2005; Carey et al. 2007; Redila and Chavkin 2008) and ethanol (Sperling et al. 2010), where stress-induced enhancement of cocaine- and ethanol-induced behaviors was attenuated by selective KOR antagonists. Recently, nor-BNI administered systemically and locally into the amygdala blocked KOR agonist-induced enhancement of nicotine-CPP when administered 1 h prior to testing (Smith et al. 2012). Similar results were observed with KOR agonists in cocaine-CPP (McLaugh lin et al. 2006). These results further show that KOR activation plays a significant role in stress-induced reinstatement of drug behaviors. Furthermore, KOR activation in the amygdala induced an anxiety-like response in mice, an effect attenuated by low doses of nicotine (Smith et al. 2012). Such results also implicate KOR pathways, specifically in the amygdala, in anxiety-like states that lead to drug-seeking behavior.
In addition to mechanisms involved in stress, the kappa opioid system mediates aspects of nicotine dependence. Dynorphin and prodynorphin mRNA levels are altered in the striatum after acute nicotine exposure (Isola et al. 2009) and after chronic nicotine exposure and nicotine withdrawal (Isola et al. 2008). Coinciding with these in vitro studies, both JDTic and nor-BNI attenuate physical and affective nicotine withdrawal signs in mice (Jackson et al. 2010), suggesting that heightened dynorphinergic tone is involved, in part, in the expression of negative affective states experienced during nicotine withdrawal. Indeed, stress and severity of the nicotine withdrawal syndrome are predictors of smoking relapse (Daughton et al. 1990; West et al. 1989). Thus, in the case of nicotine, KOR antagonists appear to have the dual effect of blocking multiple contributors to smoking relapse.
Similar to previous findings with cocaine and ethanol, nor-BNI did not block nicotine-primed reinstatement of nicotine-CPP, indicating an effect specific to stress-induced responses and implicating differential mechanisms in stress-induced vs. drug-induced reinstatement of drug relapse. The KOR antagonists JDTic and nor-BNI also had no effect on the expression of nicotine-CPP (Jackson et al. 2010), and a follow-up study from our lab revealed that JDTic, when administered to mice conditioned with a dose of nicotine that does not produce significant CPP in mice (nicotine, 0.1 mg/kg, s.c.), did not enhance nicotine-CPP (nicotine (0.1)=52±10 vs. JDTic (16) – (nicotine (0.1)=75±22), suggesting that KORs are not involved in nicotine reward pathways. However, at higher, aversive nicotine doses, nor-BNI pretreatment switched the aversive response to a place preference (Smith et al. 2012), further supporting a role for the kappa opioid receptor system in mediating aversive effects of nicotine (Jackson et al. 2010).
In summary, the KOR antagonist nor-BNI blocks stress-induced reinstatement of nicotine-CPP, but not nicotine-primed reinstatement of nicotine-CPP. Taken together with previous studies, the kappa opioid system serves as a potential therapeutic target for suppressing signaling processes associated with multiple aspects of drug abuse maintenance and relapse.
Acknowledgments
This work was supported by the National Institute of Mental Health grant MH-020030 to KJJ and NIDA DA-12610 to MID. The authors wish to thank Cindy Evans for her technical assistance with this study.
Footnotes
Conflict of interest There are no conflicts of interest to disclose for this research. The experiments in the current studies comply with the current laws in the country in which they were performed.
Contributor Information
K. J. Jackson, Department of Psychiatry, Virginia Commonwealth University, Richmond, VA 23219, USA
J. P. McLaughlin, Department of Biology, Torrey Pines Institute for Molecular Studies, Port St. Lucie, FL 34987, USA
F. I. Carroll, Organic and Medicinal Chemistry, Research Triangle Institute, Research Triangle Park, NC 27709, USA
M. I. Damaj, Email: mdamaj@vcu.edu, Department of Pharmacology & Toxicology, Virginia Commonwealth University, Richmond, VA 23219, USA
References
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183(1):118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Borozny K, Aldrich JV, McLaughlin JP. Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist, arodyn. Eur J Pharmacol. 2007;569(1–2):84–89. doi: 10.1016/j.ejphar.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Fu Y, Sharp BM. Chronic nicotine self-administration augments hypothalamic-pituitary-adrenal responses to mild acute stress. Neuropsychopharmacology. 2008;33(4):721–730. doi: 10.1038/sj.npp.1301466. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Benedict CE, Van Vunakis H, et al. The effects of smoking on the mood, cardiovascular and adrenergic reactivity of heavy and light smokers in a non-stressful environment. Biol Psychol. 1989;29(3):273–289. doi: 10.1016/0301-0511(89)90023-9. [DOI] [PubMed] [Google Scholar]
- Daughton DM, Roberts D, Patil KD, Rennard SI. Smoking cessation in the workplace: evaluation of relapse factors. Prev Med. 1990;19(2):227–223. doi: 10.1016/0091-7435(90)90023-d. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4-beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Isola R, Zhang H, Tejwani GA, Neff NH, Hadjiconstantinou M. Dynorphin and prodynorphin mRNA changes in the striatum during nicotine withdrawal. Synapse. 2008;62(6):448–455. doi: 10.1002/syn.20515. [DOI] [PubMed] [Google Scholar]
- Isola R, Zhang H, Tejwani GA, Neff NH, Hadjiconstantinou M. Acute nicotine changes dynorphin and prodynorphin mRNA in the striatum. Psychopharmacology (Berl) 2009;201(4):507–516. doi: 10.1007/s00213-008-1315-4. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Carroll FI, Negus SS, Damaj MI. Effect of the selective kappa-opioid receptor antagonist JDTic on nicotine anti-nociception, reward, and withdrawal in the mouse. Psychopharmacology (Berl) 2010;210(2):285–294. doi: 10.1007/s00213-010-1803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322(1):399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Multiple drug abuse patterns and medical consequences. In: Meltzer HY, editor. Psychopharmacology: the third generation of progress. Raven; New York: 1987. pp. 1597–1604. [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23(13):5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Shuang L, Pintar JE, Chavkin C. Prior activation of kappa-opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine-conditioned place preference conditioning. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon RC. Personality, stress, and social support in cocaine relapse prediction. J Subst Abuse Treat. 2001;21(2):77–87. doi: 10.1016/s0740-5472(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Sholar MB, Goletiani N, et al. Effects of low- and high-nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology. 2005;30(9):1751–1763. doi: 10.1038/sj.npp.1300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE. Increased desire to smoke during acute stress. Bri J Addict. 1992;87(7):1037–1040. doi: 10.1111/j.1360-0443.1992.tb03121.x. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. The interaction of alcohol and stress: a review. Neurosci Biobehav Rev. 1981;5(2):209–229. doi: 10.1016/0149-7634(81)90003-8. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF. The effects of a psychological stressor on cigarette smoking and subsequent behavioral and physiological responses. Psychophysiology. 1987;24(3):278–285. doi: 10.1111/j.1469-8986.1987.tb00295.x. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Redila VA, Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology (Berl) 2008;200(1):59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14(8):341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Ananda S, Jarvik ME. Cigarette smoking during anxiety-provoking and monotonous tasks. Addict Behav. 1983;8(4):353–359. doi: 10.1016/0306-4603(83)90035-7. [DOI] [PubMed] [Google Scholar]
- Smith JS, Schindler AG, Martinelli E, Gustin RM, Bruchas MR, Chavkin C. Stress-induced activation of the dynorphin/κ-opioid receptor system in the amygdala potentiates nicotine conditioned place preference. J Neurosci. 2012;32:1488–1495. doi: 10.1523/JNEUROSCI.2980-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP. Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology (Berl) 2010;210(2):199–209. doi: 10.1007/s00213-010-1844-5. [DOI] [PubMed] [Google Scholar]
- Todd M. Daily processes in stress and smoking: effects of negative events, nicotine dependence, and gender. Psychol Addict Behav. 2004;18(1):31–39. doi: 10.1037/0893-164X.18.1.31. [DOI] [PubMed] [Google Scholar]
- West RJ, Hajek P, Belcher M. Severity of withdrawal symptoms as a predictor of outcome of an attempt to quit smoking. Psychol Med. 1989;19(4):981–985. doi: 10.1017/s0033291700005705. [DOI] [PubMed] [Google Scholar]
- Wilkins JN, Carlson HE, Van Vunakis H, et al. Nicotine from cigarette smoking increases circulating levels of cortisol, growth hormone, and prolactin in male chronic smokers. Psychopharmacology (Berl) 1982;78(4):305–330. doi: 10.1007/BF00433730. [DOI] [PubMed] [Google Scholar]