Abstract
Tobacco smoking during adolescence has become a prominent preventable health problem faced in the United States. Addictive properties of smoking are thought to have a pronounced effect at a young age, thereby increasing vulnerability to a life-long addiction and decreasing the likelihood of smoking cessation during adulthood. Learning and memory involvement in nicotine reward was assessed in early adolescent (PND 28–34) and adult (PND 70+) male ICR mice by conducting conditioning sessions of nicotine (0.5 mg/kg) acquisition at varying time-spans, and evaluating extinction and reinstatement of nicotine preference using Conditioned Place Preference. Acquisition studies resulted in a significant preference for nicotine after 3 days of conditioning for both age groups, but not after only 1 or 2 conditioning days. In the extinction study, adolescent mice exhibited preference for nicotine 72 h after the last conditioning session, whereas preference for nicotine was extinct in adult mice by 72 h. Reinstatement studies showed adolescent mice, but not adult mice, recovering nicotine preference after a priming injection of 0.1 mg/kg nicotine on day 9 after the mice underwent extinction. No significant differences were found when nAChRs were quantified in both early adolescent and adult mice using binding techniques including cytisine sensitive, α-conotoxin-MII sensitive, and α-bungarotoxin sensitive nAChRs. Levels of striatal dopamine release were measured in both age groups using a dopamine release assay over a range of nicotine doses, which also resulted in no significant differences. More sensitive assays may facilitate in understanding the mechanisms of nicotine reward in adolescent mice.
Keywords: Nicotine, Adolescence, Reward, Conditioned place preference, Mice
1. Introduction
Tobacco smoking at a young age is an increasing problem in the United States and around the world. The rate of adolescent smoking among Americans has been rising sharply since 1992 [15]. Moreover, the age of initiation for smoking has also been declining [15]. The commencement of smoking at a young age is reported to increase addiction liability, decrease the probability of successful cessation [16], and correlate with a higher number of cigarettes smoked per day [29]. Adolescent onset of smoking is associated with several long-term consequences including a reduced probability of quitting [5,6,17,38], and a higher risk of relapse [8,39]. These studies indicate the critical nature of preventing adolescents from smoking, which in turn may reduce the likelihood of lifetime dependence.
Smoking has both positive and negative components that contribute to addiction. For example, it elicits positive and reinforcing effects due to the release of dopamine in the brain, an effect most probably due to nicotine [11]. Negative aspects, such as withdrawal and craving, prevent a smoker from successful cessation. Previous research has shown that adolescent smokers experience both positive and negative components of smoking differently than adult smokers [12]. Indeed, human studies show that adolescents may be more susceptible to the effects of various addictive substances including alcohol, nicotine, and cannabis [12,29]. Furthermore, other studies have demonstrated that early use of any drug is a strong indicator of regular drug use in adulthood [29]. In the same manner, rodent studies show age-dependent differences in the sensitivities to nicotine. Previous studies on mice and rats using conditioned place preference (CPP) and i.v. self-administration paradigms report an enhanced rewarding and reinforcing effects of nicotine in adolescent compared to adult animals [1–4,7,9,18,25,31]. On the other hand, negative effects such as aversion and withdrawal signs are attenuated [4,18,22]. This enhanced sensitivity to nicotine's rewarding effects is likely to contribute to an overall heightened vulnerability to nicotine dependence during early adolescence.
The purpose of this study was to investigate possible behavioral and molecular mechanisms involved in the increased sensitivity to nicotine's conditioned rewarding effects in early adolescent male mice (PND 28–34). We focused on this age group since it showed the most significant behavioral differences in our previous work [18]. We first studied possible behavioral factors that could contribute to the reported increase in nicotine's sensitivity in the CPP model. For example, it has been suggested that adolescents have a faster rate of learning compared to adults [27]. Since contextual learning is likely to be involved in the CPP model, nicotine preference was assessed after various numbers of conditioning sessions in both adult and early adolescent animals. In addition, memory retention is another possible factor that was examined via a CPP extinction study. This was conducted to compare how long preference for nicotine is retained, the acquisition and extinction of nicotine-induced CPP, and the effect of reinstating preference after the extinction period in both age groups. Finally, the levels of various neuronal nicotinic receptors (nAChRs) subtypes that are known to play roles in mediating nicotine rewarding and cognitive effects in relevant brain regions (α4β2* and α7 subtypes) [32] in both adult and early adolescent mice, as well as differences in nicotine-induced dopamine release within the mesolimbic reward pathway were examined in this study.
2. Materials and methods
2.1. Animals
Experimentally naïve male ICR mice were obtained from Harlan Laboratories (Indianapolis, IN). They were housed five per cage and given ad libitum access to food and water. Based on previous characterization of rodent adolescence [27,28], the following ages were used in our studies: early adolescent (PND 28–34) and adult mice (PND 70+). Animals were maintained in a facility approved by the American Association for Accreditation of Laboratory Animal Care. The study was conducted according to the guidelines of Institutional Animal Care and Use Committee of Virginia Commonwealth University.
2.2. Nicotine-induced conditioned place preference (CPP)
An unbiased CPP paradigm was utilized in all studies as reported by our group earlier [18]. Place conditioning boxes consisted of two distinct sides (20 cm × 20 cm × 20 cm). A partition separated the two sides with an opening that allowed access to either side of the chamber, and this partition could be closed for pairing days.
2.2.1. Handling habituation
On Wednesday–Friday of the week prior to the start of the place conditioning procedure, each mouse in the CPP studies was handled once per day for approximately 2 min. Handling experience plays an important role in the ability of nicotine to produce CPP [14].
2.2.2. Preconditioning phase
On the first day, animals were placed in the boxes and allowed to move freely from side to side for 15 min, and time spent in each side was recorded. These data were used to separate the animals into groups of approximately equal bias.
2.2.3. Conditioning phase
Animals were paired for 20 min, with the saline group receiving saline in both sides of the boxes and drug groups receiving nicotine (0.5 mg/kg s.c.) on one of the sides and saline on the opposite side. Drug-paired sides were randomized among all groups. In acquisition studies, conditioning lasted for 3 days, with animals in the drug group receiving drug once a day. All doses of nicotine are expressed as free base.
2.2.4. Test phase
On test day, no injections were given. Time spent on each side was recorded, and data were expressed as time spent on drug-paired side minus time spent on saline-paired side. A positive number indicated a preference for the drug-paired side, while a negative number indicated an aversion to the drug-paired side. A number at or near zero indicated no preference for either side.
2.3. CPP acquisition studies
For acquisition studies, subsets of mice were conditioned with nicotine for one, two, or three sessions and were subsequently tested for CPP the following day using the same dose of 0.5 mg/kg nicotine in both ages. This dose was chosen because it was active in both age groups as previously reported [18].
2.4. CPP extinction studies
For extinction studies, mice were tested for preference according to the test day method previously described on day 5. However animals received saline injection and were then put in the preference chambers. This test day procedure was repeated every 24 h until no significant preference was observed.
2.5. CPP reinstatement studies
After 3 days of conditioning, mice were tested for CPP as previously described in the test day procedure above. After testing, mice were returned to their home cages and tested again every 24 h until preference behavior was extinguished. Once preference was no longer evident, mice were given a low dose of nicotine (0.1 mg/kg s.c.) and re-evaluated for preference under the same test day protocol.
2.6. Nicotinic receptor binding
2.6.1. Materials
(±)-[3H]epibatidine (48 Ci/mmol) and l-[3H]nicotine (78 Ci/mmol), were purchased from Du Pont NEN (Boston, MA). α-[125I]bungarotoxin (initial specific activity = 220 Ci/mmol) plastic tritium standards and Hyperfilm-3H were purchased from Amersham (Mount Prospect, IL). NaCl, KCl, MgSO4, CaCl2, gelatin, chromium aluminum sulfate, cytisine, acetylcholine and diisopropylfluorophosphate were obtained from Sigma Chemical Co. (St. Louis, MO). Methylcarbachol chloride, (+)-epibatidine tartrate, and (–)-epibatidine tartrate were obtained from RBI (South Natick, MA). Nicotine bitartrate was a product of BDH Chemicals (Poole, England). Glass fiber filters Type A/E were obtained from Gelman Sciences (Ann Arbor, MI) and Type GB from MFS (Dublin, CA). Budget Solve scintillation fluid was obtained from RPI (Arlington Heights, IL).
2.6.2. Tissue preparation
Adult and adolescent mice were sacrificed by cervical dislocation; the brain was removed from the skull and placed on an ice-cold platform. The following four brain regions were dissected: nucleus accumbens (NAc), ventral tegmental area (VTA), hippo-campus (HIP), and prefrontal cortex (PFC). Samples were homogenized in ice-cold hypotonic buffer (NaCl, 14.4 mM; KCl, 0.2 mM; CaCl2, 0.2 mM; MgSO4, 0.1 mM, HEPES, 2.0 mM; pH 7.5) using a glass–Teflon tissue grinder. The particulate fraction was obtained by centrifugation at 20,000 × g for 20 min in a Sorvall RC-2B centrifuge. The pellet was resuspended in fresh homogenization buffer, incubated at 37 °C for 10 min, and harvested by centrifugation. Each sample was washed twice more by resuspension and centrifugation and stored as a pellet under homogenization buffer at –70 °C until use.
2.6.3. [3H]nicotine binding
The binding of [3H]nicotine (78 Ci/mmol, Du Pont NEN, Boston, MA) was measured using a modification of the method of [20]. Samples (50–200 μg, depending on brain region) were incubated in 96-well polystyrene plates with 20 nM [3H]nicotine at 22 °C for 30 min in 100 μl of binding buffer (NaCl, 144 mM; KCl, 1.5 mM; CaCl2, 2 mM; MgSO4, 1 mM; HEPES, 20 mM; pH 7.5). The binding reaction was terminated by filtration of the samples onto glass fiber filters (MFS GB top, Gelman A/E bottom) that had been soaked in binding buffer containing 0.5% polyethylenimine using an Inotech Cell Harvester (Inotech, East Lansing, MI). Samples were subsequently washed six times with ice-cold binding buffer. Nonspecific binding was determined by including 10 μM l-nicotine in the assay.
2.6.4. α-[125I]bungarotoxin binding
The binding of α-[125I]bungarotoxin (initial specific activity = 220 Ci/mmol, Amersham (Mount Prospect, IL). was measured using a modification of the method of Marks et al. [20]. The binding reaction was similar to that used for [3H]nicotine with the following changes: incubation time was 5 h, samples contained 1 nM α-[125I]bungarotoxin instead of [3H]nicotine and the binding buffer also included 0.025% bovine serum albumin. Blanks were determined by including 1 mM l-nicotine in the assay.
2.6.5. [3H]epibatidine binding
The binding of [3H]epibatidine (48 Ci/mmol, Du Pont NEN) was measured in a method analogous to that of [3H]nicotine with the following changes: incubations were in 1-ml polypropylene tubes in a 96-well format, incubation volume was 500 μl, and [3H]epibatidine rather than [3H]nicotine was used. Nonspecific binding was determined by including 100 μM l-nicotine in the assay. Nonspecific binding at all concentrations of [3H]epibatidine was less than twice background (40 dpm). The following experiments were conducted: construction of curves for inhibition of [3H]epibatidine binding in olfactory bulbs by cytisine, nicotine, acetylcholine (Sigma Chemical Co.) (using tissue treated with 10 μM diisopropylfluorophosphate during the tissue preparation), methylcarbachol (RBI, South Natick, MA), (+)-epibatidine and (–)-epibatidine (preliminary experiments indicated that inhibition in olfactory bulbs deviated markedly from that expected for a single site); construction of curves for inhibition of [3H]epibatidine binding in four brain regions by cytisine; and measurement of the concentration dependence of [3H]epibatidine binding in four brain regions. The concentration of [3H]epibatidine used for inhibition curves was about 400 pM (approximately 20 × Kd). This concentration was chosen to maintain ligand binding to the tissue to less than 5% of the total. An incubation time of 60 min was used for these experiments (equilibrium was reached in 20–30 min). For saturation curves, eight [3H]epibatidine concentrations between 6 and 800 pM were used. Incubation time for these experiments was 2 h (equilibrium was reached by 60 min for all concentrations). In these experiments a significant fraction of the [3H]epibatidine was bound to the tissue, especially at lower ligand concentrations. Free [3H]epibatidine concentration was estimated by correcting for the amount of ligand bound to the tissue at each concentration for every brain region. Protein was measured using the method of Lowry et al. [34] with bovine serum albumin as the standard.
2.6.6. Calculations
Results for saturation binding experiments were calculated using the Hill equation: , where B is the binding at free ligand concentration, L, Bmax is the maximum number of binding sites, Kd is the equilibrium dissociation constant, and n is the Hill coefficient. Values of Bmax, Kd and n were calculated using the nonlinear least squares algorithm in Sigma Plot 5 (Jandel Scientific, San Rafael, CA). Results for inhibition of epibatidine binding were calculated using the formulas for either one or two binding sites: B = B0/(1 + (I/IC50)) or B = B1/(1 + (I/IC50-1)) + B2/(1 + (I/IC50-2)), respectively, where B is ligand bound at inhibitor concentration, I, B0 is the binding in the absence of inhibitor, and B1 and B2 are the binding to two sites sensitive to inhibition with IC50-1 and IC50-2. Assuming competitive inhibition: IC50 = Ki(1 + L/Kd). Results were also calculated using the Hill equation.
2.7. Dopamine release assay
2.7.1. Membrane preparation
Adult and adolescent male mice were sacrificed by cervical dislocation. The brain was removed from the skull and immediately placed on ice for dissection. Striatum was isolated and removed from brain. Tissue was homogenized (16–20 strokes by hand) in 0.5 ml of 0.32 M sucrose buffered with 5 mM HEPES, pH 7.5. Synaptosomal pellets were then prepared by centrifugation at 1000 × g for 10 min, followed by centrifugation of resulting supernatant at 12,000 × g for 20 min. The pellets were resuspended in perfusion buffer (128 mM NaCl, 2.4 mM KCl, 3.2 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM HEPES, pH 7.5, 10 mM glucose, 1 mM ascorbic acid, and 0.01 mM pargyline). The perfusion procedure has been described previously [40]. Synaptosomes were incubated at 37 °C in perfusion buffer for 10 min before addition of 100 nM [3H]dopamine (PerkinElmer Life and Analytical Sciences, Boston, MA, 1 μCi for every 0.2 ml of synaptosomes). Aliquots of synaptosomes (80 μl) were distributed onto filters and perfused at 0.6 ml/min for 10 min before fractions were collected. [3H]dopamine was added at the same time during the last 5 min of the uptake procedure. Atropine (1 μM) was added to the perfusion buffer to inhibit muscarinic receptors. Various concentrations of nicotine were used to stimulate dopamine release. Fractions were collected every 30 s, and radioactivity was determined by scintillation counting (1600TR liquid scintillation spectrometer; Packard Instrument Co.) after addition of EconoSafe (Sigma/RBI).
2.8. Statistical analysis
Statistical analysis of CPP studies was performed with mixed-factor analysis of variance (ANOVA) with Tukey's post-hoc test when appropriate. Nicotine stimulated dopamine release was analyzed with a two-way ANOVA followed by Tukey's post hoc tests. A p value of <0.05 was considered statistically significant. nAChR binding studies were analyzed using two-way ANOVAs with significant ANOVAs further analyzed with Dunnets post hoc tests (α = 0.05) to specify differences between means. EC50 values were calculated for dopamine release assay curves.
3. Results
3.1. Nicotine-induced conditioned place preference in adolescent and adult mice
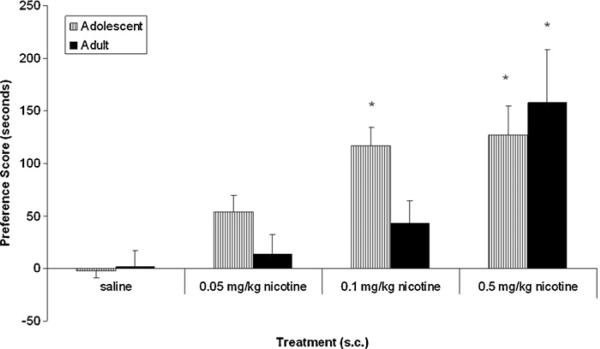
As expected, adult and early adolescent animals injected with saline in both chambers showed no preference for either chamber. For nicotine-treated mice, a significant age × nicotine dose interaction (F(3, 51) = 3.98, p < 0.05) indicated that compared to saline controls, male adult mice conditioned with the dose of 0.5 mg/kg nicotine showed a significant place preference (p < 0.05) (Fig. 1). There were no significant preferences seen with lower doses of 0.05 or 0.1 mg/kg of nicotine in adult mice. In contrast, nicotine showed a significant place preference in adolescent mice at low doses of nicotine of 0.05 and 0.1 mg/kg as well as at the 0.5 mg/kg dose (p < 0.05).
Fig. 1.
Nicotine-induced conditioned place preference in adults and adolescents. Mice were conditioned with various doses of nicotine (0.05–0.5 mg/kg) and tested on day 5. Results are expressed as the means and SEM of preference score (s) of 8–10 mice. *p < 0.05 as compared to respective saline control.
3.2. Acquisition of nicotine-induced CPP in adult and adolescent mice
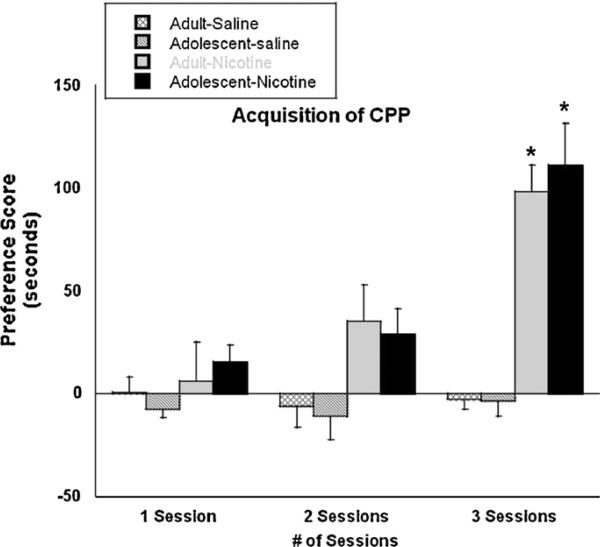
Fig. 2 shows the acquisition of nicotine-induced CPP in adult and adolescent mice at an active dose of 0.5 mg/kg nicotine. As expected, mice conditioned with saline showed no preference for either side after all numbers of conditioning sessions. Similarly, after one and two conditioning sessions, neither adult nor adolescent mice displayed significant preference for the drug-paired side. In contrast, after 3 days of conditioning, both age groups exhibited a significant preference for the nicotine-paired side. A non-significant age × number of session interaction (F(2, 81) = 2.73, p > 0.05) indicated that the intensity of preference did not differ between the age groups at the dose of nicotine used.
Fig. 2.
The rate of CPP acquisition in adult and adolescent mice. Adult (PND 70+) and adolescent (PND 28) male ICR mice were conditioned for one, two, or three sessions with 0.5 mg/kg nicotine and were subsequently evaluated for nicotine-induced preference. Bars represent the mean ± SEM of 6–8 mice. *p < 0.05 as compared to respective saline control.
3.3. Extinction of nicotine-induced CPP
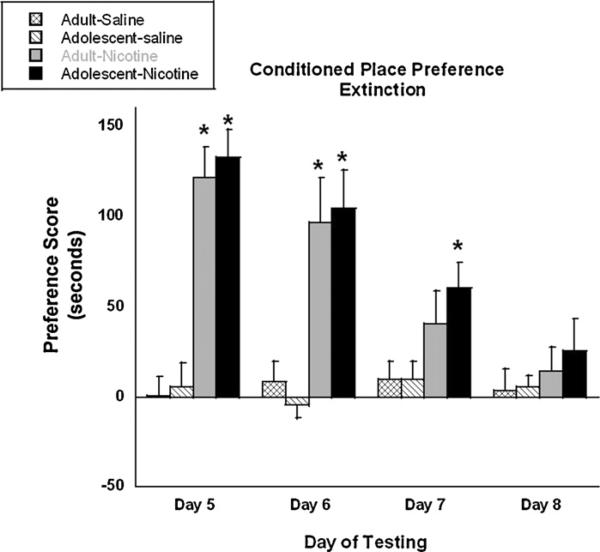
Fig. 3 shows the results from the CPP extinction study. Mice were conditioned with nicotine for 3 days and tested for preference the following day. As expected from previous studies [18], both adult and adolescent mice displayed significant preference for the nicotine-paired side. Mice were then evaluated for preference every 24 h in a drug-free state. A significant age × number of session interaction (F(3, 61) = 3.12, p < 0.05) indicated that in the same manner as day 5, both age groups exhibited significant preference for the drug-paired side on day 6. However, on day 7 (72 h after the last conditioning session), only adolescent mice displayed a significant preference for nicotine as compared to saline controls (p < 0.05). Adult mice no longer exhibited a significant preference. On day 8, neither age group showed preference for the drug-paired side.
Fig. 3.
Rate of CPP extinction in adult and adolescent mice. Adult (PND 70+) and adolescent (PND 28) male ICR were conditioned for three sessions with 0.5 mg/kg nicotine and were subsequently evaluated for extinction of nicotine-induced preference. Bars represent the mean ± SEM of 6–8 mice. *p < 0.05 as compared to respective saline control.
3.4. Reinstatement of nicotine-induced conditioned place preference
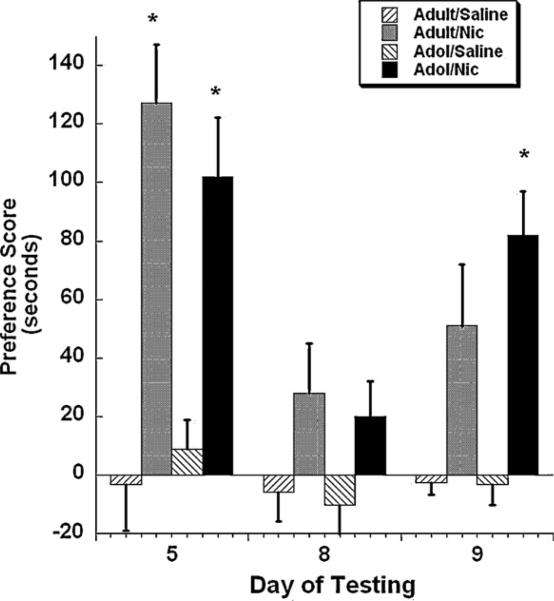
A separate group of mice were conditioned for 3 days in the CPP paradigm then tested for nicotine preference the day after. As shown in Fig. 4, a significant age × session × age × treatment interaction (F(2, 43) = 3.49, p < 0.05) indicated that both age groups displayed significant preference for the nicotine-paired side. Nicotine-induced preference was extinguished by day 8 in both age groups. On day 9, mice were given a priming injection of nicotine (0.1 mg/kg s.c.) and tested for preference in the CPP chambers. Adolescent mice showed reinstatement for nicotine preference on day 9 after priming with a nicotine dose of 0.1 mg/kg (p < 0.05), whereas adult mice did not show a significant increase for nicotine preference.
Fig. 4.
Rate of CPP reinstatement in adult and adolescent mice. Adult (PND 75+) and adolescent (PND 28–34) male ICR underwent extinction (represented by day 8) after three conditioning sessions with 0.5 mg/kg nicotine in the CPP test and then were subject to extinction procedure. At day 8, animals were subsequently evaluated for reinstatement of nicotine-induced preference via a priming injection of 0.1 mg/kg nicotine. Bars represent the mean ± SEM of 6–8 mice. *p < 0.05 as compared to respective saline control.
3.5. Nicotinic receptor binding studies
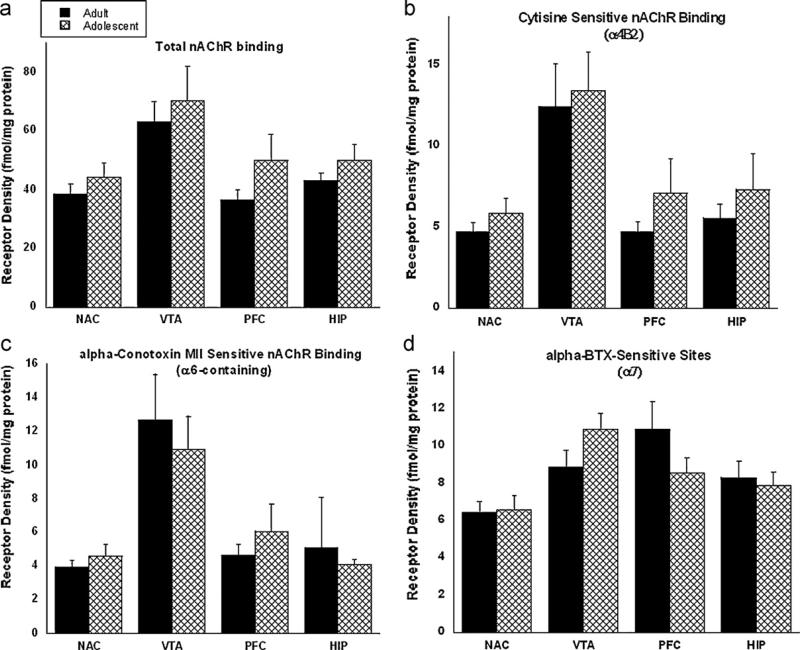
Results from the nicotinic receptor binding studies are shown in Fig. 5. Binding techniques using various nicotinic ligands in the NAc, VTA, HIP, and PFC quantified the following subtypes: (a) total nAChRs, (b) two important subtypes that are known to play roles in mediating drug reward: cytisine sensitive α4β2* nAChRs and α-conotoxin-MII sensitive α6-containing nAChRs (c), and α-bungarotoxin sensitive α7 nAChRs for its role in nicotine-induced cognitive effects. In the α4β2*, α6-containing subtypes and the total nAChR binding studies, a trend for increased nAChR binding in the adolescent mice was observed; however no significant differences were found in total nAChR binding or for a particular nAChR subtype. Similarly, no significant differences were found in the α7 subtypes.
Fig. 5.
Nicotinic acetylcholine receptor binding assays were performed on adult (PND 70+) and adolescent (PND 28) ICR mice. Various pharmacological tools were used to assess the following nAChR subtypes: (a) total nAChR binding; (b) cytisine-sensitive binding (α4β2); (c) alpha-conotoxin (α6); and (d) α-bungarotoxin (α7). Results are expressed as receptor density normalized to protein. Bars represent the mean ± SEM of 6–8 mice.
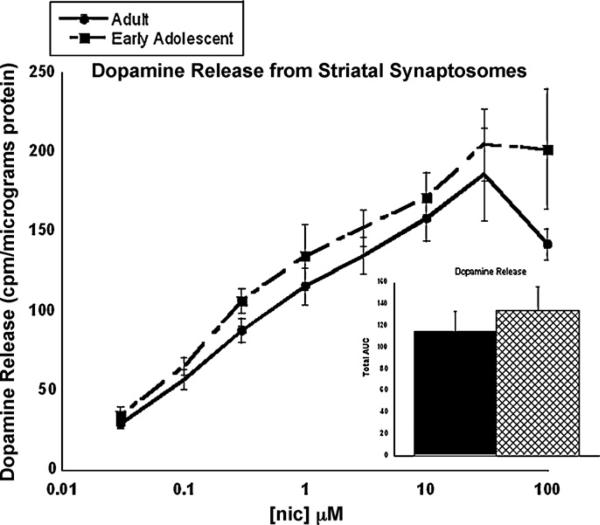
3.6. Dopamine release assay
Fig. 6 shows the results from the dopamine release assay, which examined levels of dopamine release from the striatal synaptosomes in adult and adolescent mice. Dose–response curves were calculated for both age groups over a range of nicotine doses. Nicotine-induced dopamine release is concentration dependent in both age groups. While the concentration curve in the early adolescent was slightly shifted to the left, no significant differences were found between the two ages at any concentrations of nicotine used. This was confirmed when measuring the total area under the curve.
Fig. 6.
Dopamine release from striatal synaptosomes in adult (PND 70+) and adolescent (PND 28–34) mice. Striatal synaptosomes were stimulated with various concentrations of nicotine to generate a dose–response curve. Each point represents the mean ± SEM of 6–8 mice. Total area under the curve is also presented in the graph to the right with adults represented by the solid black bar and adolescents represented by the hatched bar. *p < 0.05 from same concentration in adult.
4. Discussion
As reported previously by our group, adolescent mice displayed an enhanced sensitivity to nicotine reward as compared to adults in the CPP model (Fig. 1) [18]. This report explores behavioral and molecular mechanisms, which may underlie this age-related difference in sensitivity for nicotine preference.
The data from CPP acquisition study revealed that both adults and adolescents acquire a preference for nicotine at a similar rate. Specifically, three conditioning sessions were required for a significant preference to be developed in both age groups. These results suggest that both age groups learned to associate nicotine with a particular context at the same rate. However, our extinction data suggest that the two ages may differ in retention of nicotine preference. On the third day after the last conditioning session (day 7), preference for nicotine was lost in adult mice. However, adolescent mice retained preference for the drug-paired side even after the third day of the last conditioning session. For adolescent mice, extinction of this behavior occurred 4 days after the final conditioning session with nicotine, suggesting that memory retention may play a role in the enhanced preference for nicotine in these mice. These results suggest that adolescent mice may be able to retain drug-paired cues and contextual signs longer than adults, which may lead to increased drug-seeking in particular environments.
As for the reinstatement studies, mice underwent extinction, and day 9, they received an injection of nicotine (0.1 mg/kg) to induce nicotine preference once again. The ability of adolescent mice to reinstate preference for nicotine at a lower dose than what was given during conditioning days confirms their increased sensitivity to nicotine's effects observed in CPP [18]. Our finding is consistent with a recent study of drug-induced reinstatement using the CPP paradigm, where adolescent male rats exhibited more robust reinstatement of cocaine preference than adults [35].
Changes in nicotine preference behavior observed between adolescents and adults may be attributed to underlying molecular changes. We recently reported that adolescent mice display an increased functional response of neuronal nAChRs to nicotine stimulation as compared to adults [18]. One possibility for an increase in nAChR function is the presence of higher basal levels of nAChRs in the adolescent brain. This hypothesis was tested using various nAChR binding studies, which assess different subtypes of receptors in adolescent and adult mice. However, no significant differences in overall receptor binding levels were found in this study. In addition, receptor subtypes containing α4β2*, α6*, and α7 were also evaluated and still no significant differences were observed. In contrast to these findings, Azam et al. reported that levels of α5, α6, and α7 mRNA were higher during the adolescent period in rats [36]. However, mRNA data must be interpreted with caution in that differences in mRNA do not necessarily reflect a similar change in receptor protein expression. Another possibility is that differences in receptor stoichiometry (i.e. (α4)2(β2)3 vs. α4α5β2, etc.) could account for the lack of differences between age groups. Indeed, stoichiometric changes are not detectable by the binding methods that were used for this study.
Finally, we explored differences in post-receptor mechanisms that we thought to play an important role in nicotine reward, namely nicotine-induced dopamine release in the striatum. Since neuronal pathways are still developing in young animals, it is also possible that the adaptations in the brain during development cause the levels of dependence to change over time. For example, the dopaminergic system undergoes significant development during adolescence, and may account for behavioral observations [27]. Our hypothesis was that greater amounts of dopamine release during adolescence compared to adulthood would explain the increase in nicotine reward. However, results from our dopamine release studies did not show significant differences between the two age groups in mice. In contrast, using microdialysis, Azam et al. [36] reported that nicotine-stimulated dopamine release was significantly higher during the early adolescent period in the male rat. Furthermore, previous work has demonstrated that dopamine release is attenuated in the adult rat during withdrawal [37]. The study by Azam et al. [36] was different from this study in that they compared nicotine-evoked dopamine release in rats at younger ages (PND 7 and PND 14); whereas this study used mice at older ages (PND 27–34). More sensitive assays may be required to detect significant differences between the age groups due to the limitations of methods used in this study. Azam et al. [36] measured dopamine release using the microdialysis technique, a method that preserves neuronal connections; whereas our dopamine release studies were done on a crude preparation of synaptosomes. An alternative explanation for the behavioral responses that we have observed, may be linked to other non-nicotinic receptor types, which are known to be involved in nicotine dependence, such as glutamatergic receptors.
In summary, results suggest that behaviors such as memory retention or recollection are age-dependent, where adolescents express preference for nicotine in CPP for a longer period of time compared to adults. Results showed that differences in basal levels of various nAChR subtypes involved in nicotine reward could not explain the functional upregulation of nAChR in adolescent mice. Finally, data with striatal synaptosomes could not reveal significant differences in nicotine induced dopamine release. Thus, other unknown mechanisms might account for the observed behavioral differences in nicotine dependence among the studied age groups.
Footnotes
This study was supported by a grant from Virginia Foundation for Healthy Youth and National Institute on Drug Abuse grants # DA-12610.
References
- 1.Abreu-Villaca YA, Seidler FJ, Qiao D. Short-term exposure adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology. 2003;28:1935–49. doi: 10.1038/sj.npp.1300221. [DOI] [PubMed] [Google Scholar]
- 2.Adriani W, Deroche-Gamonet V, Le Moal M, Laviola G, Piazza PV. Preexposure during or following adolescence differently affects nicotine-rewarding properties in adult rats. Psychopharmacology (Berl) 2006;184:382–90. doi: 10.1007/s00213-005-0125-1. [DOI] [PubMed] [Google Scholar]
- 3.Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, et al. Evidence for enhanced neurobehavioral vulnerability to nicotine during peri-adolescence in rats. J Neurosci. 2003;23:4712–6. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adriani W, Macri S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27:212–24. doi: 10.1016/S0893-133X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- 5.Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug Alcohol Depend. 1995;40:9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- 6.Breslau N, Peterson EL. Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. Am J Public Health. 1996;86:214–20. doi: 10.2105/ajph.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Britton AF, Vann RE, Robinson SE. Perinatal nicotine exposure eliminates the peak in nicotinic acetylcholine receptor response in adolescent rats. J Pharmacol Exp Ther. 2007;320:871–6. doi: 10.1124/jpet.106.112730. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Millar WJ. Age of smoking initiation: implications for quitting. Health Rep. 1998;9:39–46. [PubMed] [Google Scholar]
- 9.Collins SL, Wade D, Ledon J, Izenwasser S. Neurochemical alterations produced by daily nicotine exposure in periadolescent vs. adult male rats. Eur J Pharmacol. 2004;502:75–85. doi: 10.1016/j.ejphar.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 11.Dani JA. Overview of nicotinic receptors and their roles in the central nervous system. Biol Psychiatry. 2001;49:166–74. doi: 10.1016/s0006-3223(00)01011-8. [DOI] [PubMed] [Google Scholar]
- 12.DiFranza JR. Hooked from the first cigarette. J Fam Pract. 2007;56:1017–22. [PubMed] [Google Scholar]
- 14.Grabus SD, Martin BR, Brown SE, Damaj MI. Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology. 2006;184:456–63. doi: 10.1007/s00213-006-0305-7. [DOI] [PubMed] [Google Scholar]
- 15.Johnston LD, O'Malley OM, Bachman JG. National survey results on drug use from the monitoring the future study. Vol. 1. National Institute on Drug Abuse; Washington, DC: 1998. Secondary school students. pp. 1975–97. [Google Scholar]
- 16.Kandel DB, Chen K. Extent of smoking and nicotine dependence in the United States: 1991–1993. Nicotine Tob Res. 2000;2:263–74. doi: 10.1080/14622200050147538. [DOI] [PubMed] [Google Scholar]
- 17.Khuder SA, Dayal HH, Mutgi AB. Age at smoking onset and its effect on smoking cessation. Addict Behav. 1999;24:673–7. doi: 10.1016/s0306-4603(98)00113-0. [DOI] [PubMed] [Google Scholar]
- 18.Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- 20.Marks MJ, Grady SR, Collins AC. Downregulation of nicotinic receptor function after chronic nicotine infusion. J Pharmacol Exp Ther. 1993;266:1268–76. [PubMed] [Google Scholar]
- 22.O'Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, et al. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology. 2006;186:612–9. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- 25.Shram MJ, Funk D, Li Z, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psycho-pharmacology. 2006;186:201–8. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- 27.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 28.Spear LP, Brake SC. Periadolescence: age-development behavior and psycho-pharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- 29.Taoli E, Wydner EL. Effect of the age at which smoking begins on frequency in adulthood. New Engl J Med. 1991;325:968–9. doi: 10.1056/NEJM199109263251318. [DOI] [PubMed] [Google Scholar]
- 31.Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–14. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- 32.Walters CL, Cleck JN, Kuo Y, Blendy JA. μ-Opioid receptor and CREB activation are required for nicotine reward. Neuron. 2005;46:933–43. doi: 10.1016/j.neuron.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 35.Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122:460–5. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azam L, Chen Y, Leslie FM. Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neuroscience. 2007;144:1347–60. doi: 10.1016/j.neuroscience.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hildebrand BE, Nomikos GG, Hertel P, Schilström B, Svensson TH. Reduced dopamine output in the nucleus accumbens but not in the medial prefrontal cortex in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Brain Res. 1998;779:214–25. doi: 10.1016/s0006-8993(97)01135-9. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Millar WT. Age of smoking initiation: implications for quitting. Health Rep. 1998;9:39–46. [PubMed] [Google Scholar]
- 39.Cui Y, Wen W, Moriarty CJ, Levine RS. Risk factors and their effects on the dynamic process of smoking relapse among veteran smokers. Behav Res Ther. 2006;44:967–81. doi: 10.1016/j.brat.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Grady SR, Grun EU, Marks MJ, Collins AC. Pharmacological comparison of transient and persistent [3H]dopamine release from mouse striatal synaptosomes and response to chronic L-nicotine treatment. J Pharmacol Exp Ther. 1997;282:32–43. [PubMed] [Google Scholar]