Abstract
Many pregnant women smoke cigarettes during pregnancy, but the effect of nicotine on the developing human brain is not well understood, especially in young children. This study aims to determine the effects of prenatal nicotine exposure (PNE) on brain metabolite levels in young (3–4 years old) children, using proton magnetic resonance spectroscopy (1H MRS). Twenty-six children with PNE and 24 nicotine-unexposed children (controls) were evaluated with a structured examination, a battery of neuropsychological tests, and MRI/1H MRS (without sedation). Concentrations of N-acetyl compounds (NA), total creatine (tCR), choline-containing compounds (CHO), myo-inositol (MI), and glutamate+glutamine (GLX) were measured in four brain regions. Children with PNE had similar performance to controls on neuropsychological testing. However, compared to controls, the PNE group had lower MI (repeated measures ANOVA—p=0.03) and tCr levels (repeated measures ANOVA–p=0.003), especially in the basal ganglia of the girls (−19.3%, p=0.01). In contrast, GLX was elevated in the anterior cingulate cortex of the PNE children (+9.4%, p=0.03), and those with the highest GLX levels had the poorest performance on vocabulary (r=−0.67; p<0.001) and visual motor integration (r=−0.53; p=0.01). The amount of prenatal nicotine exposure did not correlate with metabolite concentrations. These findings suggest that PNE may lead to subclinical abnormalities in glial development, especially in the basal ganglia, and regionally specific changes in other neurometabolites. These alterations were not influenced by the amount of nicotine exposure prenatally. However, the effects of PNE on energy metabolism may be sex specific, with greater alterations in girls.
Keywords: Nicotine, Pediatric, Spectroscopy, Glial, Vocabulary, Motor
Introduction
Abnormalities in neurodevelopment and neurotransmitters due to prenatal nicotine exposure (PNE) are well documented in preclinical studies (Slotkin 2008). However, data regarding how PNE might alter brain development in humans remain limited, especially in younger children who have not begun using nicotine or other drugs. Behavioral and cognitive studies of PNE demonstrate a dose–response relationship between maternal smoking rates and low birth weight (Ernst et al. 2001), and more frequent spontaneous abortions (George et al. 2006; Shea and Steiner 2008). One study of 1,872 infants found that mothers who smoked 10 or more cigarettes per day during pregnancy almost doubled the risk of their infants being non-babblers at 8 months of age (Obel et al. 1998). PNE is also associated with deficits in cognitive development, attention deficit disorder, hyperactivity, and antisocial behaviors in children and adolescents (Kandel et al. 2009).
Few neuroimaging studies have evaluated the effect of PNE on the developing brain. On functional MRI (fMRI), adolescent tobacco smokers with PNE had greater nicotine withdrawal-related deficits in visuo-spatial memory than adolescent smokers without PNE (Jacobsen et al. 2007b). PNE adolescents also showed microstructural abnormalities (elevated fractional anisotropy) in auditory pathways and frontal white matter (Jacobsen et al. 2007a). Furthermore, sex differences in brain activation and task performance were found in children with PNE (Jacobsen et al. 2007c). Specifically, girls showed lower accuracy on auditory and visual attention, especially those who had both PNE and had begun smoking (combined exposure), whereas boys with combined exposure had significantly greater deficits in auditory attention only (Jacobsen et al. 2007a). Collectively, these three studies demonstrated possible deleterious effects of nicotine on the developing brain of adolescents with PNE. However, no neuroimaging data from younger children with PNE are available. Studying younger children makes it possible to eliminate the effect of current smoking, as is common in adolescents with PNE, and minimize the amount or duration of exposure to second hand smoking, which is also common in the households of children with PNE.
Prior studies of younger children with prenatal methamphetamine (METH) exposure showed abnormalities in brain metabolites, with elevated total creatine (Smith et al. 2001) and N-acetylaspartate (Chang et al. 2009). However, since mothers who use METH during pregnancy commonly also smoke cigarettes, it was difficult to separate the effects of in utero METH exposure from those of nicotine exposure. Therefore, the current study specifically evaluated whether (1) PNE, without significant exposure to METH or other drugs, is associated with brain metabolite abnormalities in young children (ages 3–4 years); (2) there are sex differences in brain metabolite abnormalities; and (3) the amount of nicotine exposure in utero is related to alterations in brain metabolites.
Materials and methods
Research participants
Fifty healthy children [26 with a history of PNE (age 46.7± 1.6 months) and 24 unexposed controls (age 46.0± 1.3 months)] were recruited from the local community. A written informed consent, approved by our institutional review board, was signed by the parent or legal guardian of each child. Detailed interviews were completed by the parent or primary caretaker to assess the child’s birth and medical history, and the mother’s drug use, medical history, and socioeconomic status, as described previously (Chang et al. 2009). Each child completed a structured medical and neurological examination, a battery of neuropsychological tests, and an MR study without sedation. All children fulfilled the following inclusion criteria: (1) male or female of any ethnicity, age 3–4 years; (2) parental willingness for the child to participate in the study; and (3) PNE group only—any maternal self-reported cigarette use during pregnancy. Exclusion criteria for both groups were (1) congenital or genetic neurological disorder, (2) premature birth (gestational age <36 weeks), (3) history of failure to thrive within the first year of life, (4) overt TORCH [Toxoplasmosis, Other (Treponema pallidum, varicella zoster virus, parvovirus), Rubella virus, Cytomegalovirus, and Herpes simplex virus] infection at birth or major neurological disorder since birth (e.g., bacterial meningitis, epilepsy), and (5) contraindications for MR studies. Additionally, a child was excluded if the mother met any of the following criteria: (1) age <18 years at the time of giving birth, (2) non-English speaking, (3) low cognitive functioning (estimated verbal IQ <80 on National Adult Reading Test) or institutionalized for retardation, (4) seropositive for HIV-1 during pregnancy, (5) history of psychiatric illnesses that may confound the outcome measures (e.g., schizophrenia or bipolar disorder with psychosis), (6) any confounding medical condition during pregnancy that could cause alteration in brain development, and (7) history of other drug dependence or abuse during pregnancy (including cocaine, methamphetamine, marijuana, alcohol, opiates, inhalants, LSD, PCP, and barbiturates). Two of the PNE subjects’ mothers consumed <1 drink/day only during the first trimester, and one drank only on the weekends throughout her pregnancy. Two of the PNE subjects’ mothers used a total of 18 and 29 marijuana joints during the first trimester only.
Neuropsychological tests
Each child was evaluated with a battery of neuropsychological tests to evaluate the following domains: (1) Global intelligence—Stanford–Binet Abbreviated Battery IQ (ABIQ), which provided an estimated IQ based on non-verbal and verbal test performance; (2) Fluid reasoning—Stanford–Binet Routing Non-Verbal, which evaluated the ability to recognize patterns in object and picture placement; (3) Vocabulary—Stanford–Binet Verbal, which assessed spoken vocabulary for objects, pictures, and words; (4) Naming and word retrieval—Expressive One Word Picture Vocabulary Test–Revised (EOWPVT-R), which had up to 143 items that assessed the child’s abilities to name objects, actions, and concepts in illustrations, and provided an indication of the child’s speaking vocabulary; (5) Comprehension/receptive vocabulary—Peabody Picture Vocabulary Test–Third Edition (PPVT-III) had up to 175 test items to measure auditory receptive language; (6) Visual motor integration—Beery Test of Visual Motor Integration (VMI) required the child to copy up to 24 increasingly difficult geometric figures; and (7) Visual attention/visuo-motor tasks—Developmental Neuropsychological Assessment (NEPSY) Visual Attention subtest evaluated visual scanning and attention, and psychomotor speed.
MR imaging and spectroscopy
MRI studies were performed on a Siemens Trio 3.0-T scanner, using an eight- or 12-channel head array radio-frequency (RF) coil. Scanning began with three orthogonal T1-weighted localizers (echo time/relaxation time or TE/TR=5/20 ms, 10-mm slice thickness, 2-mm gap, 256-mm field of view or FOV), followed by a 3D magnetization prepared rapid acquisition by gradient echo [MP-RAGE, TE/inversion time (TI)/TR=4.47/1,000/2,200 ms, 7° flip angle, 1-mm isotropic resolution, GRAPPA with 2-fold acceleration). Next, a fluid attenuated inversion recovery sequence (TE/TI/TR=108/2,500/9,750 ms, 3-mm slices, no gap, 220-mm FOV, GRAPPA with 2-fold acceleration) was performed to screen for any brain pathology.
Localized 1H MRS
Next, voxel locations were prescribed from the coronal, transverse, and sagittal MP-RAGE images. Spectroscopic data were collected from four brain regions: right frontal white matter (FWM, 5.8 cm3), medial frontal gray matter at the anterior cingulate cortex (ACC, 8 cm3), bilateral thalamus (7.2 cm3), and right basal ganglia (5.4 cm3) (see Fig. 1). Anatomical landmarks were used to ensure consistent placement of voxels. A Point RESolved Spectroscopy (PRESS) acquisition sequence (echo time/relaxation time=3,000/30 ms, 48 averages, 2.6 min per location) was used. Metabolite concentrations corrected for the partial volume of cerebrospinal fluid were determined using the unsuppressed water signal from each voxel as a reference by measuring the T2 decay of the unsuppressed water signal from the PRESS experiment at 10 different TE and two TR values (Ernst et al. 1993; Kreis et al. 1993a). The spectral and water data were preprocessed in IDL to eventually achieve absolute quantitation. Spectral fitting was performed using the LCModel program (Provencher 1993) to determine metabolite concentrations of N-acetyl compounds (NA), total creatine (tCR), choline-containing compounds (CHO), myo-inositol (MI), and glutamate +lutamine (GLX). The LCModel basis data set included spectra from major macromolecules and lipids. Furthermore, the percentages of gray and white matter in each voxel were determined from the MP-RAGE images, and used as covariates during statistical analyses to correct for possible differences in gray–white matter proportion in voxels among subjects and groups.
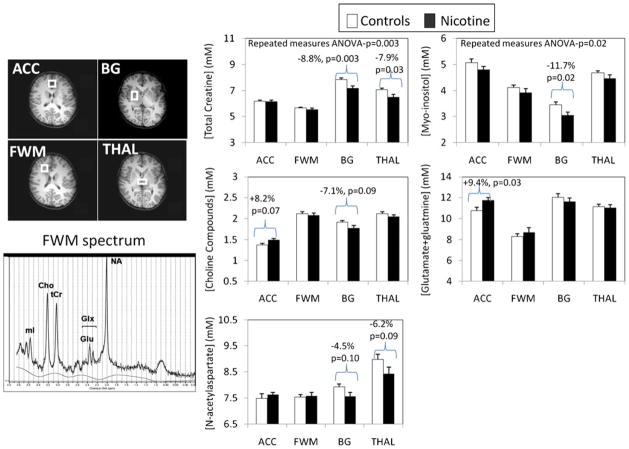
Fig. 1.
Top left: axial MR images showing the placement of the four voxels: (1) anterior cingulate cortex (ACC), which contains primarily gray matter at the anterior and medial portions of both cingulate cortices across both hemispheres and above the anterior portion of the head of caudate; (2) frontal white matter (FWM), which is confined to white matter in the right frontal lobe at the same level as the ACC; (3) right basal ganglia (BG), which contains the central portions of the putamen, the globus pallidus, and some portion of the body of the caudate; and (4) thalamus (THAL), which is confined to the gray matter structure and spans across both hemispheres, posterior to the BG voxel. Bottom left: representative MR spectrum from the frontal white matter, showing well-defined peaks for each of the brain metabolites measured (NA N-acetylaspartate, GLX glutamate+glutamine, tCr total creatine, CHO choline-containing compounds, MI myo-inositol). The solid line shows the fitted spectrum from LCModel. Right panel: brain metabolite concentrations in the four brain regions (also see Table 2) are shown for each of the metabolites of interest. The brackets indicate metabolite values that are significantly different, or show a trend for significance, between the two groups. Lower tCr and MI, and to a lesser extent CHO and NA, are found in the basal ganglia of the PNE children compared to the unexposed controls. Total creatine and trends for NA are also lower in the THAL. However, in the anterior cingulate cortex region, GLX and to a lesser extent CHO are elevated in the PNE children relative to the controls
Only 68% of the children could complete the study in one session. The primary reason for not completing the study was excessive head motion. The remaining 16 children (nine controls, seven PNE) required either one (eight controls, seven PNE) or two additional repeat scanning sessions to complete the protocol. There were no differences between subject groups in their spectral line width (full width at half maximum) in any of the four brain regions. However, the PNE group had lower signal-to-noise ratio in the ACC (−16%, p<0.001), FWM (−12%, p=0.04), and thalamus (−17%, p=0.006) compared to the unexposed controls.
Data analysis
Statistical analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA). Repeated measures analysis of variance (ANOVA) was used to assess the effect of PNE on each metabolite across the four brain regions, and post hoc t tests were then used to compare metabolite concentrations in each voxel between the two groups. Multiple linear regression was performed to examine PNE interactions with age and gender, and to adjust for potential confounders. Pearson or Spearman correlations were used to examine the relationships between clinical variables and metabolite levels that showed significant group differences. In all analyses, a two-sided p value<0.05 was considered significant. The Bonferroni procedure for multiple comparisons was used in the primary analysis with repeated measures. Post hoc t tests as well as relationships with age, gender, and other clinical variables were considered exploratory and therefore not corrected for multiple comparisons.
Results
Subject characteristics
Clinical characteristics of the research participants are shown in Table 1. The two groups had similar height, weight, and head circumference. The PNE children had slightly lower birth weights (p=0.07) with similar birth length, and hence lower BMI (p=0.02), despite greater maternal weight gain of PNE mothers during the pregnancy (p=0.02). The greater maternal weight gain in the smokers might be related to the successful smoking cessation in some of these mothers since two (8%) of the mothers stopped smoking by the second trimester and seven more mothers (total nine or 35%) stopped smoking by the third trimester. Primary caregivers of PNE children had poorer Hollingshead Index of Social Position (p=0.03) and showed trends for fewer years of education and lower verbal IQ. On average, the PNE children were exposed to nicotine cigarettes during most of the pregnancy (2.6±0.01 trimesters) but had minimal exposure to marijuana. Both groups had similar and minimal exposure to alcohol. Both groups performed similarly on the cognitive tests (Table 2); however, the PNE children tended to score lower on the NEPSY Attention task (p=0.06). Only one Nicotine subject lived with adoptive parents, and all Control children lived with their biological parents.
Table 1.
Clinical characteristics of the research participants (mean±SEM)
| Nicotine exposed (n=26) | Unexposed (n=24) | p value | |
|---|---|---|---|
| Characteristics of the children | |||
| # of children (% males/% females) | 19 (73%)/7 (27%) | 11 (46%)/13 (54%) | 0.09 |
| Age (months) | 46.7±1.6 | 46.0±1.3 | 0.70 |
| Race | 0.45 | ||
| Asian | 2 (8%) | 5 (21%) | |
| Mixed | 17 (65%) | 11 (46%) | |
| Native Hawaiian/other Pacific Islander | 6 (23%) | 7 (29%) | |
| White | 1 (4%) | 1 (4%) | |
| Current height (cm) | 101.1±1.4 | 99.4±1.1 | 0.33 |
| Current weight (kg) | 17.3±0.6 | 16.7±0.6 | 0.44 |
| Current BMI | 16.9±0.3 | 16.8±0.4 | 0.81 |
| Current head circumference (cm) | 51.0±0.3 | 51.7±1.1 | 0.57 |
| Gestational age at birth (weeks) | 39.3±0.3 | 39.5±0.3 | 0.69 |
| Activity, Pulse, Grimace, Appearance, and Respiration (APGAR) score at 5 min | 9.0±0 | 8.6±0.3 | 0.20 |
| Birth weight (kg) | 3.2±0.1 | 3.5±0.1 | 0.07 |
| Birth length (cm) | 49.2±1.7 | 51.1±0.6 | 0.30 |
| Birth BMI | 12.4±0.3 | 13.4±0.2 | 0.02 |
| Characteristics of the mothers | |||
| Age at time of child’s birth (years) | 27.8±2.4 | 26.5±1.1 | 0.65 |
| Maternal weight gain (kg) | 24.6±2.5 | 17.3±1.6 | 0.02 |
| Cigarettes smoked during pregnancy | 1,865 (560, 5,460)a 2,973±654 |
||
| # Trimesters with nicotine exposure | 2.6±0.1 | ||
| # Trimesters with alcohol exposure | 0.5±0.2 | 0.2±0.1 | 0.14 |
| # Trimesters with marijuana exposure | 0.4±0.2 | ||
| Caffeine during pregnancy (mg) | 6,825 (0, 49,140)a | 1,300 (0, 11,700) | 0.17 |
| Characteristics of the primary caregiver (parent or legal guardian) | |||
| Education (years) | 12.5±0.4 | 13.5±0.4 | 0.12 |
| Estimate verbal intelligence quotient | 95.4±1.2 | 97.7±1.1 | 0.15 |
| Beck depression inventory | 10.5±2.3 | 8.9±1.8 | 0.57 |
| Socioeconomic status (index of social position) | 57.8±3.2 | 49.1±3.3 | 0.03 |
Median with first and third quartile in parentheses
Table 2.
Cognitive function of the research participants (mean±SEM)
| Nicotine exposed (n=26) | Unexposed (n=24) | p value | |
|---|---|---|---|
| Stanford–Binet Non-verbal (Fluid Reasoning) | 9.8±0.7 | 9.0±0.6 | 0.39 |
| Stanford–Binet Verbal (Vocabulary) | 7.8±0.4 | 8.4±0.5 | 0.38 |
| Stanford–Binet Verbal ABIQ | 93.1±2.4 | 92.9±2.4 | 0.96 |
| Expressive one word picture vocabulary test | 81.2±3.4 | 82.0±3.4 | 0.86 |
| Beery visual motor integration | 99.4±3.2 | 104.2±3.8 | 0.34 |
| Peabody picture vocabulary test—III | 89.2±3.3 | 84.0±3.1 | 0.26 |
| NEPSY attention | 9.7±0.6 | 11.2±0.5 | 0.06 |
MRI and metabolite abnormalities in PNE children
All brain MRI scans were reviewed by a neurologist (L.C.) to ensure that there were no structural abnormalities. Across the four brain regions, PNE children showed lower tCr (p=0.003) and MI (p=0.03) than unexposed children (using repeated measures ANOVA). After Bonferroni correction, tCr remained significant at the 0.01 level (0.05/5 metabolites). However, decreased tCr concentrations in PNE children were significant only in the basal ganglia and thalamus due to an interaction between region and PNE status (p=0.03 for interaction).
In post hoc analysis, PNE children had lower tCr (−8.8%, p=0.003) and lower MI (−11.7%, p=0.02) in the basal ganglia compared to controls (Fig. 1, Table 3). Children with PNE also had lower tCr (−7.9%, p=0.03) in the thalamus. In contrast, in the ACC, GLX was higher (+9.4%, p=0.03) in the PNE compared to unexposed children. Excluding the one subject with the light but highest alcohol exposure, the findings remained the same (basal ganglia tCr, p=0.004; basal ganglia MI, p=0.03; frontal gray matter GLX, p=0.03; thalamus tCr, p=0.03). Results were similar after covarying for the gray–white matter proportion in each voxel or for gender, birth BMI, socioeconomic status of the primary caregiver, maternal weight gain, and caffeine intake (one covariate at a time in all models); the p value for basal ganglia tCr ranged from 0.002 to 0.007. Alternatively, when all the covariates were included in the model and variables were removed starting with the least significant ones, only the PNE effect remained significant for basal ganglia tCr.
Table 3.
Brain metabolite concentrations in four regions
| Nicotine exposed | Unexposed | p value | |
|---|---|---|---|
| Basal ganglia | |||
| tCr | 7.17±0.18 | 7.85±0.12 | 0.003 |
| NA | 7.57±0.17 | 7.92±0.13 | 0.10 |
| CHO | 1.78±0.06 | 1.91±0.05 | 0.09 |
| MI | 3.05±0.13 | 3.45±0.11 | 0.02 |
| GLX | 11.43±0.33 | 12.05±0.36 | 0.21 |
| Frontal white matter | |||
| tCr | 5.56±0.10 | 5.66±0.08 | 0.48 |
| NA | 7.58±0.15 | 7.54±0.10 | 0.83 |
| CHO | 2.08±0.06 | 2.11±0.05 | 0.71 |
| MI | 3.92±0.16 | 4.11±0.11 | 0.33 |
| GLX | 8.70±0.40 | 8.30±0.28 | 0.42 |
| Frontal gray matter | |||
| tCr | 6.15±0.13 | 6.17±0.11 | 0.92 |
| NA | 7.63±0.09 | 7.49±0.18 | 0.48 |
| CHO | 1.49±0.04 | 1.38±0.05 | 0.07 |
| MI | 4.80±0.13 | 5.08±0.14 | 0.16 |
| GLX | 11.74±0.29 | 10.74±0.33 | 0.03 |
| Thalamus | |||
| tCr | 6.50±0.20 | 7.05±0.14 | 0.03 |
| NA | 8.43±0.25 | 8.99±0.20 | 0.09 |
| CHO | 2.05±0.06 | 2.12±0.05 | 0.40 |
| MI | 4.47±0.13 | 4.68±0.09 | 0.22 |
| GLX | 11.05±0.29 | 11.14±0.23 | 0.81 |
Values in bold indicate statistical significance
CHO levels in the ACC showed age-dependent decline in the control children (r=−0.52, p=0.02), but not in PNE children (age×PNE status interaction, p=0.02; Fig. 2). The ACC MI showed a similar trend for lower levels in the older controls (r=−0.42, p=0.06) but not in PNE children (age×PNE interaction, p=0.09). The GLX level in the thalamus showed a positive relationship with age in the controls (r=0.34, p=0.12) but a negative relationship in the PNE children (r=−0.29, p=0.17; age×PNE status interaction, p=0.05). However, both groups showed a negative relationship with age and CHO in the FWM (r=−0.33, p=0.03). No group difference in N-acetylaspartate (NAA) was observed, with only trends for lower levels of NAA in the thalamus and the basal ganglia.
Fig. 2.
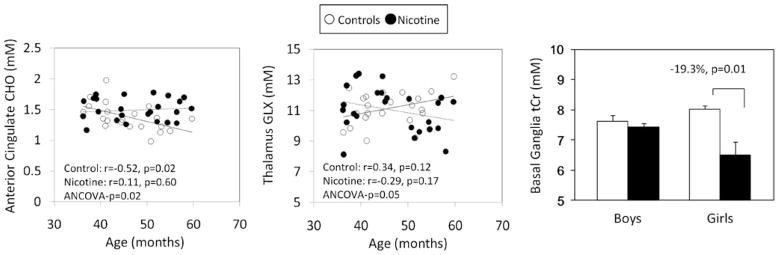
Graphs show dependence of the metabolite concentrations on age and sex. Left: older PNE children show higher, but older non-PNE children show lower, ACC–choline concentrations with age (age×PNE interaction, p=0.02). Middle: older PNE children show lower thalamic GLX levels, while the opposite is true in non-PNE children (age×PNE interaction, p=0.05). Right: graph shows lower tCr in the basal ganglia of the girls, but not boys with PNE (boys—n=18; girls—n=7) compared to those unexposed to nicotine (boys—n=10; girls—n=12), resulting in a sex by PNE interaction (p=0.003)
Sex differences in PNE effects were observed in basal ganglia tCr (Sex×PNE status interaction, p=0.0028). In fact, the lower tCr concentration observed in the PNE children was due primarily to the lower levels in the female PNE children compared to female controls (−19.3%, p=0.01; Fig. 2).
Correlations between metabolite abnormalities, cognitive tests, and amount of cigarettes used
On neuropsychological tests, PNE children with higher GLX levels in the ACC scored worse on the EOWPVT-R (r=−0.67, p<0.001) and VMI (r=−0.53, p=0.01). In contrast, the unexposed group did not show correlations between these measures (interaction p values<0.05, Fig. 3).
Fig. 3.
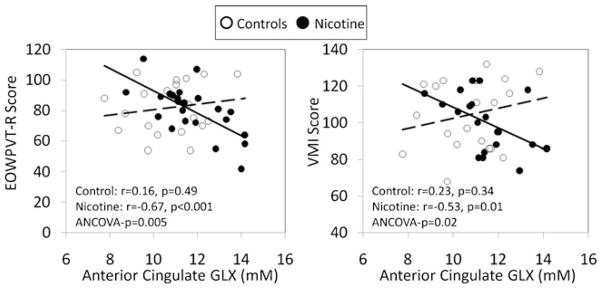
Negative relationships of ACC-GLX levels with vocabulary (EOWPVT-R score, left scatter plot) and with visual motor integration (right scatter plot) are observed in the PNE group, but not in the non-exposed children. The ANCOVA p value reflects the significance of the interaction between PNE status and ACC GLX on outcome measures
There were no correlations between metabolite levels and gestational nicotine exposure measures (total number of cigarettes smoked during pregnancy, the average number of cigarettes smoked per day during the pregnancy, or the duration since last in utero exposure to nicotine). Furthermore, we found no correlations between the metabolite concentrations and the amount of cigarettes smoked by mothers after the children were born (second-hand smoke).
Discussion
A group of young children with prenatal nicotine exposure had lower tCr and lower MI levels than unexposed children, especially in the basal ganglia, which suggests abnormalities in glial development. The lower tCr concentration in the basal ganglia and in the thalamus also indicates abnormalities in energy metabolism in these regions. The lower basal ganglia tCr was significant in girls but not boys, suggesting that girls may be more vulnerable to the effects of PNE. These metabolite abnormalities are found despite relatively normal cognitive performance in the PNE children, except for a tendency for poorer attention. Therefore, these findings suggest subclinical neurometabolite abnormalities in this group of young children with PNE.
Metabolic alterations in children with PNE
Since MI measured with MRS is a putative glial marker (Brand et al. 1993), lower MI suggests reduced glial content or altered glial development. In humans, MI levels in newborn infants are typically high and rapidly decline with age toward levels similar to those in adults by 2–3 years of age (Kreis et al. 1993b). Therefore, lower MI levels in young children with PNE, especially in the basal ganglia, may reflect an accelerated but altered glial development. Likewise, lower tCr might in part be due to lower glial content since the tCr concentration is approximately 3-fold higher in glia than in neurons (Brand et al. 1993). Such altered glial development might be due to nicotine’s effects on astrocytes and oligodendrocyte precursor cells, which have functional nicotinic receptors (Hosli et al. 2001; Rogers et al. 2001). In vitro studies of isolated neonatal rat optic nerve treated with nicotine indeed showed irreversible disruption of signal conduction in glia but not in neurons (Constantinou and Fern 2009).
The notion of altered glial development in the PNE children is supported further by a trend for higher CHO and the lack of age-related decline in CHO in the ACC of the PNE children compared to unexposed controls. The CHO peak on MRS reflects metabolites involved in cell membrane metabolism, and is two to three times higher in glia, such as oligodendrocytes, than in neurons (Urenjak et al. 1993). The higher CHO in the ACC may also be due to greater cellularity and possible delayed cellular reorganization, as seen in preclinical studies of rodents and non-human primates treated with nicotine (Roy et al. 2002; Slotkin et al. 2006a). These findings with CHO levels suggest a delay in cellular reorganization in the PNE group, and are consistent with findings in adult tobacco smokers who began smoking in adolescence and showed a positive correlation between their pack years of smoking and ACC CHO levels (Gallinat et al. 2007). However, unlike the adult smokers, we did not observe a correlation between CHO concentrations and the dose of nicotine exposure (i.e., from the amount of gestational nicotine exposed).
Furthermore, glutamate+glutamine (GLX) in the ACC was altered in children with PNE, with higher levels being associated with poorer performance on vocabulary and visual motor integration. Like higher CHO levels, elevated GLX may reflect increased cellularity and metabolism in this brain region. However, nicotine can also directly influence the glutamatergic system (Broide and Leslie 1999), particularly disrupting NMDA receptors in the auditory cortex of rodents (Aramakis and Metherate 1998; Aramakis et al. 2000; Hsieh et al. 2002). Furthermore, human studies in periadolescents with PNE found microstructural (DTI) abnormalities in the genu of the corpus callosum, near the ACC, and in the auditory pathway (Jacobsen et al. 2007a), as well as deficits in auditory attention (Fried et al. 1997, 2003; Jacobsen et al. 2007c). Therefore, the association between higher GLX in the ACC and poorer vocabulary in our children with PNE might be related to poorer auditory processing. Future studies evaluating the relationship between GLX in the ACC and auditory pathways or function are needed.
Another study that used MRS to evaluate GLX in 16 alcohol-dependent men during a monitored abstinence period, at approximately 1 week and 1 month of medication-free sobriety, also found higher GLX, but normal NAA levels, in smokers than non-smokers (Mason et al. 2006). However, the investigators did not provide data for these individuals’ history of PNE. They also did not find group difference on brain CHO between smokers and non-smokers. Contrary to this study and to our study of young children with PNE, another preliminary study of nine women who participated in a smoking cessation program found that those who “slipped” and resumed smoking had lower brain glutamate/tCr level in the ACC, but also normal NAA level, compared to those who remained abstinent (Mashhoon et al. 2011). Given the small sample size of these studies and the difference in the developing brain of young children compared to the matured adult brain, the effects of nicotine on neurometabolites are not comparable between these populations. Nevertheless, both the adult smoker studies and ours demonstrate that the glutamatergic system may be affected by nicotine. Finally, since the ACC is a critical node in the neural network for attention and executive function, ACC abnormalities might lead to future deficits in these cognitive domains. We indeed observe poorer attentional abilities in these young children, but the effect was not significant, perhaps due to the relatively small sample size. Deficits in visual and auditory attention as well as in verbal and working memory are also well documented in adolescent tobacco smokers (Jacobsen et al. 2005); those with additional PNE had even greater cognitive deficits and abnormalities on functional MRI than smokers without PNE (Jacobsen et al. 2007c).
Sex differences
Lower tCr in the basal ganglia was found in girls, but not boys, with PNE. Likewise, in utero exposure to methamphetamine affected brain metabolism in girls more than boys; however, girls with prenatal methamphetamine exposure showed elevation of the frontal white matter neuronal marker NA and tCr (Chang et al. 2009). The greater vulnerability of girls than boys to the effects of PNE is also consistent with a prior fMRI study that found reduction in both visual and auditory attention in female adolescent smokers with PNE, but only deficits in auditory attention in the males (Jacobsen et al. 2007c). Furthermore, PNE resulted in lower hemicholinium-3 (HC3) binding to the choline transporter in striatum of female rats (Slotkin et al. 2007) and lower 5-HT receptor binding in female, but not in male rats (Slotkin et al. 2006b). Understanding these sex differences in the effects of PNE is important since PNE might predispose individuals to nicotine use, particularly young women (Kandel et al. 1994).
The relatively young age (3–4 years) of our children allowed us to reduce potential environmental effects on brain development after birth or longer periods of exposure to second-hand smoking which would occur in older children, and also to minimize variance in brain measures due to age. However, the relatively narrow age range also makes it more difficult to determine age-associated changes. Furthermore, despite our efforts to match the socioeconomic status of the two subject groups, the PNE group had poorer index of social position, which might have impacted these children’s brain development.
Overall, similar to children with prenatal methamphetamine exposure (Chang et al. 2009), the current group of children with PNE had higher GLX in the ACC, which suggests altered neuronal metabolism in this region. However, unlike the children with prenatal methamphetamine exposure (Chang et al. 2009), our children with PNE did not show higher levels of neuronal marker NAA, but had trends for lower levels in the thalamus and the basal ganglia. NAA was found to be lower in the frontal white matter of adult smokers who were also recovering alcoholics (Wang et al. 2009) but not in the other studies of adult men or women smokers (Mason et al. 2006; Mashhoon et al. 2011). Therefore, our findings are consistent with studies that found no evidence of neuronal loss (cell numbers in adult rats) in hippocampus or cerebellum after exposed to nicotine prenatally or both prenatally and postnatally (Chen et al. 2006). Although we did not observe the most severe form of brain injury, with neuronal loss, the absence of age-related decline in CHO in the ACC may indicate a delay in cellular reorganization in the PNE group. Furthermore, lower MI and tCr in the PNE children suggest abnormalities in glial development and energy metabolism. These abnormalities could reflect a cellular response to brain insult, such as the exposure to nicotine smoking, which may be subclinical since these children did not have significant abnormalities on cognitive performance, but only trends for poorer attention. However, the subclinical effect in this group of young children may not apply to all children with PNE. Girls with PNE had greater alteration in brain metabolites than boys, similar to findings in methamphetamine-exposed children; however, the detailed patterns of metabolite alterations differ between PNE and prenatal methamphetamine exposure.
Future studies should compare brain metabolite levels in young children with those in neonates with PNE since neonates have even less or no second-hand smoke exposure. Given the difficulty of imaging young children or infants without sedation, the development of motion-compensated imaging techniques is needed to allow studies of brain development in even younger children or in neonates without second-hand smoking. Longitudinal follow-up evaluation of our young children is ongoing and will allow determination of whether the abnormalities found on the initial examination will persist or normalize with continued development, and if the metabolite alterations affect cognitive or behavioral measures when these children are older. Likewise, long-term longitudinal follow-up would make it possible to determine whether other sex differences on brain development might emerge since others have postulated that the inhibition of aromatase by nicotine, which disrupts estrogen synthesis by the placenta, may interfere with sexual differentiation in the male brains and may change the timing of puberty in adolescent boys with PNE (Fried et al. 2001). Correlation of our MRS findings with other brain imaging measures, such as morphometry or DTI, will provide further assessments of how brain development is altered by PNE.
Acknowledgments
We would like to thank Drs. Helenna Nakama, Mary Ricardo-Dukelow, Daniel Alicata, and Michael Watters for some of the subject evaluations; Brooke Hedemark, Christopher Dunlap, and Lynn Anderson for coordinating participant visits and data entry; and Riley Kitamura and Eric Cunningham for technical support and MRS processing. We are grateful to our research subjects who participated in this study.
Footnotes
Disclosure/conflict of interest Portions of these data were presented at the 2009 College on Problems of Drug Dependence, Reno NV, USA. Study supported by NIH (1R01 DA21016; 2 K24-DA16170; K01-DA021203; K02-DA16991; 1U54-NS56883; 1P20-RR11091); not industry sponsored/supported. All authors report no competing interests. Dr. Chang was funded by NIH grant # R01-MH061427-08, R01-DA021016-05, R24-DA027318-02, 2 K24-DA016170-07, U01-DA013045-11S1, U54-NS056883-04, and RC2-DA029475-02. Dr. Cloak received an ACNP travel award supported by Eli Lilly, was funded by NIH grant # K01-DA021203, and received research support from the Queen Emma Research Foundation and NIH grant # 2U54-NS039406. Ms. Jiang was funded by NIH grant # 5U54-NS056883 and 5R21-DA02444. Mr. Hoo was funded by NIH grant # 1R01-DA21016. Ms. Hernandez was funded by NIH grant # 1U54-NS56883. Dr. Ernst was funded by NIH grant # U54-NS056883-04, RC2-DA029475-02, R01-DA021146-04, G12-RR003061-25, R21-CA139712-02, and R01-NS36524.
Contributor Information
Linda Chang, Email: lchang@hawaii.edu, Division of Neurology, Department of Medicine, John A. Burns School of Medicine, University of Hawai’i at Manoa, 7th floor University Tower, 1356 Lusitana Street, Honolulu, HI 96813, USA. The Queen’s Medical Center, Honolulu, HI, USA.
Christine C. Cloak, Division of Neurology, Department of Medicine, John A. Burns School of Medicine, University of Hawai’i at Manoa, 7th floor University Tower, 1356 Lusitana Street, Honolulu, HI 96813, USA
Caroline S. Jiang, Division of Neurology, Department of Medicine, John A. Burns School of Medicine, University of Hawai’i at Manoa, 7th floor University Tower, 1356 Lusitana Street, Honolulu, HI 96813, USA
Aaron Hoo, Division of Neurology, Department of Medicine, John A. Burns School of Medicine, University of Hawai’i at Manoa, 7th floor University Tower, 1356 Lusitana Street, Honolulu, HI 96813, USA.
Antonette B. Hernandez, Division of Neurology, Department of Medicine, John A. Burns School of Medicine, University of Hawai’i at Manoa, 7th floor University Tower, 1356 Lusitana Street, Honolulu, HI 96813, USA
Thomas M. Ernst, Division of Neurology, Department of Medicine, John A. Burns School of Medicine, University of Hawai’i at Manoa, 7th floor University Tower, 1356 Lusitana Street, Honolulu, HI 96813, USA
References
- Aramakis VB, Metherate R. Nicotine selectively enhances NMDA receptor-mediated synaptic transmission during postnatal development in sensory neocortex. J Neurosci. 1998;18(20):8485–8495. doi: 10.1523/JNEUROSCI.18-20-08485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramakis VB, Hsieh CY, Leslie FM, Metherate R. A critical period for nicotine-induced disruption of synaptic development in rat auditory cortex. J Neurosci. 2000;20(16):6106–6116. doi: 10.1523/JNEUROSCI.20-16-06106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15:289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- Broide RS, Leslie FM. The alpha7 nicotinic acetylcholine receptor in neuronal plasticity. Mol Neurobiol. 1999;20(1):1–16. doi: 10.1007/BF02741361. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Jiang CS, Farnham S, Tokeshi B, Buchthal S, Hedemark B, Smith LM, Ernst T. Altered neurometabolites and motor integration in children exposed to methamphetamine in utero. Neuroimage. 2009;48(2):391–397. doi: 10.1016/j.neuroimage.2009.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WJ, King KA, Lee RE, Sedtal CS, Smith AM. Effects of nicotine exposure during prenatal or perinatal period on cell numbers in adult rat hippocampus and cerebellum: a stereology study. Life Sci. 2006;79(23):2221–2227. doi: 10.1016/j.lfs.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Constantinou S, Fern R. Conduction block and glial injury induced in developing central white matter by glycine, GABA, noradrenalin, or nicotine, studied in isolated neonatal rat optic nerve. Glia. 2009;57(11):1168–1177. doi: 10.1002/glia.20839. [DOI] [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry. 2001;40(6):630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Ernst T, Kreis R, Ross BD. Absolute quantitation of water and metabolites in the human brain. I: compartments and water. J Magn Reson B. 1993;102:1–8. [Google Scholar]
- Fried PA, Watkinson B, Siegel LS. Reading and language in 9- to 12-year olds prenatally exposed to cigarettes and marijuana. Neurotoxicol Teratol. 1997;19(3):171–183. doi: 10.1016/s0892-0362(97)00015-9. [DOI] [PubMed] [Google Scholar]
- Fried PA, James DS, Watkinson B. Growth and pubertal milestones during adolescence in offspring prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2001;23(5):431–436. doi: 10.1016/s0892-0362(01)00161-1. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2003;25(4):427–436. doi: 10.1016/s0892-0362(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Lang UE, Jacobsen LK, Bajbouj M, Kalus P, von Haebler D, Seifert F, Schubert F. Abnormal hippocampal neurochemistry in smokers: evidence from proton magnetic resonance spectroscopy at 3 T. J Clin Psychopharmacol. 2007;27(1):80–84. doi: 10.1097/JCP.0b013e31802dffde. [DOI] [PubMed] [Google Scholar]
- George L, Granath F, Johansson AL, Anneren G, Cnattingius S. Environmental tobacco smoke and risk of spontaneous abortion. Epidemiology. 2006;17(5):500–505. doi: 10.1097/01.ede.0000229984.53726.33. [DOI] [PubMed] [Google Scholar]
- Hosli E, Jurasin K, Ruhl W, Luthy R, Hosli L. Colocalization of androgen, estrogen and cholinergic receptors on cultured astrocytes of rat central nervous system. Int J Dev Neurosci. 2001;19(1):11–19. doi: 10.1016/s0736-5748(00)00082-4. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Leslie FM, Metherate R. Nicotine exposure during a postnatal critical period alters NR2A and NR2B mRNA expression in rat auditory forebrain. Brain Res Dev Brain Res. 2002;133(1):19–25. doi: 10.1016/s0165-3806(01)00314-5. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57 (1):56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Picciotto MR, Heath CJ, Frost SJ, Tsou KA, Dwan RA, Jackowski MP, Constable RT, Mencl WE. Prenatal and adolescent exposure to tobacco smoke modulates the development of white matter microstructure. J Neurosci. 2007a;27(49):13491–13498. doi: 10.1523/JNEUROSCI.2402-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biol Psychiatry. 2007b;61(1):31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Slotkin TA, Mencl WE, Frost SJ, Pugh KR. Gender-specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology. 2007c;32(12):2453–2464. doi: 10.1038/sj.npp.1301398. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Wu P, Davies M. Maternal smoking during pregnancy and smoking by adolescent daughters. Am J Public Health. 1994;84(9):1407–1413. doi: 10.2105/ajph.84.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Griesler PC, Schaffran C. Educational attainment and smoking among women: risk factors and consequences for offspring. Drug Alcohol Depend. 2009;104(Suppl 1):S24–S33. doi: 10.1016/j.drugalcdep.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis R, Ernst T, Ross BD. Absolute quantitation of water and metabolites in the human brain. II: metabolite concentrations. J Magn Reson B. 1993a;102:9–19. [Google Scholar]
- Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993b;30:424–437. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- Mashhoon Y, Janes AC, Jensen JE, Prescot AP, Pachas G, Renshaw PF, Fava M, Evins AE, Kaufman MJ. Anterior cingulate proton spectroscopy glutamate levels differ as a function of smoking cessation outcome. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(7):1709–1713. doi: 10.1016/j.pnpbp.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason GF, Petrakis IL, de Graaf RA, Gueorguieva R, Guidone E, Coric V, Epperson CN, Rothman DL, Krystal JH. Cortical gamma-aminobutyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biol Psychiatry. 2006;59(1):85–93. doi: 10.1016/j.biopsych.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Obel C, Henriksen TB, Hedegaard M, Secher NJ, Ostergaard J. Smoking during pregnancy and babbling abilities of the 8-month-old infant. Paediatr Perinat Epidemiol. 1998;12(1):37–48. [PubMed] [Google Scholar]
- Provencher S. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Rogers SW, Gregori NZ, Carlson N, Gahring LC, Noble M. Neuronal nicotinic acetylcholine receptor expression by O2A/oligodendrocyte progenitor cells. Glia. 2001;33(4):306–313. [PubMed] [Google Scholar]
- Roy TS, Seidler FJ, Slotkin TA. Prenatal nicotine exposure evokes alterations of cell structure in hippocampus and somatosensory cortex. J Pharmacol Exp Ther. 2002;300(1):124–133. doi: 10.1124/jpet.300.1.124. [DOI] [PubMed] [Google Scholar]
- Shea AK, Steiner M. Cigarette smoking during pregnancy. Nicotine Tob Res. 2008;10(2):267–278. doi: 10.1080/14622200701825908. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol Teratol. 2008;30(1):1–19. doi: 10.1016/j.ntt.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Pinkerton KE, Seidler FJ. Perinatal environmental tobacco smoke exposure in rhesus monkeys: critical periods and regional selectivity for effects on brain cell development and lipid peroxidation. Environ Health Perspect. 2006a;114(1):34–39. doi: 10.1289/ehp.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Prenatal nicotine exposure alters the responses to subsequent nicotine administration and withdrawal in adolescence: serotonin receptors and cell signaling. Neuropsychopharmacology. 2006b;31(11):2462–2475. doi: 10.1038/sj.npp.1300988. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Ryde IT, Seidler FJ. Separate or sequential exposure to nicotine prenatally and in adulthood: persistent effects on acetylcholine systems in rat brain regions. Brain Res Bull. 2007;74 (1–3):91–103. doi: 10.1016/j.brainresbull.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T. Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology. 2001;57(2):255–260. doi: 10.1212/wnl.57.2.255. [DOI] [PubMed] [Google Scholar]
- Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci. 1993;13(3):981–989. doi: 10.1523/JNEUROSCI.13-03-00981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JJ, Durazzo TC, Gazdzinski S, Yeh PH, Mon A, Meyerhoff DJ. MRSI and DTI: a multimodal approach for improved detection of white matter abnormalities in alcohol and nicotine dependence. NMR Biomed. 2009;22(5):516–522. doi: 10.1002/nbm.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]