Abstract
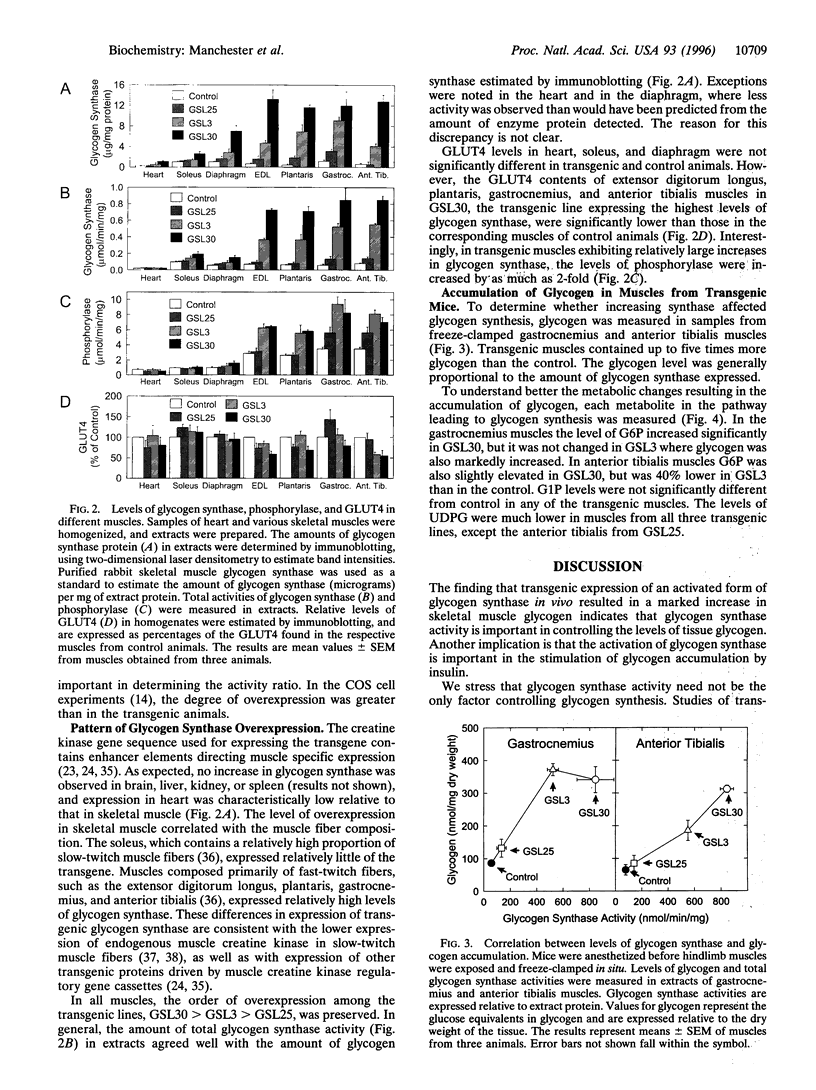
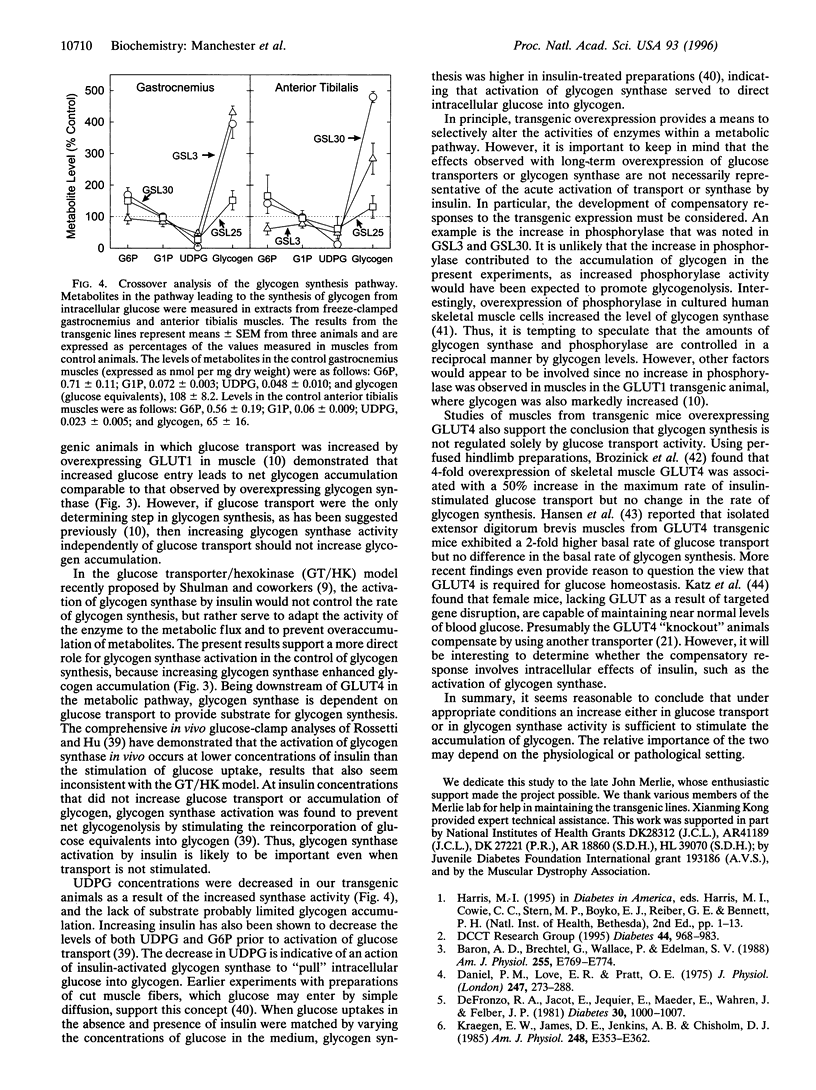
To investigate the role of glycogen synthase in controlling glycogen accumulation, we generated three lines of transgenic mice in which the enzyme was overexpressed in skeletal muscle by using promoter-enhancer elements derived from the mouse muscle creatine kinase gene. In all three lines, expression was highest in muscles composed primarily of fast-twitch fibers, such as the gastrocnemius and anterior tibialis. In these muscles, glycogen synthase activity was increased by as much as 10-fold, with concomitant increases (up to 5-fold) in the glycogen content. The uridine diphosphoglucose concentrations were markedly decreased, consistent with the increase in glycogen synthase activity. Levels of glycogen phosphorylase in these muscles increased (up to 3-fold), whereas the amount of the insulin-sensitive glucose transporter 4 either remained unchanged or decreased. The observation that increasing glycogen synthase enhances glycogen accumulation supports the conclusion that the activation of glycogen synthase, as well as glucose transport, contributes to the accumulation of glycogen in response to insulin in skeletal muscle.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Azpiazu I., Saltiel A. R., DePaoli-Roach A. A., Lawrence J. C. Regulation of both glycogen synthase and PHAS-I by insulin in rat skeletal muscle involves mitogen-activated protein kinase-independent and rapamycin-sensitive pathways. J Biol Chem. 1996 Mar 1;271(9):5033–5039. doi: 10.1074/jbc.271.9.5033. [DOI] [PubMed] [Google Scholar]
- BROWN D. H., PARK C. R., DAUGHADAY W. H., CORNBLATH M. The influence of preliminary soaking on glucose utilization by diaphragm. J Biol Chem. 1952 May;197(1):167–174. [PubMed] [Google Scholar]
- Baqué S., Guinovart J. J., Gómez-Foix A. M. Overexpression of muscle glycogen phosphorylase in cultured human muscle fibers causes increased glucose consumption and nonoxidative disposal. J Biol Chem. 1996 Feb 2;271(5):2594–2598. doi: 10.1074/jbc.271.5.2594. [DOI] [PubMed] [Google Scholar]
- Baron A. D., Brechtel G., Wallace P., Edelman S. V. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol. 1988 Dec;255(6 Pt 1):E769–E774. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brozinick J. T., Jr, Yaspelkis B. B., 3rd, Wilson C. M., Grant K. E., Gibbs E. M., Cushman S. W., Ivy J. L. Glucose transport and GLUT4 protein distribution in skeletal muscle of GLUT4 transgenic mice. Biochem J. 1996 Jan 1;313(Pt 1):133–140. doi: 10.1042/bj3130133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANFORTH W. H. GLYCOGEN SYNTHETASE ACTIVITY IN SKELETAL MUSCLE. INTERCONVERSION OF TWO FORMS AND CONTROL OF GLYCOGEN SYNTHESIS. J Biol Chem. 1965 Feb;240:588–593. [PubMed] [Google Scholar]
- Daniel P. M., Love E. R., Pratt O. E. Insulin-stimulated entry of glucose into muscle in vivo as a major factor in the regulation of blood glucose. J Physiol. 1975 May;247(2):273–288. doi: 10.1113/jphysiol.1975.sp010931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981 Dec;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- DePaoli-Roach A. A., Ahmad Z., Camici M., Lawrence J. C., Jr, Roach P. J. Multiple phosphorylation of rabbit skeletal muscle glycogen synthase. Evidence for interactions among phosphorylation sites and the resolution of electrophoretically distinct forms of the subunit. J Biol Chem. 1983 Sep 10;258(17):10702–10709. [PubMed] [Google Scholar]
- Donoviel D. B., Shield M. A., Buskin J. N., Haugen H. S., Clegg C. H., Hauschka S. D. Analysis of muscle creatine kinase gene regulatory elements in skeletal and cardiac muscles of transgenic mice. Mol Cell Biol. 1996 Apr;16(4):1649–1658. doi: 10.1128/mcb.16.4.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboe D. P., Larson K. L., Nuttall F. Q. Radioactive method for the assay of glycogen phosphorylases. Anal Biochem. 1972 May;47(1):20–27. doi: 10.1016/0003-2697(72)90274-6. [DOI] [PubMed] [Google Scholar]
- Hansen P. A., Gulve E. A., Marshall B. A., Gao J., Pessin J. E., Holloszy J. O., Mueckler M. Skeletal muscle glucose transport and metabolism are enhanced in transgenic mice overexpressing the Glut4 glucose transporter. J Biol Chem. 1995 Jan 27;270(4):1679–1684. doi: 10.1074/jbc.270.5.1679. [DOI] [PubMed] [Google Scholar]
- Hintz C. S., Lowry C. V., Kaiser K. K., McKee D., Lowry O. H. Enzyme levels in individual rat muscle fibers. Am J Physiol. 1980 Sep;239(3):C58–C65. doi: 10.1152/ajpcell.1980.239.3.C58. [DOI] [PubMed] [Google Scholar]
- Jaynes J. B., Johnson J. E., Buskin J. N., Gartside C. L., Hauschka S. D. The muscle creatine kinase gene is regulated by multiple upstream elements, including a muscle-specific enhancer. Mol Cell Biol. 1988 Jan;8(1):62–70. doi: 10.1128/mcb.8.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. E., Wold B. J., Hauschka S. D. Muscle creatine kinase sequence elements regulating skeletal and cardiac muscle expression in transgenic mice. Mol Cell Biol. 1989 Aug;9(8):3393–3399. doi: 10.1128/mcb.9.8.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jue T., Rothman D. L., Tavitian B. A., Shulman R. G. Natural-abundance 13C NMR study of glycogen repletion in human liver and muscle. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1439–1442. doi: 10.1073/pnas.86.5.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E. B., Stenbit A. E., Hatton K., DePinho R., Charron M. J. Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature. 1995 Sep 14;377(6545):151–155. doi: 10.1038/377151a0. [DOI] [PubMed] [Google Scholar]
- Klip A., Marette A. Acute and chronic signals controlling glucose transport in skeletal muscle. J Cell Biochem. 1992 Jan;48(1):51–60. doi: 10.1002/jcb.240480109. [DOI] [PubMed] [Google Scholar]
- Kraegen E. W., James D. E., Jenkins A. B., Chisholm D. J. Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. Am J Physiol. 1985 Mar;248(3 Pt 1):E353–E362. doi: 10.1152/ajpendo.1985.248.3.E353. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence J. C., Jr Signal transduction and protein phosphorylation in the regulation of cellular metabolism by insulin. Annu Rev Physiol. 1992;54:177–193. doi: 10.1146/annurev.ph.54.030192.001141. [DOI] [PubMed] [Google Scholar]
- Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994 Feb 1;219(3):713–725. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Holman G. Homeostasis without a GLUT. Nature. 1995 Sep 14;377(6545):100–101. doi: 10.1038/377100a0. [DOI] [PubMed] [Google Scholar]
- NORMAN D., MENOZZI P., REID D., LESTER G., HECHTER O. Action of insulin on sugar permeability in rat diaphragm muscle. J Gen Physiol. 1959 Jul 20;42(6):1277–1299. doi: 10.1085/jgp.42.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J. M., Marshall B. A., Gulve E. A., Gao J., Johnson D. W., Holloszy J. O., Mueckler M. Evidence from transgenic mice that glucose transport is rate-limiting for glycogen deposition and glycolysis in skeletal muscle. J Biol Chem. 1993 Aug 5;268(22):16113–16115. [PubMed] [Google Scholar]
- Roach P. J. Control of glycogen synthase by hierarchal protein phosphorylation. FASEB J. 1990 Sep;4(12):2961–2968. [PubMed] [Google Scholar]
- Robinson R., Robinson L. J., James D. E., Lawrence J. C., Jr Glucose transport in L6 myoblasts overexpressing GLUT1 and GLUT4. J Biol Chem. 1993 Oct 15;268(29):22119–22126. [PubMed] [Google Scholar]
- Rodnick K. J., Piper R. C., Slot J. W., James D. E. Interaction of insulin and exercise on glucose transport in muscle. Diabetes Care. 1992 Nov;15(11):1679–1689. doi: 10.2337/diacare.15.11.1679. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Hu M. Skeletal muscle glycogenolysis is more sensitive to insulin than is glucose transport/phosphorylation. Relation to the insulin-mediated inhibition of hepatic glucose production. J Clin Invest. 1993 Dec;92(6):2963–2974. doi: 10.1172/JCI116919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield M. A., Haugen H. S., Clegg C. H., Hauschka S. D. E-box sites and a proximal regulatory region of the muscle creatine kinase gene differentially regulate expression in diverse skeletal muscles and cardiac muscle of transgenic mice. Mol Cell Biol. 1996 Sep;16(9):5058–5068. doi: 10.1128/mcb.16.9.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman R. G., Bloch G., Rothman D. L. In vivo regulation of muscle glycogen synthase and the control of glycogen synthesis. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8535–8542. doi: 10.1073/pnas.92.19.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurat A. V., Peng H. L., Chang H. Y., Cannon J. F., Roach P. J. Rate-determining steps in the biosynthesis of glycogen in COS cells. Arch Biochem Biophys. 1996 Apr 15;328(2):283–288. doi: 10.1006/abbi.1996.0174. [DOI] [PubMed] [Google Scholar]
- Skurat A. V., Roach P. J. Phosphorylation of sites 3a and 3b (Ser640 and Ser644) in the control of rabbit muscle glycogen synthase. J Biol Chem. 1995 May 26;270(21):12491–12497. doi: 10.1074/jbc.270.21.12491. [DOI] [PubMed] [Google Scholar]
- Skurat A. V., Wang Y., Roach P. J. Rabbit skeletal muscle glycogen synthase expressed in COS cells. Identification of regulatory phosphorylation sites. J Biol Chem. 1994 Oct 14;269(41):25534–25542. [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Wang Y., Roach P. J. Inactivation of rabbit muscle glycogen synthase by glycogen synthase kinase-3. Dominant role of the phosphorylation of Ser-640 (site-3a). J Biol Chem. 1993 Nov 15;268(32):23876–23880. [PubMed] [Google Scholar]
- Yamashita K., Yoshioka T. Profiles of creatine kinase isoenzyme compositions in single muscle fibres of different types. J Muscle Res Cell Motil. 1991 Feb;12(1):37–44. doi: 10.1007/BF01781172. [DOI] [PubMed] [Google Scholar]




