Abstract
In vitro culture of Plasmodium falciparum gametocytes is essential for studying sexual development of the parasite. Here we describe a simple method for producing synchronous gametocyte culture without contamination of asexual stages. This method employs heparin’s activity in blocking merozoite invasion of erythrocytes to eliminate asexual stage parasites from gametocyte culture. We show that following induction of gametocyte formation, addition of heparin in culture medium for four days effectively eliminates asexual stages and produces pure, synchronous cultures of gametocytes. Compared with the commonly used N-acetylglucosamine treatment method, heparin treatment requires shorter time to eliminate asexual stages and causes significantly less hemolysis in late stage gametocyte cultures.
Keywords: Plasmodium falciparum, gametocyte, in vitro culture, heparin, synchronization
1. Introduction
The life cycle of the human malaria parasites involves two hosts, Anopheles mosquito vectors and humans. The parasite sexual stages in the human blood, or gametocytes, are essential for continued transmission of the parasites to the mosquito vectors (Carter and Graves, 1988, Sinden, 1983). Because sexual development is obligatory for the life cycle, it is the target of transmission blocking vaccines and drugs. Although systems for in vitro culture of Plasmodium falciparum gametocytes have been established a long time ago (Ponnudurai et al., 1986, Ponnudurai et al., 1982), studies on gametocyte stages have been relatively slow, which is largely due to difficulties in procuring sufficient amounts of materials from in vitro cultures. During asexual multiplication of P. falciparum in erythrocytes, only a small proportion of the parasites initiate sexual development to produce gametocytes, and it takes approximately 10 days for gametocytes to reach maturity. Therefore, two important goals for in vitro culture of P. falciparum gametocytes are to increase the gametocytogenesis rates and to achieve higher levels of synchrony in gametocyte development.
Although the exact mechanism of gametocytogenesis remains poorly understood, it is often thought as a stress response of the parasite to deteriorating conditions in the infected blood. The observation that spent culture medium stimulates sexual development (Carter and Miller, 1979, Dyer and Day, 2003, Williams, 1999) has led to the inclusion of spent medium as one of the conditions to induce gametocytogenesis (Fivelman et al., 2007). A recent, improved gametocyte culture method using conditioned medium is able to induce high-level gametocytogenesis with relatively good synchrony (Fivelman et al., 2007). Asexual erythrocytic stages are eliminated in order to obtain pure gametocyte culture. Depletion of asexual stages also improved the synchronization of gametocytes (Fivelman et al., 2007, Saul et al., 1990) and elevated gametocytemia (Roncales et al., 2012). Petmitr et al. used 5% D-sorbitol treatment for two consecutive days, which could reduce ~97% asexual stages (Petmitr et al., 1995, Petmitr et al., 1997). Yet, this method is not able to eliminate all asexual stages (Saul et al., 1990), probably because this sugar could only selectively lyse the trophozoite stage. To date, the most commonly used method to remove asexual stages is to supplement the gametocyte culture medium with 50 mM N-acetylglucosamine (GlcNAc) (Fivelman et al., 2007, Ponnudurai et al., 1986). For complete removal of asexual stages, GlcNAc is added from the time of gametocyte induction and continued for at least five days (Fivelman et al., 2007). The function of GlcNAc in eliminating asexual stages may involve both toxicity to asexual stage parasites and blocking activity of erythrocyte invasion. At <20 mM, asexual parasite development is normal, and the growth inhibitory effect of GlcNAc is mainly due to blocking merozoite invasion (Gupta et al., 1985, Jungery et al., 1983). It has been found that BSA-coupled GlcNAc is >100,000 times more effective than GlcNAc alone in blocking parasite invasion (Jungery et al., 1983). At 50 mM, GlcNAc is toxic for trophozoites and schizonts without affecting gametocyte development (Gupta et al., 1985, Ponnudurai et al., 1986). To obtain pure early stages of gametocytes, treatment with 5% sorbitol is recommended. However, timing of the treatment is critical, since asexual ring stage parasites are not affected by sorbitol, but early gametocyte stages can be vulnerable to sorbitol treatment (Fivelman et al., 2007).
Interactions between malaria parasite-derived proteins and host cell surface proteoglycans such as the blood group A antigen, heparin sulfate (HS)-like glycosaminoglycans, and chondroitin sulfate are involved in parasite invasion (for both sporozoites and merozoites) and responsible for rosetting and adhesion of infected red blood cells (RBCs). A number of glycosaminoglycans and sulfated glycoconjugates have been reported to interfere with rosetting and adhesion and are potential therapies for severe malaria (Rowe et al., 2009). Heparin and other sulfated polysaccharides such as dextran sulfate, curdlan sulfate, and fucoidan are found to inhibit merozoites invasion of RBCs (Clark et al., 1997, Dalton et al., 1991, Evans et al., 1998, Xiao et al., 1996). Heparin and HS are structurally very similar and composed of alternating glucosamine and uronic acid residues in a repeating disaccharide unit (-4GlcAβ1-4GlcNAcα1-) with varying degrees of N- and O-sulfation, but heparin is more extensively and uniformly sulfated. It has been established recently that merozoite surface protein 1 (MSP1) and erythrocyte binding antigen-140 (also known as BAEBL) are the parasite invasion ligands that bind to HS-like molecules on the RBC surface (Boyle et al., 2010, Kobayashi et al., 2010). The invasion blocking activity of the heparin-like molecules requires two or more sulfate groups per disaccharide unit (Boyle et al., 2010). Heparin has a higher degree of sulfation, and thus, is very effective in blocking merozoite invasion regardless of the invasion pathways used by the parasites (Boyle et al., 2010). It has been found that heparin interacts with C-terminal 42 kDa processed fragment of merozoite surface protein 1 (msp1), a molecule that is essential for merozoites invasion (Boyle et al., 2010). Here, we sought to employ the activity of heparin in blocking merozoites invasion of erythrocyte to eliminate asexual-stage parasites from gametocyte culture. We show that adding heparin in the culture medium for four days allows us to obtain pure cultures of synchronous gametocytes even at early developmental stages.
2. Materials and methods
2.1. Chemicals
GlcNAc and heparin sodium salt from porcine intestinal mucosa were purchased from Sigma (St. Louis, MO). Stock solutions of GlcNAc and heparin were made in water at 500 mM and 20 units (U) /μl (approximate 100 μg /ml), respectively. The heparin unit is a measure of the anticoagulant property of a given heparin product as it acts on antithrombin III. One unit is defined as the amount of heparin required to keep 1 ml of cat’s blood fluid for 24 h at 0°C (Ferro et al., 1990). Prior to gametocyte culture, complete medium was prepared to include either heparin 20 U/ml or GlcNAc at a final concentration of 20 U/ml and 50 mM, respectively.
2.2. Culture of P. falciparum
P. falciparum strains 3D7, NF54, Dd2, HB3, D10, 7G8 and 3D7-luc (a 3D7 transgenic clone with constitutive expression of the firefly luciferase (Cui et al., 2008)) were cultured in type O+ human RBCs in a complete medium supplemented with 10% AB human serum under an atmosphere of 90% N2/5% O2/3% CO2 (Trager and Jensen, 1976). Synchronization of the asexual stage culture was done by treatment of the ring stage culture with 5% D-sorbitol (Lambros and Vanderberg, 1979). Gametocyte induction was performed following an established protocol (Fivelman et al., 2007) with some modifications. Briefly, synchronous parasites at the trophozoite stage were set up at a parasitemia between 2–3% in T75 flasks with 3 ml of fresh 50% RBCs in 50 ml complete medium at day -3. On the next day (day -2), 20–30 ml of spent medium was replaced by fresh complete medium to the parasite culture (~10% parasitemia at ring stage). Stressed schizont cultures (day -1) including spent medium were transferred into T225 flasks with fresh RBCs and complete medium to 3% parasitemia and 5% hematocrit. Fresh complete medium were changed daily and Giemsa-stained smears were examined to monitor development for 12 days.
2.3. Inhibition of RBC invasion by heparin
To confirm the inhibitory effect of heparin on the invasion of RBCs by P. falciparum merozoites (Boyle et al., 2010), synchronized parasite culture of late ring stage at 1% parasitemia was incubated in a complete medium with heparin at serially diluted concentrations. Parasite growth at 48 h later in the next cycle was quantified, which should reflect the efficiency of parasite invasion. For the 3D7-luc parasite, parasite lysates were prepared and luciferase activities were measured as described (Cui et al., 2008). For other strains (3D7, NF54, HB3, D10, 7G8 and Dd2), parasitemia was determined by the SYBR Green I method (Smilkstein et al., 2004). Each treatment had three biological replications. The 50% maximal inhibitory concentration (IC50) was calculated using the GraphPad Prism software (La Jolla, CA).
2.4. Depletion of asexual stage parasites in gametocyte culture by heparin
Gametocytogenesis was induced by the aforementioned method (Fivelman et al., 2007). The culture was split into two aliquots on day 0. In one aliquot, the culture medium was replaced with the heparin (20 U/ml) medium from day 1 for 4 days, whereas in the other aliquot the culture medium was replaced with the GlcNAc (50 mM) medium from day 1 for 5 days. Parasitemia (asexual and sexual stages) was enumerated daily by counting about 10,000 cells on thin smears of culture until day 12. Three biological replicates were performed. To determine whether heparin treatment could synchronize sexual development, 10 ml of culture from both treatments on day 4 were layered on a Percoll gradient (35% over 75% Percoll) to enrich gametocytes. The percentage of stage II gametocytes was counted from ~1,000 parasites to determine the degree of synchrony of the gametocyte culture.
2.5. Determination of hemolysis in gametocyte culture
Aliquots of 100 μl culture medium from gametocyte culture treated with either GlcNAc or heparin were collected from day 7 to 12. The concentration of hemoglobin in the culture medium was analyzed using the Drabkin’s reagent with a hemoglobin standard (Sigma) as the reference (Drabkin, 1946). The experiment was done with three biological replicates.
2.6. Statistical analysis
We used two-way ANOVAs to test for statistical differences between treatment groups (heparin and GluNAC) and days for gametocytemia, parasitemia, and hemoglobin levels. We used Bartlett’s tests to look for significant differences in variances as well as Shapiro-Wilk normality tests to test for normality of the data. The parasitemia and hemoglobin data were non-normal. We then used a Box-Cox transformation and found that log-transformations made the data normal.
3. Results
3.1. Heparin inhibits erythrocyte invasion of parasites at low concentrations
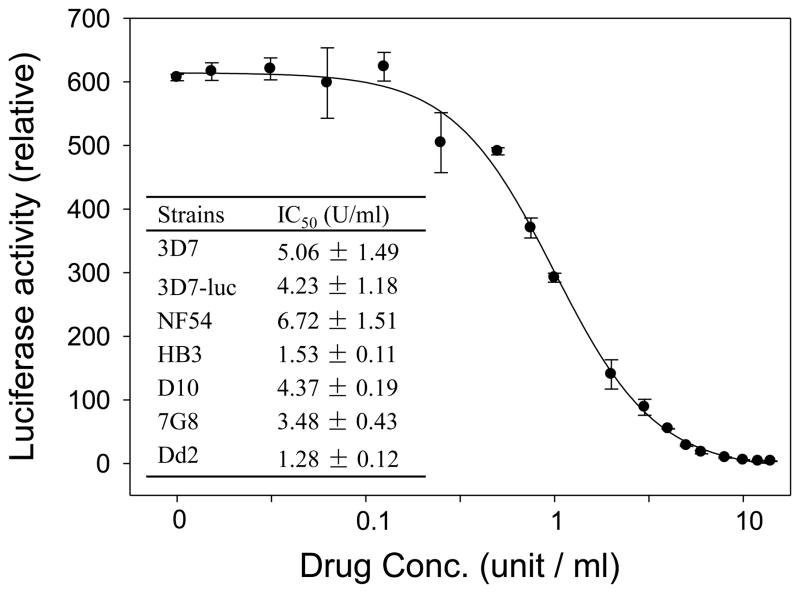
Heparin has been found to effectively inhibit merozoite invasion of several genetically different parasite lines (3D7, W2mef, D10, E8B, XIE, HCS3) (Boyle et al., 2010). To confirm this finding, we performed in vitro invasion inhibition using the SYBR Green I assay on additional parasite strains (NF54, HB3, 7G8 and Dd2). The results show that the IC50 values of heparin in the six strains tested were all within a narrow range of 1–7 U/ml (Figure 1). The IC50 value for 3D7 was similar to that estimated in the earlier study (3.8 U/ml) (Boyle et al., 2010). The invasion inhibition assay was also performed in a transgenic P. falciparum line expressing luciferase (3D7-luc) using a more sensitive bioluminescence assay (Cui et al., 2008). The IC50 value of heparin in 3D7-luc from the luciferase assay (1.09 ± 0.07 U/ml) was much lower than that from the SYBR Green I assay (Fig. 1). Taken together, heparin inhibits parasite invasion of RBCs at low concentrations. Accordingly, we used the 20 U/ml heparin concentration to eliminate asexual stages in gametocyte cultures. It is noteworthy that 30 U/ml of heparin showed no adverse effects on parasite growth (Boyle et al., 2010).
Figure 1.
Heparin inhibits P. falciparum parasite invasion of RBCs. A response curve of 3D7-luc parasite line to the inhibitory activity of heparin measured using the luciferase assay. Inset: IC50 values of heparin (mean ± standard deviation) for different parasite strains measured using the SYBR Green I assay.
3.2. Heparin clears asexual-stage parasite more rapidly than GlcNAc
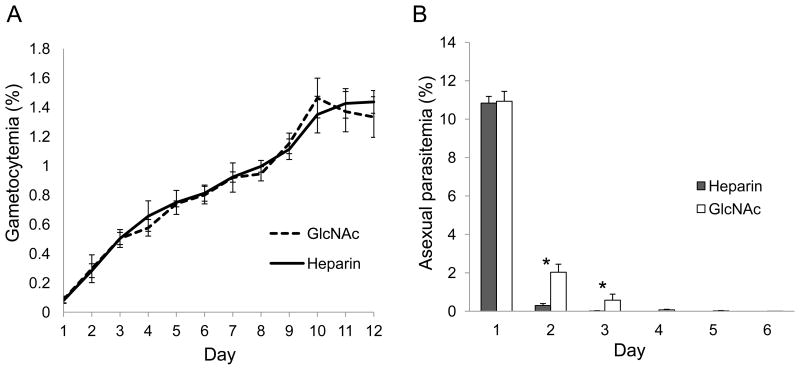
We compared the effect of heparin and GlcNAc on gametocyte culture for the elimination of asexual stages. After induction of gametocytogenesis, 3D7 gametocyte cultures were incubated with 20 U/ml of heparin for 4 days or 50 mM GlcNAc for 5 days. Asexual stage parasitemia and gametocytemia were determined daily. The daily gametocytemias between heparin and GlcNAc treated cultures were not significantly different (P = 0.533) (Fig. 2A), suggesting that both compounds have no obvious negative impact on gametocyte development. Daily asexual parasitemia indicated that heparin treatment cleared asexual stages more efficiently than GlcNAc (Fig. 2B). Heparin treatment for three days completely eliminated asexual stage parasites from the gametocyte culture. In addition, stage II gametocytes on day 4 accounted for 87.3% total parasites from heparin treated culture versus 84.7% from GlcNAc treated culture, suggesting that heparin treatment could achieve similar levels of synchrony of gametocyte development (Fig. 3).
Figure 2.
Parasitemias in culture aliquots upon gametocyte induction treated with 20 U/ml heparin for four days or 50 mM GlcNAc for five days. A. Daily gametocytemias; B. Daily asexual stage parasitemias. Single and double Asterisks in B indicates there were significant differences between GlcNAc and heparin treated culture (* P <0.01, ** P<0.001, ANOVA).
Figure 3.
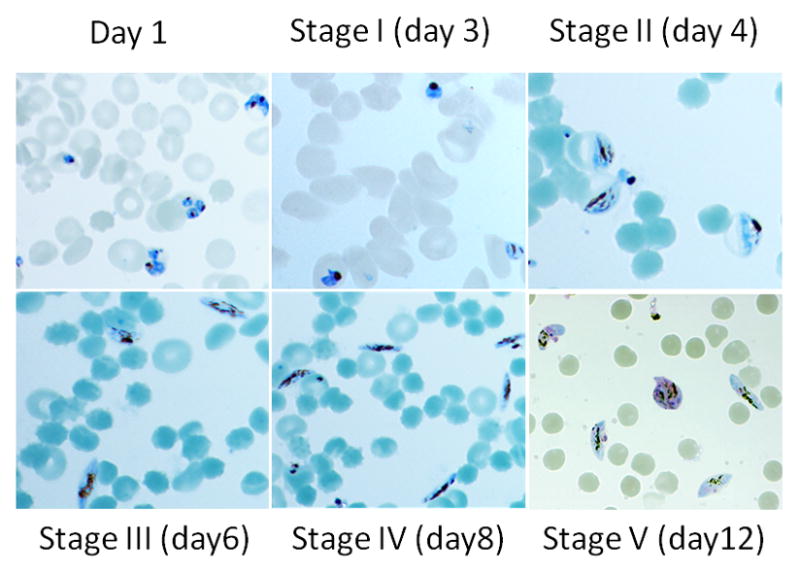
Images of Giemsa-stained gametocytes from heparin-treated gametocyte cultures to show the degrees of synchrony of the culture and morphology of gametocytes.
3.3. GlcNAc causes higher levels of hemolysis
Certain degrees of hemolysis are observed during the lengthy culture of P. falciparum gametocytes and it appears to be more severe in GlcNAc treated culture. To quantify this event, we measured the concentrations of hemoglubin in the medium of gematocyte culture from day 7 to day 12. The results showed that the hemoglobin concentrations in culture medium were significantly higher in GlcNAc-treated cultures than heparin-treated cultures (P <0.0001) on day 9, 10, 11, and 12, suggesting that GlcNAc increased hemolysis (Fig. 4).
Figure 4.
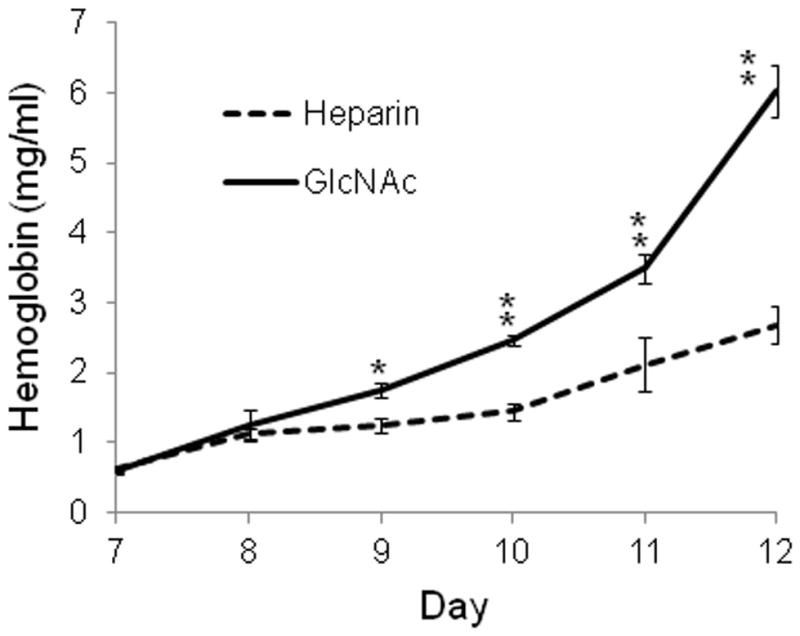
Hemoglobin concentrations in the medium of heparin- or GlcNAc- treated gametocyte cultures. Asterisks indicate significant differences in daily hemoglobin concentration between the two treatment methods (* P < 0.01, ANOVA).
4. Discussion
Heparin is a commonly used anticoagulant, and a potential therapy for severe malaria because of its anti-adhesion activity (Rowe et al., 2009). Heparin has recently been found to interact with the MSP1 molecule during the P. falciparum invasion of RBCs (Boyle et al., 2010). Here we employed the invasion-blocking activity of heparin for clearing the asexual stage parasites to obtain pure synchronous gametocyte culture of P. falciparum. We found that treatment with 20 U/ml of heparin for four days after induction of gametocytogenesis could completely eliminate asexual stage parasites without obvious negative effects on the developing gametocytes.
The commonly used method for removing asexual stages during gametocyte culture is GlcNAc treatment. The recommended treatment regimen is 50 mM GlcNAc for five consecutive days after gametocyte induction. However, with this method it is difficult to obtain highly pure parasites at early gametocyte stages. The higher efficiency of heparin in eliminating asexual-stage parasite than GlcNAc provides a better way to procure pure early stage gametocytes. Whereas both treatments do not apparently affect gametocyte development, GlcNAc is associated with higher levels of hemolysis at later stage gametocyte culture. Based on this finding and the comparable gametocytemias between the two treatments, the actual number of gametocytes in a GlcNAc-treated culture should be lower than that in a heparin-treated culture. This also suggests that higher ratios of gametocytes might have been lysed at the late gametocyte stages in GlcNAc-treated cultures.
In conclusion, low concentrations of heparin (20 U/ml) added during the early stages of P. falciparum gametocyte culture for four days can effectively clear asexual stage parasite, which allows one to obtain pure, highly synchronized gametocytes, even at relatively early stages of gametocyte development.
Highlights.
Heparin can effectively block invasion of erythrocytes by P. falciparum merozoites
Heparin is used to eliminate asexual stages and obtain pure gametocyte culture
Heparin causes less hemolysis in late stage gametocyte cultures
Acknowledgments
This work was supported by grants (U19 AI089672 and R01 AI064553) from the National Institute of Allergy and Infectious Diseases, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boyle MJ, Richards JS, Gilson PR, Chai W, Beeson JG. Interactions with heparin-like molecules during erythrocyte invasion by Plasmodium falciparum merozoites. Blood. 2010;115:4559–4568. doi: 10.1182/blood-2009-09-243725. [DOI] [PubMed] [Google Scholar]
- 2.Boyle MJ, Wilson DW, Richards JS, Riglar DT, Tetteh KK, Conway DJ, Ralph SA, Baum J, Beeson JG. Isolation of viable Plasmodium falciparum merozoites to define erythrocyte invasion events and advance vaccine and drug development. Proc Natl Acad Sci U S A. 2010;107:14378–14383. doi: 10.1073/pnas.1009198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter R, Graves PM. Gametocytes. In: Wernsdorfer WH, Sir McGregor I, editors. Malaria: Principles and Practice of Malariology. Churchill Livingstone; London: 1988. pp. 253–305. [Google Scholar]
- 4.Carter R, Miller LH. Evidence for environmental modulation of gametocytogenesis in Plasmodium falciparum in continuous culture. Bull World Health Organ. 1979;57(Suppl 1):37–52. [PMC free article] [PubMed] [Google Scholar]
- 5.Clark DL, Su S, Davidson EA. Saccharide anions as inhibitors of the malaria parasite. Glycoconj J. 1997;14:473–479. doi: 10.1023/a:1018551518610. [DOI] [PubMed] [Google Scholar]
- 6.Cui L, Miao J, Wang J, Li Q, Cui L. Plasmodium falciparum: development of a transgenic line for screening antimalarials using firefly luciferase as the reporter. Exp Parasitol. 2008;120:80–87. doi: 10.1016/j.exppara.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalton JP, Hudson D, Adams JH, Miller LH. Blocking of the receptor-mediated invasion of erythrocytes by Plasmodium knowlesi malaria with sulfated polysaccharides and glycosaminoglycans. Eur J Biochem. 1991;195:789–794. doi: 10.1111/j.1432-1033.1991.tb15767.x. [DOI] [PubMed] [Google Scholar]
- 8.Drabkin DL. Spectrophotometric studies; the crystallographic and optical properties of the hemoglobin of man in comparison with those of other species. J Biol Chem. 1946;164:703–723. [PubMed] [Google Scholar]
- 9.Dyer M, Day KP. Regulation of the rate of asexual growth and commitment to sexual development by diffusible factors from in vitro cultures of Plasmodium falciparum. Am J Trop Med Hyg. 2003;68:403–409. [PubMed] [Google Scholar]
- 10.Evans SG, Morrison D, Kaneko Y, Havlik I. The effect of curdlan sulphate on development in vitro of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1998;92:87–89. doi: 10.1016/s0035-9203(98)90969-5. [DOI] [PubMed] [Google Scholar]
- 11.Ferro DR, Provasoli A, Ragazzi M, Casu B, Torri G, Bossennec V, Perly B, Sinay P, Petitou M, Choay J. Conformer populations of L-iduronic acid residues in glycosaminoglycan sequences. Carbohydr Res. 1990;195:157–167. doi: 10.1016/0008-6215(90)84164-p. [DOI] [PubMed] [Google Scholar]
- 12.Fivelman QL, McRobert L, Sharp S, Taylor CJ, Saeed M, Swales CA, Sutherland CJ, Baker DA. Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Mol Biochem Parasitol. 2007;154:119–123. doi: 10.1016/j.molbiopara.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Gupta SK, Schulman S, Vanderberg JP. Stage-dependent toxicity of N-acetyl-glucosamine to Plasmodium falciparum. J Protozool. 1985;32:91–95. doi: 10.1111/j.1550-7408.1985.tb03020.x. [DOI] [PubMed] [Google Scholar]
- 14.Jungery M, Pasvol G, Newbold CI, Weatherall DJ. A lectin-like receptor is involved in invasion of erythrocytes by Plasmodium falciparum. Proc Natl Acad Sci U S A. 1983;80:1018–1022. doi: 10.1073/pnas.80.4.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi K, Kato K, Sugi T, Takemae H, Pandey K, Gong H, Tohya Y, Akashi H. Plasmodium falciparum BAEBL binds to heparan sulfate proteoglycans on the human erythrocyte surface. J Biol Chem. 2010;285:1716–1725. doi: 10.1074/jbc.M109.021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 17.Petmitr P, Pongvilairat G, Wilairat P. Development of cultivation technique for pure Plasmodium falciparum gametocytes. Southeast Asian J Trop Med Public Health. 1995;26:606–610. [PubMed] [Google Scholar]
- 18.Petmitr P, Pongvilairat G, Wilairat P. Large scale culture technique for pure Plasmodium falciparum gametocytes. Southeast Asian J Trop Med Public Health. 1997;28:18–21. [PubMed] [Google Scholar]
- 19.Ponnudurai T, Lensen AH, Meis JF, Meuwissen JH. Synchronization of Plasmodium falciparum gametocytes using an automated suspension culture system. Parasitology. 1986;93 ( Pt 2):263–274. doi: 10.1017/s003118200005143x. [DOI] [PubMed] [Google Scholar]
- 20.Ponnudurai T, Meuwissen JH, Leeuwenberg AD, Verhave JP, Lensen AH. The production of mature gametocytes of Plasmodium falciparum in continuous cultures of different isolates infective to mosquitoes. Trans R Soc Trop Med Hyg. 1982;76:242–250. doi: 10.1016/0035-9203(82)90289-9. [DOI] [PubMed] [Google Scholar]
- 21.Roncales M, Vidal-Mas J, Leroy D, Herreros E. Comparison and Optimization of Different Methods for the In Vitro Production of Plasmodium falciparum Gametocytes. J Parasitol Res. 2012;2012:927148. doi: 10.1155/2012/927148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowe JA, Claessens A, Corrigan RA, Arman M. Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: molecular mechanisms and therapeutic implications. Expert Rev Mol Med. 2009;11:e16. doi: 10.1017/S1462399409001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saul A, Graves P, Edser L. Refractoriness of erythrocytes infected with Plasmodium falciparum gametocytes to lysis by sorbitol. Int J Parasitol. 1990;20:1095–1097. doi: 10.1016/0020-7519(90)90056-s. [DOI] [PubMed] [Google Scholar]
- 24.Sinden RE. Sexual development of malarial parasites. Adv Parasitol. 1983;22:153–216. doi: 10.1016/s0065-308x(08)60462-5. [DOI] [PubMed] [Google Scholar]
- 25.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother. 2004;48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 27.Williams JL. Stimulation of Plasmodium falciparum gametocytogenesis by conditioned medium from parasite cultures. Am J Trop Med Hyg. 1999;60:7–13. doi: 10.4269/ajtmh.1999.60.7. [DOI] [PubMed] [Google Scholar]
- 28.Xiao L, Yang C, Patterson PS, Udhayakumar V, Lal AA. Sulfated polyanions inhibit invasion of erythrocytes by plasmodial merozoites and cytoadherence of endothelial cells to parasitized erythrocytes. Infect Immun. 1996;64:1373–1378. doi: 10.1128/iai.64.4.1373-1378.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]