Abstract
Objective
Chylomicron and very low-density lipoprotein remnants are cleared from the circulation in the liver by heparan sulfate proteoglycan (HSPG) receptors (syndecan-1), the low-density lipoprotein receptor (LDLR), and LDLR-related protein-1 (LRP1), but the relative contribution of each class of receptors under different dietary conditions remains unclear.
Approach and Results
Triglyceride-rich lipoprotein clearance was measured in AlbCre+Ndst1f/f, Ldlr−/−, and AlbCre+Lrp1f/f mice and mice containing combinations of these mutations. Triglyceride measurements in single and double mutant mice showed that HSPGs and LDLR dominate clearance under fasting conditions and postprandial conditions, but LRP1 contributes significantly when LDLR is absent. Mice lacking hepatic expression of all three receptors (AlbCre+Ndst1f/f Lrp1f/f Ldlr−/−) displayed dramatic hyperlipidemia (870 ± 270 mg triglyceride/dL; 1300 ± 350 mg of total cholesterol/dL) and exhibited persistent elevated postprandial triglyceride levels due to reduced hepatic clearance. Analysis of the particles accumulating in mutants showed that HSPGs preferentially clear a subset of small triglyceride-rich lipoproteins (~20-40 nm diameter), while LDLR and LRP1 clear larger particles (~40-60 nm diameter). Finally, we show that HSPGs play a major role in clearance of TRLs in mice fed normal chow or under postprandial conditions but appear to play a less significant role on a high fat diet.
Conclusion
These data show that HSPGs, LDLR, and LRP1 clear distinct subsets of particles, that HSPGs work independently from LDLR and LRP1, and that HSPGs, LDLR, and LRP1 are the three major hepatic TRL clearance receptors in mice.
Keywords: Triglycerides, cholesterol, heparan sulfate, Syndecan-1, LDLR, LRP1, lipoproteins
Introduction
Hypertriglyceridemia is characterized by the accumulation of triglyceride-rich lipoproteins (TRLs) in the blood. This condition affects 10–20% of the population in Western countries and increases the risk of atherosclerosis, coronary artery disease, and pancreatitis1-4. TRLs consist of intestinal chylomicrons derived from dietary fats and very low density lipoproteins (VLDL) from the liver, as well as remnant particles resulting from the lipolytic processing of these lipoproteins in the peripheral circulation. Clearance of TRL remnants occurs in the liver via a multi-step process5-7. The particles first pass through the fenestrated endothelium of the liver sinusoids and become sequestered in the perisinusoidal space of Disse, where they undergo further processing by lipoprotein lipase and hepatic lipase6. Particles are then cleared by endocytic receptors on the surface of hepatocytes, leading to their lysosomal catabolism. Several receptors for remnant lipoproteins have been identified, including the low-density lipoprotein receptor (LDLR) 8, members of the LDLR-related protein family (LRP1 and LRP5) 9,10, Syndecan-111, a type of heparan sulfate proteoglycan (HSPG), the very low density lipoprotein receptor (VLDLR)12, scavenger receptor B1 (SR-B1)13, and lipolysis stimulated receptor (LSR)14. Understanding the relative contribution of these receptors to clearance, their coordinate regulation, and their role in maintaining plasma lipids under different dietary conditions is important because the information could help focus future drug development efforts to reduce hypertriglyceridemia.
In the present study, we sought to understand the relative contributions of HSPGs, LDLR, and LRP1 to clearance of remnant lipoproteins under various dietary conditions in mice. To this end, we interbred mice deficient in Ldlr, Lrp1 and the gene N-acetylglucosamine N-deacetylase-N-sulfotransdferase-1 (Ndst1), which encodes an enzyme that affects the synthesis of the heparan sulfate chains on syndecan-1 and other hepatic proteoglycans, producing double mutants lacking various combinations of the three receptors, and the triple mutant lacking all three receptors. HSPGs and LDLR are the two dominant receptors mediating TRL clearance under fasting conditions and in the postprandial state, but LRP1 can substitute for LDLR in LDLR-deficient mice. No coordinate regulation of the three receptors was noted under these conditions demonstrating their independence. HSPGs appear to bind and internalize a distinct subset of particles compared to LDLR and LRP1. Interestingly, when fed a high-fat Western diet, mice rely more on LDLR and LRP1 for remnant TRL clearance.
Results
Inactivation of Ldlr, Lrp1 and Ndst1
We previously described mice in which the Cre transgene under the control of the albumin promoter was used to inactivate the heparan sulfate biosynthetic enzyme N-acetylglucosamine N-deacetylase N-sulfotransferase-1 (Ndst1) selectively in hepatocytes15. Ndst1 affects the sulfation of all HSPGs including syndecan-1 (Sdc1), the primary hepatic proteoglycan receptor11. Like Sdc1−/− mice, AlbCre+Ndst1f/f mice display a two-fold accumulation of fasting triglycerides and have delayed clearance of postprandial triglycerides due to loss of sulfation of the heparan sulfate chains located on syndecan-1 specifically in the liver11. Syndecan-1 knockout mice were not used in this study because a conditional mutant has not been described. Liver-specific AlbCre+Ndst1f/f mice are designated by genotype or as HSPG receptor-deficient throughout the rest of this study.
HSPG receptor-deficient mice were bred with Ldlr systemic knockout mice (Ldlr−/−) or mice bearing a floxed allele of Lrp1 (Lrp1f/f) to produce all combinations of double and triple receptor-deficient mice. Compound mutant mice were viable, fertile and produced litters of normal size and with the expected Mendelian ratios of genotypes. To differentiate the contribution of the LDLR family of receptors and HSPGs we chose to focus on four genotypes: Cre−Ndst1f/f (wildtype), Cre+Ndst1f/f (HSPG receptor-deficient), Cre+Lrp1f/fLdlr−/− (HSPG receptor-only), and Cre+Ndst1f/fLrp1f/fLdlr−/− (triple receptor-deficient).
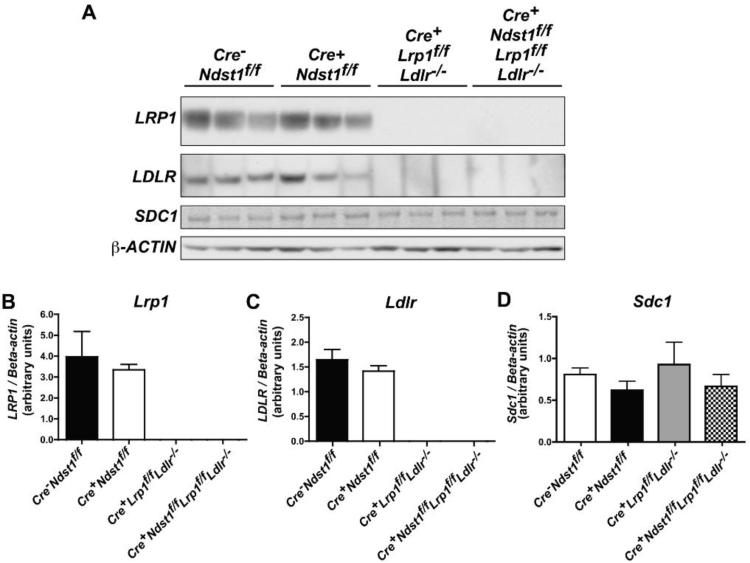
To verify that the Cre transgene was sufficient to mediate recombination of two separate floxed genes (Ndst1f/f and Lrp1f/f), we analyzed several markers. First, we purified heparan sulfate from isolated hepatocytes and determined its disaccharide composition using mass spectrometry16. Glucosamine N-sulfation was decreased from 43 N-sulfate groups/100 disaccharides in wildtype hepatocytes (sum of D0S0, D0S6, D2S0, and D2S6 in Table 1) to 19 sulfates/100 disaccharides in triple receptor-deficient hepatocytes. The reduction in sulfation was similar to that observed previously and in hepatocytes from Cre+Ndst1f/f mice (Table 1 and 15). Next, we showed by Western blotting that LRP1 expression was ablated in isolated hepatocytes from Cre+Lrp1f/fLdlr−/− (HSPG receptor-only) and Cre+Ndst1f/fLrp1f/fLdlr−/− mice (Fig. 1a). LRP1 and LDLR expression exhibited some variability in wildtype and HSPG receptor-deficient mice. However, when normalized to β-actin their levels of expression were not significantly different in any of the strains, indicating that altering HSPG receptor activity by inactivation of Ndst1 did not cause any compensatory changes in these receptors (Figs. 1b and 1c). Similarly, hepatic Syndecan-1 expression was not affected by inactivation of Ldlr, Lrp1 or Ndst1 and any combination of mutations in these genes (Fig. 1d). Thus, the expression of Syndecan-1 and LDLR-family members does not appear to occur in a coordinated manner in hepatocytes under these conditions.
Table 1.
Disaccharide analysis of hepatocyte heparan sulfate
| Strain | Disaccharides (mole %) | |||||||
|---|---|---|---|---|---|---|---|---|
| D0A0 | D0S0 | D0A6 | D0S6 | D2A0 | D2S0 | D2A6 | D2S6 | |
|
Cre
−
Ndst1f/f
(Wildtype) |
40.1 | 16.5 | 15.9 | 9.6 | 0.8 | 6.3 | 0.1 | 10.7 |
|
Cre+Ndst1f/f
(HSPG receptor-deficient) |
63.3 | 3.9 | 17.8 | 11.6 | 0.1 | 1.5 | 0.0 | 1.9 |
|
Cre+Lrp1f/fLdlr−/−
(HSPG receptor-only) |
43.8 | 18.7 | 14.7 | 7.9 | 0.7 | 6.5 | 0.0 | 7.7 |
|
Cre+Ndst1f/fLrp1f/fLdlr−/−
(Triple receptor-deficient) |
65.1 | 3.2 | 16.1 | 13.9 | 0.1 | 0.6 | 0.1 | 1.1 |
Heparan sulfate purified from isolated hepatocytes was digested with heparin lyases I, II, and III. The resulting disaccharides were derivatized with isotopically labeled aniline and quantified by mass spectrometry (see methods). Disaccharides are designated using the code established in 40. Those containing the letter S contain a N-sulfate group.
Figure 1.
Inactivation of Ldlr and Lrp1 in compound mutant mice. (A) Fresh hepatocytes were isolated from 12 week-old male mice and solubilized in RIPA buffer. Samples were separated by gradient SDS-PAGE and transferred to PVDF membranes. The membrane was probed with antibodies against Ldlr, Lrp1, Sdc1 andβ-actin. Each lane represents a different mouse (n = 3 per genotype). (B-D) Densitometry analysis of the Western blots presented in A. Lrp1 and Ldlr protein expression is not detected in the relevant mutants, whereas syndecan-1 is detected at comparable levels in all of the strains.
Hyperlipidemia in compound mutant mice
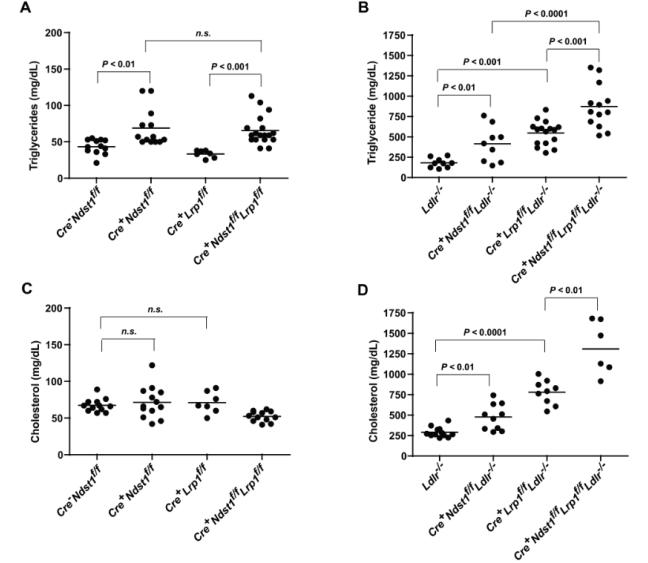
To study the relative contribution of the three receptors to clearance of TRLs, we first analyzed plasma triglyceride and cholesterol in blood drawn from overnight-fasted animals raised on a normal chow diet (Fig. 2, and Table 2). HSPG receptor-deficient mice (Cre+Ndst1f/f) accumulated triglycerides compared to wildtype animals (Fig. 2a, 69 ± 26 mg/dL vs. 43 ± 10 mg/dL respectively, P < 0.01), as shown previously15. In contrast, mice lacking hepatic LRP1 (Cre+Lrp1f/f) did not accumulate plasma triglycerides (Fig. 2a). Double mutant mice deficient for both the HSPG receptors and LRP1 (Cre+Ndst1f/f Lrp1f/f) also did not accumulate fasting triglycerides (66 ± 20 mg/dL) beyond that observed in Cre+Ndst1f/f mice (P = 0.7). Mice lacking LDLR (Ldlr−/−) accumulated triglycerides approximately 4-fold over wildtype (Fig. 2b, 181 ± 59 mg/dL [n = 9]; P < 0.0001). Inactivation of Ndst1 and LDL, i.e., the mutant expressing only LRP1 (Cre+Ndst1f/f Ldlr−/−), resulted in further elevation in plasma triglyceride compared to either Ldlr−/− or Ndst1−/− (415 ± 218 mg/dL [n = 9]). Mice expressing only HSPG receptors (Cre+Lrp1f/fLdlr−/− also exhibited elevated plasma triglycerides (548 ± 140 mg/dL [n = 9]) compared to Ldlr−/− and Lrp−/− mice (Fig. 2b, P < 0.0001). The triple receptor mutant (Cre+Ndst1f/fLrp1f/f Ldlr−/−) accumulated even greater levels of triglycerides (874 ± 270 mg/dL, [n = 14], P < 0.001 compared to Cre+Ndst1f/fLdlr−/− or Cre+Lrp1f/fLdlr−/−). These findings suggest that LRP1 does not play a major role in TRL clearance when LDLR is present, consistent with previous studies9, 17.
Figure 2.
Fasting lipids in compound mutant mice. After a 12-hour fast (10 pm – 10 am), blood was drawn via retroorbital sinus. Fasting triglycerides (A, B) and cholesterol (C, D) were measured in plasma from the various strains (n = 6-19 per genotype, see text). Horizontal bars indicate mean values. Statistical significance was determined by one-way ANOVA, P < 0.05.
Table 2.
Fasting lipid levels on a chow diet
| Genotype | Triglyceride | Cholesterol |
|---|---|---|
| (mg/dL plasma) | ||
|
Cre
−
Ndst1f/f
(Wildtype) |
43 ± 10 | 67 ± 9 |
|
Cre+Ndst1f/f
(HSPG receptor-deficient) |
69 ± 26 | 71 ± 22 |
|
Cre+Lrp1f/f
(LRP1-deficient) |
33 ± 5 | 71 ± 15 |
|
Ldlr−/−
(LDLR-deficient) |
181 ± 59 | 291 ± 63 |
|
Cre+Ndst1f/fLrp1f/f
(LDLR-only) |
66 ± 20 | 52 ± 7 |
|
Cre+Ndst1f/fLdlr−/−
(LRP1-only) |
415 ±218 | 477 ±160 |
|
Cre+Lrp1f/fLdlr−/−
HSPG receptor-only |
548 ±148 | 779 ±149 |
|
Cre+Ndst1f/fLrp1f/fLdlr−/−
(Triple receptor-deficient) |
874 ± 270 | 1308±346 |
All values are expressed as average ± standard error. Statistical significance was determined by two-tailed t-test. n.s., not significant
Heparan sulfate proteoglycans are not thought to contribute to clearance of cholesterol-rich lipoprotein particles based on the observation that HSPG receptor-deficient mice (Cre+Ndst1f/f) do not exhibit elevated plasma cholesterol levels compared to wildtype mice (Fig. 2b, 67 ± 9 mg/dL in wildtype [n = 12] vs. 71 ± 22 mg/dL in Cre+Ndst1f/f [n = 13], P = 0.6)15. LRP1 deficient mice (Cre+Lrp1f/f) also did not exhibit elevated plasma cholesterol (71 ± 15 mg/dL [n = 7]) compared to the wildtype (P = 0.5), presumably due to compensation by LDLR. Mice lacking the HSPG receptors and LRP1 (Cre+Ndst1f/f Lrp1f/f) also did not accumulate plasma cholesterol compared to wildtype (52 ± 7 mg/dL [n = 12], P < 0.001). However, mice lacking HSPG receptors and LDLR (Cre+Ndst1f/f Ldlr−/−) had approximately 2-fold higher cholesterol (477 ± 160 mg/dL [n = 10]) than Ldlr−/− mice (291 ± 63 mg/dL, P < 0.01). Triple receptor-deficient mice (Cre+Ndst1f/f Lrp1f/f Ldlr−/−) exhibited higher fasting plasma cholesterol (1308 ± 346 mg/dL [n = 6]) than HSPG receptor-only mice (Cre+Lrp1f/f Ldlr−/−, 779 ± 149 mg/dL [n = 9], P < 0.01). Thus, HSPGs can also mediate clearance of cholesterol-rich lipoproteins that LDLR family members normally clear.
Heparan sulfate proteoglycans and LDL receptor family members mediate clearance of TRLs of unique size
The accumulation of TRLs in single mutants deficient either in HSPGs or LDLR suggested that these receptors can clear different subsets of TRLs. We recently showed that HSPG receptor-deficient mice accumulate particles enriched in apoAV, whereas animals lacking LDLR and LRP1 do not18. To study the composition of these particles in greater detail, we analyzed samples by agarose electrophoresis, gel filtration, ultracentrifugation, and electron microscopy.
First, we analyzed TRLs (δ < 1.006 g/ml) using non-denaturing agarose gel electrophoresis, which separates particles dependent on size and charge (Fig. 3a). Wildtype TRLs migrated furthest, while the migration of TRLs that accumulated in HSPG receptor-deficient mice (Cre+Ndst1f/f) was retarded. The migration of TRLs from HSPG receptor-only animals (Cre+Lrp1f/fLdlr−/−) was even more delayed. Migration of TRLs from triple receptor mutant mice was intermediate between that observed in samples from the HSPG receptor-only and HSPG receptor-deficient mice. These findings confirm that particles of unique size and charge properties accumulate in each mutant and are consistent with an earlier study showing that mutants lacking LDLR accumulate large TRLs.8
Figure 3.
Sizing of accumulated particles. (A) Lipoproteins of δ < 1.006 g/ml (pooled from n = 3 mice per each genotype) were isolated by buoyant density ultracentrifugation and were analyzed by non-denaturing agarose electrophoresis. Asterisk indicates location of the origin. (B-C) Lipoproteins were analyzed by gel filtration FPLC, and the amount of (B) triglyceride and (C) cholesterol in each fraction was measured. The elution positions of human CR/VLDL, IDL/LDL, and HDL are indicated. (D) TRLs (δ < 1.006 g/ml) were negatively stained with 1% uranyl acetate and particle size was analyzed using images obtained by transmission electron microscopy. The diameters of particles present in 5-6 fields (1000-2000 particles per genotype) were measured using ImageJ software. The difference in distribution of particle size was significant between all groups by Kruskal-Wallis test except between the Cre+Ndst1f/fLrp1f/fLdlr−/− and Cre+Lrp1f/fLdlr−/− mice (P < 0.001). (E) Samples of pooled lipoproteins of δ < 1.006 g/ml (n = 3 per genotype) were analyzed by gradient SDS-PAGE, and the individual lipoproteins were visualized by silver staining. The location of ApoB-48, ApoB-100, ApoE and ApoCs was deduced by Mr values. (F) Samples of purified lipoproteins of δ < 1.006 g/ml were analyzed by gradient SDS-PAGE, and the individual lipoproteins were visualized by western blotting using antibodies against ApoB, ApoE, and ApoAV. Representative images from the same gel are shown (n = 3 mice per genotype).
To analyze the size of the accumulated particles, we fractionated whole plasma from fasted mice by gel filtration fast-phase liquid chromatography (FPLC). HSPG receptor-deficient mice (Cre+Ndst1f/f) accumulated large TRLs consistent in size with chylomicron, VLDL, and their remnants. Similarly sized triglyceride-rich particles were recovered from HSPG receptor-only mice (Cre+Lrp1f/fLdlr−/−) as well as particles resembling low density or intermediate density or lipoproteins (LDL/IDL) in size. The accumulation of LDL/IDL-like particles was further accentuated in the triple receptor-deficient mutants. No significant change in HDL triglycerides or cholesterol was noted in any of the mutants by this method (Fig. 3b and 3c).
Higher resolution was achieved by evaluating the size of TRLs (δ < 1.006 g/ml) by transmission electron microscopy (Fig. 3d). Mice lacking HSPG receptors accumulated a range of particles, predominantly 20-50 nm in diameter. In contrast, the mutant lacking both LDLR family members (Cre+Lrp1f/fLdlr−/−) accumulated somewhat larger TRLs (30-60 nm diameter). The TRLs from triple receptor deficient mice essentially behaved like the particles derived from Cre+Lrp1f/fLdlr−/− mice. The altered distribution of particle size was significant between all groups except between triple receptor-deficient and Cre+Lrp1f/f Ldlr−/− mice (Kruskal-Wallis test, P < 0.001). When we compared the protein to triglyceride ratio in the TRLs we observed that the ratio was smaller in Cre+Ndst1f/f mice compared to Cre+Lrp1f/fLdlr−/− and the triple receptor-deficient mice (0.38, 0.48 and 0.47 respectively). Thus, Cre+Ndst1f/f mice accumulate smaller TRL particles with lower protein content.
Finally, purified TRLs (d < 1.006 g/mL) were analyzed by gradient SDS-PAGE, and the individual apolipoproteins were visualized by silver staining. In all mutants, the TRLs contained apoB-48, apoB-100, apoE, and various apoCs (Figure 3e). In Cre+Ndst1f/f mice, both apoB-100 and apoB-48 containing lipoproteins accumulated. Combined inactivation of Ldlr and Lrp1 (Cre+Lrp1f/fLdlr−/− and Cre+Ndst1f/fLrp1f/fLdlr−/−) led to accumulation of apoB-48 containing lipoproteins similar to the observation by Rohlmann et al.9 Particles containing apoE accumulated in Cre+Ndst1f/f, Cre+Lrp1f/fLdlr−/− and triple receptor-deficient mice. The apoE accumulation was more profound in Cre+Lrp1f/fLdlr−/− and triple receptor-deficient mice compared to the Cre+Ndst1f/f mutant. We further characterized the apolipoprotein composition of accumulated particles by Western blot (Figures 3f). Both Cre+Ndst1f/f and triple receptor-deficient mutants accumulated ApoAV-containing lipoproteins, whereas Cre+Lrp1f/fLdlr−/− mice did not. These findings are consistent with recent studies showing that HSPGs clear lipoprotein particles containing ApoAV.18
Together, these data suggest that HSPGs preferentially clears a subset of small TRL particles enriched in ApoAV and ApoE, while members of the LDLR family of receptors clear large particles, enriched in ApoE, as reported previously8.
Postprandial triglyceride clearance and liver uptake is significantly delayed in compound mutant mice
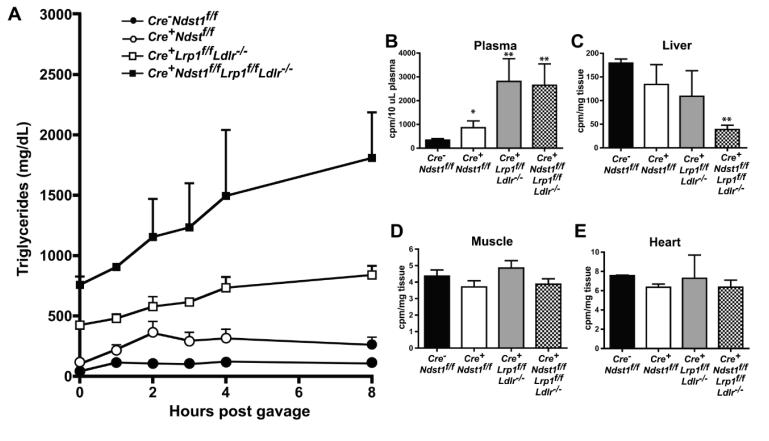
We next examined how receptor inactivation affected postprandial clearance. Fasted mice were given a bolus of corn oil by oral gavage, and blood was sampled at various time points post-gavage to measure appearance and disappearance of triglycerides in the circulation. As shown in Fig. 4a, HSPG receptor-deficient mice (Cre+Ndst1f/f) had delayed clearance of postprandial triglycerides compared to the wildtype (AUC = 2244 vs. 864 respectively). HSPG receptor-only mice (Cre+Lrp1f/f Ldlr−/−) had an even greater delay in clearance (AUC = 5406). Triple receptor-deficient mice had severely impaired clearance (AUC = 11033), and triglyceride levels remained high even 8 hours post-gavage.
Figure 4.
Postprandial clearance and triglyceride uptake in compound mutant mice. (A) To measure triglyceride clearance rates in mutants, overnight fasted mice were given 200 μL of corn oil by oral gavage. After 0, 1, 2, 3, 4, and 8 hours, mice were bled via the tail vein and triglycerides in plasma were measured. (B-E) Overnight fasted mice were given a bolus of [3H]retinol mixed with corn oil by oral gavage. After 8 hours, blood was obtained by cardiac puncture and plasma was assayed for radioactivity by liquid scintillation counting (B). Subsequently, the mice were sacrificed and dissected. Organs were weighed, homogenized in SOLVABLE, and assayed for radioactivity. Counts per milligram of tissue are reported for (C) liver, (D) muscle, and (E) heart. No statistically significant difference in uptake was observed in muscle or heart among the mutants (n = 3 mice per genotype).
To verify that this delay in postprandial clearance was due to altered liver clearance, we performed a [3H]retinol excursion study. In this experiment, the animals were gavaged with [3H]retinol mixed with corn oil. In the intestine, [3H]retinol is packaged into chylomicrons and these radioactive particles are subsequently cleared from the circulation in the liver in a time-dependent manner. At 8-hours post-gavage, counts remained high in plasma from the HSPG receptor-deficient mice and even greater in Cre+Lrp1f/f Ldlr−/− and triple receptor-deficient mice (Fig. 4b). In a separate experiment, counts remained significantly elevated in plasma from the triple receptor-deficient mice even 24 hours post-gavage. Correspondingly, liver uptake of the radioactive TRLs was decreased compared to the wildtype, with very low uptake occurring in livers from triple receptor-deficient mice (Fig. 4c). There was no significant difference in uptake of radioactive counts into skeletal muscle or heart between the wildtype and any of the mutants (Fig. 4d and 4e), confirming that that the observed persistence of postprandial triglycerides was due to defective clearance by hepatic receptors.
A high-fat diet shifts the relative contribution of HSPG receptors and LDL receptor family members to TRL clearance
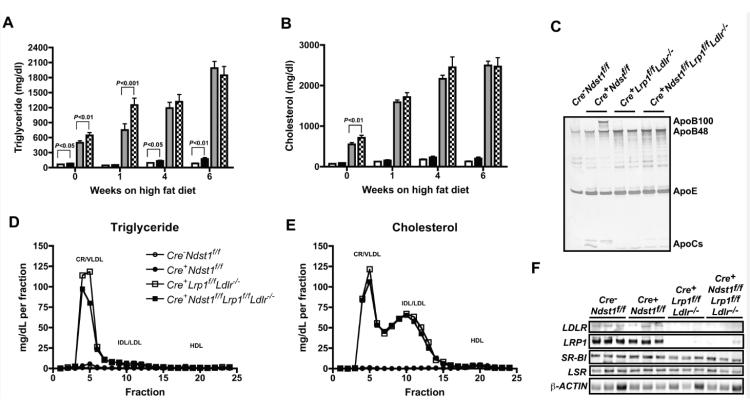
In a final set of experiments we examined how the various mutants would respond to a Western diet, high in fat. Twelve week-old mice were put on the Western diet and blood was taken for lipid analysis at 0, 1, 4, and 6 weeks after a short fasting period (Fig. 5 and Table 3). The high-fat diet induced little to no change in triglyceride levels of wildtype mice (65 ± 3 mg/dL at t = 0 to 82 ± 10 mg/dL after 6 weeks on the Western diet, P > 0.1). HSPG receptor-deficient mice exhibited a two-fold increase in triglyceride levels after 6-weeks on the Western diet (from 82 ± 7 to 172 ± 30 mg/dL, P < 0.05). This change was mild in comparison to the increase seen in mice lacking LDLR and LRP1. In the absence of these receptors plasma triglycerides increased at all time points, from 497 ± 39 at the start of the Western diet to 1990 ± 135 mg/dL after 6 weeks. Inactivation of all three receptor increased plasma triglyceride to a similar level from 644 ± 57 to 1850 ± 170 mg/dL.
Figure 5.
Fasting lipids in compound mutant mice on a high fat diet. After a 6-hour fast, triglycerides (A) and cholesterol (B) were measured in plasma from the various mutant mice (n = 5-6 per genotype) fed a high fat diet for 1, 4, and 6 weeks. (C) After 4-weeks on the HFD samples of pooled lipoproteins of δ < 1.006 g/ml (n = 3 per genotype) were analyzed by gradient SDS-PAGE, and the individual lipoproteins were visualized by silver staining. The location of ApoB-48, ApoB-100, ApoE and ApoCs was deduced by Mr values. After 6-weeks on the HFD, plasma lipoproteins were analyzed by gel filtration FPLC, and the amount of (D) triglyceride and (E) cholesterol in each fraction was measured. The elution positions of human CR/VLDL, IDL/LDL, and HDL are indicated. Horizontal bars indicate mean values (n = 5-6 per genotype). Statistical significance was determined by one-way ANOVA. (F) After 6-weeks on the HFD, livers were isolated from mice and solubilized in RIPA buffer. Samples were separated by gradient SDS-PAGE and transferred to PVDF membranes. The membrane was probed with antibodies against LDLR, LRP1, SR-BI, LSR and β-actin. Each lane represents a different mouse (n = 3 per genotype).
Table 3.
Fasting plasma lipid levels on a high fat diet
| Weeks on Diet |
Cre−Ndst1f/f (Wildtype) |
Cre+Ndst1f/f (HSPG receptor- deficient) |
Cre+Lrp1f/f
Ldlr− /− (HSPG receptor-only) |
Cre+Ndst1f/f
Lrp1f/f
Ldlr−/− (Triple receptor- deficient) |
|---|---|---|---|---|
| Triglycerides (mg/dL) | ||||
| 0 | 65 ± 3 | 82 ± 7 | 498 ± 39 | 644 ± 57 |
| 1 | 45 ± 6 | 51 ± 9 | 751 ±127 | 1251 ±138 |
| 4 | 94 ± 6 | 133 ± 14 | 1189±116 | 1317±144 |
| 6 | 82 ± 10 | 172 ± 30 | 1989±135 | 1847±173 |
| Cholesterol (mg/dL) | ||||
| 0 | 72 ± 4 | 90 ± 8 | 549 ± 44 | 710 ± 63 |
| 1 | 126 ± 9 | 152 ± 18 | 1588±56 | 1716 ±111 |
| 4 | 176 ± 16 | 229 ± 29 | 2167±89 | 2450 ± 256 |
| 6 | 128 ± 14 | 201 ± 37 | 2496±106 | 2466 ± 224 |
All values are expressed as average ± standard error.
The high fat diet also increased plasma cholesterol in Cre+Lrpf/fLdlr−/− mice from 550 ± 40 mg/dL at the start of the experiment to 2500 ± 110 mg/dL after 6 weeks (P < 0.001). The triple mutant was affected similarly (710 ± 60 mg/dL increasing to 2500 ± 220 mg/dL (P < 0.001). The majority of the cholesterol was associated with the LDL fraction based on gel filtration (Fig. 5e). Plasma cholesterol levels increased slightly during high fat feeding in both wildtype (72 ± 4 to 130 ± 14) and HSPG receptor-deficient mice (90 ± 8 to 200 ± 37) (Fig. 5b). Apolipoprotein analysis of purified TRLs (d < 1.006 g/mL) by gradient SDS-PAGE and silver staining showed that the Cre+Ndst1f/f mutant accumulated apoB-100 and apoE-containing lipoproteins (Fig. 5c), whereas mutants lacking Ldlr (Cre+Lrp1f/fLdlr−/− and Cre+Ndst1f/fLrp1f/fLdlr−/−) accumulated apoB-48 and apoE-containing lipoproteins.
High fat diet feeding did not induce changes in hepatic LDLR and LRP1 expression in HSPG receptor-deficient mice (Fig. 5e). Examination of other receptors showed that the high fat diet did not affect hepatic SR-BI expression in single, double and triple mutants (Cre−Ndst1f/f, 0.66 ± 0.16 AU, n=3; Cre+Ndst1f/f, 0.92 ± 0.05 AU, n=3; Cre+Lrp1f/fLdlr−/−, 0.60 ± 0.16 AU, n=3; Cre+Ndst1f/fLrp1f/fLdlr−/−, 0.53 ± 0.16 AU, n=3; Fig. 5e). LSR expression was also not altered (Cre−Ndst1f/f, 0.49 ± 0.19 AU, n=3; Cre+Ndst1f/f, 0.68 ± 0.07 AU, n=3; Cre+Lrp1f/fLdlr−/−, 0.46 ± 0.13 AU, n=3; Cre+Ndst1f/fLrp1f/fLdlr−/−, 0.34 ± 0.11 AU, n=3; Fig. 5e). Together the data suggest that the LDLR family members play a dominant role in TRL clearance when mice consume a high fat/high cholesterol-diet, whereas HSPGs play a more significant role when the animals are fed normal chow.
Discussion
A large body of work has established a role for HSPGs in hepatic clearance of triglyceride-rich lipoprotein remnants. In cultured cells, HSPGs can interact directly with apolipoproteins and lipases and facilitate their internalization by endocytosis19-21. Reduced plasma clearance rates and reduced hepatic uptake of labeled-VLDL has been observed in mice after intravenous infusion with agents that neutralize heparan sulfate such as heparinase, heparin, suramin or lactoferrin22-24. ApoE variants have been described in which patients exhibit severe hyperlipoproteinemia, which has been associated with altered binding to heparan sulfate22, 25, 26. Recently, we showed that Cre-mediated inactivation of Ndst1 or uronyl 2-O-sulfotransferase (Hs2st) in hepatocytes results in the accumulation of fasting triglycerides and delayed clearance of intestinally derived lipoproteins due to undersulfation of heparan sulfate15, 27. In subsequent studies, we identified syndecan-1 as the primary HSPG receptor for triglyceride-rich lipoproteins in mouse and human hepatocytes11, 28.
Members of the LDL receptor family have also has been demonstrated to act as receptors for remnant lipoprotein clearance. LDLR was originally characterized as a receptor for cholesterol-rich apoB100- and apoE-containing particles such as LDL. Mice, rabbits, and humans deficient for LDLR have only modest accumulation of triglycerides, suggesting a less dominant role for LDLR in TRL metabolism as compared to LDL metabolism8, 29, 30. Hepatocytes also express LRP1, which was thought to participate in clearance of apoE-enriched apoB48-bearing lipoproteins, including TRLs31. However, hepatocyte-specific ablation of LRP1 does not result in accumulation of fasting triglycerides or cholesterol (Fig. 1 and Table 2). A role for LRP1 becomes evident when LDLR is deficient (e.g. in Cre+Lrp1f/fLdlr−/− mice and when LRP1 was inactivated by injection of Adenoviral-Cre9). In other studies, in vivo inhibition of LDLR was achieved using tail vein injection of a blocking antibody32. This treatment reduced plasma clearance of TRLs by approximately 45%, whereas injection of the receptor-associated protein (RAP), an inhibitor of LRP1, reduced plasma clearance by 55%. When the LDLR blocking antibody and RAP were administered together, plasma removal was only decreased by 60%, an incremental effect at best. In a different study, mice with systemic deletions of the VLDL receptor (VLDLR) and LDLR were bred to mice in which LRP1 was inactivated by an inducible Cre33. VLDLR is not expressed in the liver, and has been shown to mediate TRL clearance in tissues active in fatty acid metabolism (heart, adipose, and skeletal muscle). Notably, heparin blocked the association of DiI-labeled lipoproteins to hepatocytes from the deficient mice. These findings are consistent with our conclusions that HSPG receptors are a major contributor to clearance in the liver, and that the two receptor families do not fully compensate for one another.
Our genetic analysis of the relative contribution of HSPG receptors and LDLR and LRP1 to TRL clearance has several important implications. Based on fasting triglyceride data from double and triple mutant mice (Fig. 2), we conclude that LDLR, LRP1, and HSPGs are the major TRL clearance receptors in mice, although the role of LRP1 only becomes apparent in the absence of LDLR. Postprandial clearance data also support this conclusion (Fig. 4). In triple receptor-deficient mice, triglyceride levels remain elevated and liver uptake remains low even 8 hours post-gavage, suggesting that other TRL receptors, such as LSR, LRP5, VLDLR, or SR-B1, do not compensate for the absence of these three major receptors. Nevertheless, we cannot exclude the possibility that these other receptors play a role in clearance under other conditions that were not addressed in our studies10, 13, 34.
Our findings also suggest that the different receptor subtypes clear distinct subsets of lipoprotein particles. Based on EM studies of particles that accumulate in the mutants, HSPG receptors apparently clear a subset of smaller TRLs, while members of the LDLR family of receptors clear larger particles (Fig. 3). The idea that particle size partially determines the affinity of lipoprotein particles for their receptors has been suggested 35, and may be the result of apolipoprotein conformation or content. In this study we provide additional evidence showing that HSPGs preferentially clear particles that are enriched in ApoE and ApoAV18. Based on our findings, we suggest that the small particles preferentially cleared by HSPG receptors are enriched for apoE and apoAV. Whether these particles have other unique attributes is unknown. Conceivably, these particles might originate from lipolysis of a unique pool of VLDL or chylomicrons in the vasculature.
HSPGs can act as independent endocytic receptors for numerous ligands36-38. Nevertheless, the idea that HSPGs act independently of other receptors in TRL clearance has been questioned6, 39. Biochemical data has been presented suggesting that these two proteins act as co-receptors for lipoprotein particles or participate in a “handoff” process whereby HSPGs trap lipoprotein particles in the space of Disse and subsequently transfer them to LRP1 for endocytosis6. Given our findings that the receptors clear different subsets of particles and that syndecan-1 expression is not affected by the loss of LDLR and LRP1 in hepatocytes (and vice versa), it seems likely that each receptor family acts independently.
The prominent role of syndecan-1 in clearance under fasting and postprandial conditions predicted that the proteoglycans would play an important role in clearing remnant lipoproteins in animals fed a high-fat diet. Surprisingly, triglyceride and cholesterol levels in HSPG receptor-deficient mice hardly changed when fed a high fat diet. This is in sharp contrast to LDLR/LRP1-deficient mice in which high fat feeding drastically increased both cholesterol and triglyceride levels. Furthermore, our data show that this discrepancy could not be explained by drastic changes in apolipoprotein composition of TRLs or changes in hepatic expression of LDLR, LRP1, SR-BI and LSR in HSPG receptor-deficient mice on the high fat diet. One interpretation of these findings is that hepatic HSPG receptors are active under basal (chow-fed) conditions and in response to acute dietary changes, i.e. postprandial lipid loading, but do not recognize the particles that accumulate under chronic high fat feeding. Conceivably, high levels of circulating LDL particles that accumulate under these conditions could decrease the availability of apoE and/or apoAV or shift particle size towards substrates poorly recognized by syndecan-1. We cannot exclude that changes in TRL lipid composition or the increase in blood lipids per se are contributing to this intriguing observation. Additional studies are clearly warranted to determine if increasing the expression of apoE and or apoAV pharmacologically stimulates HSPG-mediated clearance under high-fat diet conditions3.
The triple receptor-deficient mouse represents a unique hyperlipidemic model that can be used to study triglyceride and cholesterol metabolism under conditions where clearance is greatly delayed. Additionally, the triple receptor mutant may prove to be a valuable tool for elaborating the mechanism of action of compounds that reduce or prevent atherosclerosis independent of hepatic TRL clearance.
Materials and Methods
Mice and animal husbandry
Lrp1f/f, Ldlr−/− , and AlbCre+ mice were purchased from The Jackson Laboratory. Ndst1f/fAlbCre+ mice were generated and genotyped as described1. All mice were backcrossed more than 10 generations on a C57BL/6 background. Mice were housed and bred in vivaria approved by the Association for Assessment and Accreditation of Laboratory Animal Care located in the School of Medicine at the University of California, San Diego, following standards and procedures approved by the local Institutional Animal Care and Use Committee. Mice were weaned at 3 weeks, maintained on a 12-hour-light cycle, and fed ad libitum with water and standard rodent chow (Harlan Teklad) or a high fat/high cholesterol diet (TD.88137 from Harlan Teklad; 21% anhydrous milkfat (butterfat), 34% sucrose, and a total of 0.2% cholesterol).
Lipid analysis
Blood was drawn via the retroorbital sinus from mice fasted for 6-12 hr. Total cholesterol and triglyceride levels in plasma were determined using kits (Genzyme) against the Precipath L lipoprotein standard (Roche Diagnostics).
Ultracentrifugation studies
Plasma was pooled from several mice (70 μL per mouse, n = 3 mice per genotype). Lipoprotein fractions were separated by buoyant density ultracentrifugation according to established methods2. Briefly, 210 μL of pooled plasma was loaded into micro-ultracentrifuge tubes (Beckman). The samples were centrifuged 12 hours in a 42.2 Ti rotor at 38,000 rpm at 18°C. The top 50 μL containing VLDL and chylomicron remnants (δ < 1.006 g/ml) was removed and used for analysis. TRLs were analyzed by SDS-PAGE on 4-12% Bis-Tris gradient gels (NuPage, Invitrogen). Proteins were visualized by silver staining (Pierce) or after transfer to Immobilon-FL PVDF membrane (Millipore) as previously described3.
Heparan sulfate purification and disaccharide analysis
Hepatocytes were isolated and allowed to recover in culture overnight 4. The cells were then treated overnight with Pronase (2 mg/ml; Roche Diagnostics) to degrade proteins, followed by purification of the glycopeptides by anion exchange chromatography using DEAE Sephacel (Amersham Biosciences). Columns were washed with low-salt buffer (0.15 M NaCl, 20mM sodium acetate; pH 6.0) and eluted with 1 M NaCl. Samples were desalted using PD-10 columns and then lyophilized. The glycosaminoglycans were digested with heparin lyases I, II, and III. The resulting disaccharides were derivatized with isotopically labeled aniline and quantified by mass spectrometry as described previously5. Molar percentages were calculated based on the relative area under each peak compared to standards.
Electron microscopy
Lipoprotein particles isolated by buoyant density ultracentrifugation (δ < 1.006 g/ml) were subjected to negative staining. Briefly, particles were diluted to a concentration of 0.5-1.5 mg/ml triglyceride in water, and sucrose was added to a final concentration of 0.1%. The particles were then allowed to adhere to a Formvar carbon-coated grid and stained with 1% uranyl acetate before visualization on a FEI Tecnai Spirit G2 BioTWIN transmission electron microscope equipped with a 4K Eagle digital camera.
Fast performance liquid chromatography (FPLC)
Pooled plasma samples were separated by gel filtration FPLC. Samples were loaded on a GE Superose 6 10/30 GL column (Cat# 175172-01) in 0.15 M sodium chloride containing 1 mM ethylenediaminetetraacetic acid and 0.02% sodium azide, pH 7.4. Fractions (0,5 mL) were collected (0.5 mL/min). Total cholesterol and triglyceride levels were determined enzymatically using an automated reader (Cobas Mira; Roche Diagnostics) and kits: Cholesterol High-Performance Reagent (Roche Diagnostics) and Triglyceride-SL (Diagnostic Chemicals Ltd.).
Western blots
In Figure 1, freshly isolated hepatocytes were immediately solubilized in RIPA buffer plus protease inhibitors (Sigma). In Figure 5, freshly isolated livers were solubilized in RIPA buffer plus protease inhibitors (Sigma). For each sample, 20 μg protein was resolved on a 4-12% Bis-Tris NuPage gel (Invitrogen) and transferred to polyvinlyidene fluoride membrane (Bio-Rad Laboratories). The membrane was blocked with Super-Block buffer (Pierce). Blots were incubated with antibodies against LDLR (AbCam), Lrp-1 (AbCam), Syndecan-1 (Pharmingen), SR-BI (Novus Biologicals), LSR (Sigma) or β-actin (Sigma) and appropriate HRP-conjugated secondary antibodies (Santa Cruz). Reactive bands were visualized by chemiluminescence.
Agarose electrophoresis
Lipoprotein samples (δ < 1.006 g/ml) were loaded onto a TITAN Gel (Helena Laboratories), electrophoresed, and stained per the manufacturer’s instructions.
Postprandial clearance studies
Mice were fasted for 12 hours. At 10 am, they were given a 200 μL bolus of corn oil (Sigma) by oral gavage. At the indicated time points, mice were sedated with isofluorane and bled via the tail vein. Triglyceride and cholesterol levels were measured as described above.
[3H]Retinol organ uptake experiments
Clearance of chylomicrons derived from dietary triglyceride was measured by Vitamin A excursion essentially as described6. Briefly, 27 μCi of [11,12-3H]-retinol (Perkin Elmer; 44 Ci/mmol) in ethanol was mixed with 1 mL of corn oil (Sigma-Aldrich) and administered to overnight fasted mice by oral gavage (200 μL/mouse). Blood was obtained 8-hours post-gavage by cardiac puncture and counts remaining in the serum were assayed in duplicate by liquid scintillation counting. The mice were dissected, and organs were removed. Approximately 100-200 mg of tissue was solubilized in 1 mL of SOLVABLE (Perkin Elmer) at 55°C, and 3H-counts in the tissue were assayed by liquid scintillation counting.
Statistical analysis
Statistical analyses were performed using Prism 4.0c (GraphPad Software) using the indicated tests. Significance was taken as P < 0.05. Data are expressed as average ± standard deviation.
Significance.
Elevated plasma triglyceride concentrations contribute to increased risk of cardiovascular disease. This increase is a result from elevated triglyceride production in the liver and intestine or through decreased clearance from the circulation. In this paper we identified that heparan sulfate proteoglycan, LDLR and LRP1 are the three main receptors responsible for hepatic catabolism of triglyceride-rich lipoproteins. We establish that heparan sulfate proteoglycans act independently of LDLR and LRP1 and clear a distinct subset of small triglyceride-rich particles. Interestingly, heparan sulfate proteoglycans play a major role when fed a balanced diet but are less crucial when consuming a high fat diet. The data provide a better understanding of the relative contribution of the receptors to hepatic clearance and its dynamic interactions with genetic and environmental factors. The current findings are valuable, as they shed new light on the already complex etiology of hypertriglyceridemia.
Acknowledgements
We would like to thank Jennifer Pattison and Joe Juliano for their assistance with FPLC and lipid analysis. We would also like to thank Timo Merloo and Marilyn Farquhar for technical assistance with electron microscopy. We acknowledge Carlos Lameda Diaz for his kind assistance with glycosaminoglycan isolation. We are grateful to Joe Witztum for helpful discussions.
Sources of Funding This work was supported by NIH grant GM33063 (to J.D.E.), European Community FP7 Award PIOF-GA-2010-273994 (to P.L.S.M.G) and training grant 5T32CA067754-17 (to E.M.F).
Abbreviations
- HSPG
heparan sulfate proteoglycans
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- LDLR
low density lipoprotein receptor
- LRP
low density lipoprotein receptor-related protein
- NDST1
GlcNAc N-deacetylase/N-sulfotransferase 1
- TRL
triglyceride-rich lipoprotein
- VLDL
very low density lipoprotein
Footnotes
Disclosures: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 2.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 3.Yuan G, Al-Shali KZ, Hegele RA. Hypertriglyceridemia: its etiology, effects and treatment. Cmaj. 2007;176:1113–1120. doi: 10.1503/cmaj.060963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stalenhoef AF, de Graaf J. Association of fasting and nonfasting serum triglycerides with cardiovascular disease and the role of remnant-like lipoproteins and small dense LDL. Curr Opin Lipidol. 2008;19:355–361. doi: 10.1097/MOL.0b013e328304b63c. [DOI] [PubMed] [Google Scholar]
- 5.Foley EM, Esko JD. Hepatic heparan sulfate proteoglycans and endocytic clearance of triglyceride-rich lipoproteins. Prog Mol Biol Transl Sci. 2010;93:213–233. doi: 10.1016/S1877-1173(10)93010-X. [DOI] [PubMed] [Google Scholar]
- 6.Mahley RW, Huang Y. Atherogenic remnant lipoproteins: role for proteoglycans in trapping, transferring, and internalizing. J Clin Invest. 2007;117:94–98. doi: 10.1172/JCI30889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams KJ, Chen K. Recent insights into factors affecting remnant lipoprotein uptake. Curr Opin Lipidol. 2010;21:218–228. doi: 10.1097/MOL.0b013e328338cabc. [DOI] [PubMed] [Google Scholar]
- 8.Ishibashi S, Perrey S, Chen Z, Osuga J, Shimada M, Ohashi K, Harada K, Yazaki Y, Yamada N. Role of the low density lipoprotein (LDL) receptor pathway in the metabolism of chylomicron remnants. A quantitative study in knockout mice lacking the LDL receptor, apolipoprotein E, or both. J Biol Chem. 1996;271:22422–22427. doi: 10.1074/jbc.271.37.22422. [DOI] [PubMed] [Google Scholar]
- 9.Rohlmann A, Gotthardt M, Hammer RE, Herz J. Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. J Clin Invest. 1998;101:689–695. doi: 10.1172/JCI1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujino T, Asaba H, Kang MJ, Ikeda Y, Sone H, Takada S, Kim DH, Ioka RX, Ono M, Tomoyori H, Okubo M, Murase T, Kamataki A, Yamamoto J, Magoori K, Takahashi S, Miyamoto Y, Oishi H, Nose M, Okazaki M, Usui S, Imaizumi K, Yanagisawa M, Sakai J, Yamamoto TT. Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc Natl Acad Sci USA. 2003;100:229–234. doi: 10.1073/pnas.0133792100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanford KI, Bishop JR, Foley EM, Gonzales JC, Niesman IR, Witztum JL, Esko JD. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in mice. J Clin Invest. 2009;119:3236–3245. doi: 10.1172/JCI38251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goudriaan JR, Espirito Santo SM, Voshol PJ, Teusink B, van Dijk KW, van Vlijmen BJ, Romijn JA, Havekes LM, Rensen PC. The VLDL receptor plays a major role in chylomicron metabolism by enhancing LPL-mediated triglyceride hydrolysis. J Lipid Res. 2004;45:1475–1481. doi: 10.1194/jlr.M400009-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Van Eck M, Hoekstra M, Out R, Bos IS, Kruijt JK, Hildebrand RB, Van Berkel TJ. Scavenger receptor BI facilitates the metabolism of VLDL lipoproteins in vivo. J Lipid Res. 2008;49:136–146. doi: 10.1194/jlr.M700355-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Mann CJ, Khallou J, Chevreuil O, Troussard AA, Guermani LM, Launay K, Delplanque B, Yen FT, Bihain BE. Mechanism of activation and functional significance of the lipolysis-stimulated receptor. Evidence for a role as chylomicron remnant receptor. Biochemistry. 1995;34:10421–10431. doi: 10.1021/bi00033a014. [DOI] [PubMed] [Google Scholar]
- 15.MacArthur JM, Bishop JR, Wang L, Stanford KI, Bensadoun A, Witztum JL, Esko JD. Liver heparan sulfate proteoglycans mediate clearance of triglyceride-rich lipoproteins independently of LDL receptor family members. J Clin Invest. 2007;117:153–164. doi: 10.1172/JCI29154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence R, Olson SK, Steele RE, Wang L, Warrior R, Cummings RD, Esko JD. Evolutionary differences in glycosaminoglycan fine structure detected by quantitative glycan reductive isotope labeling. J Biol Chem. 2008;283:33674–33684. doi: 10.1074/jbc.M804288200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espirito Santo SM, Pires NM, Boesten LS, Gerritsen G, Bovenschen N, van Dijk KW, Jukema JW, Princen HM, Bensadoun A, Li WP, Herz J, Havekes LM, van Vlijmen BJ. Hepatic low-density lipoprotein receptor-related protein deficiency in mice increases atherosclerosis independent of plasma cholesterol. Blood. 2004;103:3777–3782. doi: 10.1182/blood-2003-11-4051. [DOI] [PubMed] [Google Scholar]
- 18.Waern I, Karlsson I, Thorpe M, Schlenner SM, Feyerabend TB, Rodewald HR, Abrink M, Hellman L, Pejler G, Wernersson S. Mast cells limit extracellular levels of IL-13 via a serglycin proteoglycan-serine protease axis. Biol Chem. 2012;393:1555–1567. doi: 10.1515/hsz-2012-0189. [DOI] [PubMed] [Google Scholar]
- 19.Williams KJ, Fless GM, Petrie KA, Snyder ML, Brocia RW, Swenson TL. Mechanisms by which lipoprotein lipase alters cellular metabolism of lipoprotein(a), low density lipoprotein, and nascent lipoproteins. Roles for low density lipoprotein receptors and heparan sulfate proteoglycans. J Biol Chem. 1992;267:13284–13292. [PubMed] [Google Scholar]
- 20.Ji ZS, Brecht WJ, Miranda RD, Hussain MM, Innerarity TL, Mahley RW. Role of heparan sulfate proteoglycans in the binding and uptake of apolipoprotein E-enriched remnant lipoproteins by cultured cells. J Biol Chem. 1993;268:10160–10167. [PubMed] [Google Scholar]
- 21.Ji ZS, Dichek HL, Miranda RD, Mahley RW. Heparan sulfate proteoglycans participate in hepatic lipase- and apolipoprotein E-mediated binding and uptake of plasma lipoproteins, including high density lipoproteins. J Biol Chem. 1997;272:31285–31292. doi: 10.1074/jbc.272.50.31285. [DOI] [PubMed] [Google Scholar]
- 22.Ji ZS, Sanan DA, Mahley RW. Intravenous heparinase inhibits remnant lipoprotein clearance from the plasma and uptake by the liver: in vivo role of heparan sulfate proteoglycans. J Lipid Res. 1995;36:583–592. [PubMed] [Google Scholar]
- 23.Mortimer BC, Beveridge DJ, Martins IJ, Redgrave TG. Intracellular localization and metabolism of chylomicron remnants in the livers of low density lipoprotein receptor-deficient mice and apoE-deficient mice. Evidence for slow metabolism via an alternative apoE-dependent pathway. J Biol Chem. 1995;270:28767–28776. doi: 10.1074/jbc.270.48.28767. [DOI] [PubMed] [Google Scholar]
- 24.Windler E, Greeve J, Robenek H, Rinninger F, Greten H, Jackle S. Differences in the mechanisms of uptake and endocytosis of small and large chylomicron remnants by rat liver. Hepatology. 1996;24:344–351. doi: 10.1053/jhep.1996.v24.pm0008690403. [DOI] [PubMed] [Google Scholar]
- 25.Mann WA, Lohse P, Gregg RE, Ronan R, Hoeg JM, Zech LA, Brewer HB., Jr Dominant expression of type III hyperlipoproteinemia. Pathophysiological insights derived from the structural and kinetic characteristics of ApoE-1 (Lys146-->Glu) J Clin Invest. 1995;96:1100–1107. doi: 10.1172/JCI118096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mann WA, Meyer N, Weber W, Meyer S, Greten H, Beisiegel U. Apolipoprotein E isoforms and rare mutations: parallel reduction in binding to cells and to heparin reflects severity of associated type III hyperlipoproteinemia. J Lipid Res. 1995;36:517–525. [PubMed] [Google Scholar]
- 27.Stanford KI, Wang L, Castagnola J, Song D, Bishop JR, Brown JR, Lawrence R, Bai X, Habuchi H, Tanaka M, Cardoso WV, Kimata K, Esko JD. Heparan sulfate 2-O-sulfotransferase is required for triglyceride-rich lipoprotein clearance. J Biol Chem. 2010;285:286–294. doi: 10.1074/jbc.M109.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng Y, Foley EM, Gonzales JC, Gordts PL, Li Y, Esko JD. Shedding of syndecan-1 from human hepatocytes alters very low density lipoprotein clearance. Hepatology. 2012;55:277–286. doi: 10.1002/hep.24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kita T, Goldstein JL, Brown MS, Watanabe Y, Hornick CA, Havel RJ. Hepatic uptake of chylomicron remnants in WHHL rabbits: a mechanism genetically distinct from the low density lipoprotein receptor. Proc Natl Acad Sci USA. 1982;79:3623–3627. doi: 10.1073/pnas.79.11.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubinsztein DC, Cohen JC, Berger GM, van der Westhuyzen DR, Coetzee GA, Gevers W. Chylomicron remnant clearance from the plasma is normal in familial hypercholesterolemic homozygotes with defined receptor defects. J Clin Invest. 1990;86:1306–1312. doi: 10.1172/JCI114839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.May P, Woldt E, Matz RL, Boucher P. The LDL receptor-related protein (LRP) family: an old family of proteins with new physiological functions. Ann Med. 2007;39:219–228. doi: 10.1080/07853890701214881. [DOI] [PubMed] [Google Scholar]
- 32.de Faria E, Fong LG, Komaromy M, Cooper AD. Relative roles of the LDL receptor, the LDL receptor-like protein, and hepatic lipase in chylomicron remnant removal by the liver. J Lipid Res. 1996;37:197–209. [PubMed] [Google Scholar]
- 33.Espirito Santo SM, Rensen PC, Goudriaan JR, Bensadoun A, Bovenschen N, Voshol PJ, Havekes LM, van Vlijmen BJ. Triglyceride-rich lipoprotein metabolism in unique VLDL receptor, LDL receptor, and LRP triple-deficient mice. J Lipid Res. 2005;46:1097–1102. doi: 10.1194/jlr.C500007-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Yen FT, Roitel O, Bonnard L, Notet V, Pratte D, Stenger C, Magueur E, Bihain BE. Lipolysis stimulated lipoprotein receptor: a novel molecular link between hyperlipidemia, weight gain, and atherosclerosis in mice. J Biol Chem. 2008;283:25650–25659. doi: 10.1074/jbc.M801027200. [DOI] [PubMed] [Google Scholar]
- 35.Rensen PC, Herijgers N, Netscher MH, Meskers SC, van Eck M, van Berkel TJ. Particle size determines the specificity of apolipoprotein E-containing triglyceride-rich emulsions for the LDL receptor versus hepatic remnant receptor in vivo. J Lipid Res. 1997;38:1070–1084. [PubMed] [Google Scholar]
- 36.Elson-Schwab L, Garner OB, Schuksz M, Crawford BE, Esko JD, Tor Y. Guanidinylated neomycin delivers large, bioactive cargo into cells through a heparan sulfate-dependent pathway. J Biol Chem. 2007;282:13585–13591. doi: 10.1074/jbc.M700463200. [DOI] [PubMed] [Google Scholar]
- 37.Belting M. Heparan sulfate proteoglycan as a plasma membrane carrier. Trends Biochem Sci. 2003;28:145–151. doi: 10.1016/S0968-0004(03)00031-8. [DOI] [PubMed] [Google Scholar]
- 38.Williams KJ, Fuki Cell-surface heparan sulfate proteoglycans: dynamic molecules mediating ligand catabolism. Curr Opin Lipidol. 1997;8:253–262. doi: 10.1097/00041433-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Wilsie LC, Orlando RA. The low density lipoprotein receptor-related protein complexes with cell surface heparan sulfate proteoglycans to regulate proteoglycan-mediated lipoprotein catabolism. J Biol Chem. 2003;278:15758–15764. doi: 10.1074/jbc.M208786200. [DOI] [PubMed] [Google Scholar]
- 40.Lawrence R, Lu H, Rosenberg RD, Esko JD, Zhang L. Disaccharide structure code for the easy representation of constituent oligosaccharides from glycosaminoglycans. Nat Methods. 2008;5:291–292. doi: 10.1038/nmeth0408-291. [DOI] [PubMed] [Google Scholar]
- 41.Kelley JL, Kruski AW. Density gradient ultracentrifugation of serum lipoproteins in a swinging bucket rotor. Methods Enzymol. 1986;128:170–181. doi: 10.1016/0076-6879(86)28067-2. [DOI] [PubMed] [Google Scholar]
References
- 1.MacArthur JM, Bishop JR, Wang L, Stanford KI, Bensadoun A, Witztum JL, Esko JD. Liver heparan sulfate proteoglycans mediate clearance of triglyceride-rich lipoproteins independently of ldl receptor family members. J Clin Invest. 2007;117:153–164. doi: 10.1172/JCI29154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelley JL, Kruski AW. Density gradient ultracentrifugation of serum lipoproteins in a swinging bucket rotor. Methods Enzymol. 1986;128:170–181. doi: 10.1016/0076-6879(86)28067-2. [DOI] [PubMed] [Google Scholar]
- 3.Gonzales JC, Gordts PL, Foley EM, Esko JD. Apolipoproteins e and av mediate lipoprotein clearance by hepatic proteoglycans. J Clin Invest. 2013;123:2742–2751. doi: 10.1172/JCI67398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng Y, Foley EM, Gonzales JC, Gordts PL, Li Y, Esko JD. Shedding of syndecan-1 from human hepatocytes alters very low density lipoprotein clearance. Hepatology. 55:277–286. doi: 10.1002/hep.24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence R, Olson SK, Steele RE, Wang L, Warrior R, Cummings RD, Esko JD. Evolutionary differences in glycosaminoglycan fine structure detected by quantitative glycan reductive isotope labeling. J Biol Chem. 2008;283:33674–33684. doi: 10.1074/jbc.M804288200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanford KI, Bishop JR, Foley EM, Gonzales JC, Niesman IR, Witztum JL, Esko JD. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in mice. J Clin Invest. 2009;119:3236–3245. doi: 10.1172/JCI38251. [DOI] [PMC free article] [PubMed] [Google Scholar]