Abstract
Persistent exposure to environmental stressors causes dysregulation of the limbic-hypothalamic-pituitary-adrenal (LHPA) axis and alters GABAA receptor (GABAAR) levels throughout the brain. Social subordination in socially housed female rhesus results in distinctive stress-related physiological and behavioral phenotypes that are dependent on the ovarian hormone estradiol (E2). In the present study, we utilized ovariectomized adult female rhesus monkeys undergoing hormone replacement with E2 to test the hypothesis that the chronic psychosocial stress of subordination alters GABAAR binding potential (GABAAR BPND) in limbic regions implicated in emotional processing including the prefrontal cortex, temporal lobe (amygdala and hippocampus), and hypothalamus. Furthermore, we tested the hypothesis that peripheral administration of a corticotropin-releasing hormone receptor (CRHR) antagonist (astressin B) would reverse the alterations in GABAAR binding within these regions in subordinate females. After subjects received astressin B or saline for three consecutive days, GABAAR BPND was determined by positron emission tomography (PET) using 18F-flumazenil as a radioligand. T1-weighted structural MRI scans were also acquired for PET scan co-registration, in order to perform a region of interest analysis using the pons as a reference region. Compared to socially dominant females, subordinate females exhibited increased GABAAR BPND in the prefrontal cortex but not in the temporal lobe or the hypothalamus. Administration of astressin B eliminated the status difference in GABAAR BPND in the prefrontal cortex, suggesting that the chronic stressor of social subordination modulates GABAergic tone via effects on CRH and the LHPA axis, at least in prefrontal regions.
Keywords: estradiol, social subordination, stress, flumazenil, Astressin B, GABAA receptor, monkeys
Introduction
Exposure to psychosocial stressors is implicated in the etiology of psychopathologies in humans. These illnesses, including depression and anxiety, are often associated with alterations in the regulation and function of the limbic-hypothalamic-pituitary-adrenal (LHPA) axis (Juster et al, 2010). Furthermore, stress-induced psychopathologies occur in women twice as often as they do in men (Weissman and Olfson, 1995), implicating a role for gonadal steroid hormones in vulnerability to stress-induced adverse health outcomes. Indeed, the major ovarian hormone, estradiol (E2), plays a key role not only in the control of reproductive function in females, but also in emotional reactivity and the expression of prosocial behavior (Pfaff et al, 2000). E2 modulates both cognitive and affective behavior (Bodo et al, 2006; McEwen et al, 1997) and influences the activity of the LHPA axis under both basal and stress-induced conditions throughout the course of the menstrual cycle across species (Altemus et al, 2001; Giussani et al, 2000; Roy et al, 1999; Wilson et al, 2005). E2 also increases the expression of corticotropin-releasing hormone (CRH) in the hypothalamus in female rhesus monkeys (Roy et al, 1999). Importantly, exposure to stressors in female rodents and monkeys alters both behavioral and physiological sensitivity to E2 (Michopoulos et al, 2009; Uphouse et al, 2005; White and Uphouse, 2004) but the mechanism responsible for this stress-induced change in sensitivity is poorly understood.
The γ-aminobutyric acid (GABA) neurotransmitter system has widespread regulatory function on systems that regulate physiology and behavior, and is significantly modulated by E2. For example, it has been shown that E2 increases the expression of GABA and the GABA synthesizing enzyme, glutamic acid decarboxylase (GAD), in the cortex and hippocampus (Tan et al, 2012). Additionally, E2 treatment in rodents increases GABAA receptor levels (GABAAR) in the olfactory bulb (Guerra-Araiza et al, 2008) as well as alters subunit organization of GABAARs in the hypothalamus and bed nucleus of the stria terminalis (BNST) (Herbison and Fenelon, 1995). The GABAergic system is also regulated by the activity of the LHPA axis (Bowers et al, 1998; Cullinan et al, 2008) and GABAAR levels throughout the brain are altered following stress exposure (Serra et al, 2000; Skerritt et al, 1981; Skilbeck et al, 2010). Recent studies in humans have shown GABAAR binding is decreased in brain regions involved in emotional regulation and the control of the LHPA axis in individuals with posttraumatic stress disorder and depression (Cameron et al, 2007; Geuze et al, 2008; Klumpers et al, 2010).
Social subordination in subordinate female rhesus monkeys results in altered physiological responses to E2, including enhanced E2 negative feedback inhibition of luteinizing hormone (LH) (Michopoulos et al, 2009) and an attenuated ability of E2 to decrease body weight (Michopoulos and Wilson, 2011c). Furthermore, social subordination also impairs the ability of E2 to produce socio-sexual (Reding et al, 2012) and anxiolytic behavior (Michopoulos et al, 2011b), changes E2-regulated modulation of the central serotonergic system (Asher et al, 2012), and alters E2-induced activation in the prefrontal cortex (unpublished data) in adult female rhesus monkeys. Thus, the goal of the current study was to assess whether the psychosocial stressor of social subordination in ovariectomized adult female rhesus monkeys alters E2’s ability to modify GABAAR levels in the medial and dorsolateral prefrontal cortex, the anterior cingulate cortex, the orbitofrontal cortex, the amygdala and the hippocampus, and the hypothalamus, all of which are brain regions that have been implicated in the regulation of emotional and stress-related behavior and the LHPA axis and express GABAAR (Herman et al, 2004; Mody and Maguire, 2011; Sarkar et al, 2011; Serra et al, 2000; Skerritt et al, 1981; Skilbeck et al, 2010).
In addition, because CRH release from the paraventricular nucleus of the hypothalamus is modulated, in part, by projections for the prefrontal cortex (Sullivan and Gratton, 2002), and because it has been shown that E2 can increase CRH release in brain regions that mediate emotional behavior (Jasnow et al, 2006; Lunga and Herbert, 2004), we assessed whether acute treatment with astressin B, a mixed CRH receptor type 1 and type 2 (CRHR1/2) antagonist (Broadbear et al, 2004), would eliminate any status differences in E2’s ability to modulate GABAAR binding within these brain regions in subordinate females. Positron emission tomography (PET) using a 18F-flumazenil (benzodiazepine antagonist) (Geuze et al, 2008) was undertaken to test the hypothesis that subordinate female monkeys would have decreased GABAAR binding compared to dominant females in the prefrontal cortex, temporal lobe and hypothalamus in response to E2 administration, and that administration of astressin B would abolish status differences in E2-induced changes in GABAAR binding. The data from this study will elucidate whether exposure to psychosocial stressors change GABAA receptor binding potential in response to E2 replacement and whether these changes are corrected by the administration of a CRH receptor antagonist.
Methods
Subjects
Adult ovariectomized female rhesus macaques (n=17) receiving hormone replacement via estradiol benzoate injections and living in indoor/outdoor enclosures, measuring 3.8 by 3.8 by 3.8 m, at the Yerkes National Primate Research Center (YNPRC) Field Station were subjects for the current study. Subjects were members of small social groups of 4 and 5 females each. Animals were fed Purina monkey chow (diet 5038, PMI, St Louis, MO) ad libitum twice daily and had continuous access to water. In addition, seasonal fruits and vegetables were provided daily as a nutritional supplement. The Emory University Institutional Animal Care and Use Committee in accordance with the Animal Welfare Act and the U.S. Department of Health and Human Services “Guide for Care and Use of Laboratory Animals” approved all procedures.
Female rhesus monkeys represent an appropriate translational model to investigate the effects of psychosocial stress exposure and changes in behavior and physiology (Michopoulos et al, 2012a; Michopoulos et al, 2012b; Shively and Kaplan, 1984). Female macaques, when housed socially, form linear dominance hierarchies wherein dominant females constantly harass lower ranking females (Bernstein and Gordon, 1974a; Bernstein et al, 1974b). Subordinate female macaques show dysregulation of the LHPA axis (Arce et al, 2010; Collura et al, 2009; Michopoulos et al, 2012b; Wilson et al, 2008) and alterations in behavior and physiology (Abbott et al, 2003; Michopoulos et al, 2012a), including reproductive dysfunction (Kaplan et al, 2010; Michopoulos et al, 2009), immune compromise (Paiardini et al, 2009; Tung et al, 2012), emotional feeding (Michopoulos et al, 2012c), impaired cardiovascular function (Kaplan and Manuck, 1999) and altered reward pathways (Grant et al, 1998; Morgan et al, 2002). Importantly for the purposes of this study, subordinate female rhesus monkeys show enhanced sensitivity to E2 negative feedback inhibition of the reproductive axis (Michopoulos et al, 2009) and altered sensitivity to E2’s anxiolytic (Michopoulos et al, 2011b) and affiliative effects (Reding et al, 2012).
The formation of the small social groups, as previously described (Jarrell et al, 2008), occurred three years previous to the initiation of the current study. Females were ovariectomized (Michopoulos et al, 2011a) prior to new group formation (Jarrell et al, 2008) as they were part of a series of experiments investigating the effects of social status on a number of behavioral and physiological phenotypes regulated by E2 replacement (Michopoulos et al, 2009; Michopoulos et al, 2011a; Michopoulos et al, 2011b). The dominance hierarchy of each group was confirmed from the outcome of dyadic agonistic interactions in which subordinate females emit an unequivocal submissive behavior towards another animal (Bernstein et al, 1974a; Bernstein et al, 1974b). Behavior was assessed via two 30-min behavioral observations during the beginning of each phase of the study to define social ranking (Jarrell et al, 2008). Females ranked as 1 and 2 were categorized as dominant and females ranked 3–5 were classified as subordinate in accordance with previously established convention (Michopoulos et al, 2012a; Michopoulos et al, 2012b; Shively, 1998). The current study sample consisted of four alpha females (highest ranked in each group), four beta females (second-highest ranked in each group), two gamma ranked females (third-highest ranked in each group), four delta females (fourth-highest ranked in each group), and three epsilon females (lowest-ranked in each group). Thus, in total, eight dominant females and nine subordinate females were subjects.
Treatment conditions
In order to mimic the hormonal milieu of the follicular phase, females were studied under hormone replacement with estradiol benzoate in two conditions, saline and astressin B. All females received estradiol benzoate injections (1 μg/kg IM) (Karsch et al, 1973) on each of the two days leading up to her PET scan and on the morning of her scan. A dose of 0.45 mg/kg/day, sc, of astressin B (Vulliemoz et al, 2008) or saline was administered on these same days. Astressin B was used in the current study because it decreases peripheral cortisol levels in female rhesus monkeys (Michopoulos et al, 2010).
PET imaging
The radioligand 2′-[18F]Fluoroethylflumazenil ([18F]FFMZ; 18F-flumazenil) was used to assess the binding potential of central GABAAR (Grunder et al, 2001). The YNPRC Radiochemistry Laboratory synthesized the 18F-flumazenil with a radiochemical purity of over 99%. Binding potential (BPND) was defined as the ratio at equilibrium of bound flumazenil to that of nondisplacable flumazenil in the tissue of each region. BPND is a measurement used by our group previously (Embree et al, 2012) that correlates receptor density to experimental conditions (Innis et al, 2007).
Each female received two PET scans (saline vs. astressin B in a counterbalanced manner) separated by one month using 18F-flumazenil. PET scans for 12 of the total 17 subjects were performed at the YNPRC Imaging Center on a Siemens Focus 220 microPET scanner (Concorde Microsystems, Knoxville, TN, USA; 26-cm transaxial field of view (FOV), 8-cm axial FOV; 2.1-mm isotropic reconstructed resolution). Subjects were transported from the YNPRC Field Station to the YNPRC Imaging Center the morning of their scan. All procedures were standardized across subjects to minimize the stress-inducing effects of temporary social separation and transport. All subjects had an IV catheter placed for radioligand infusion and hydration fluids and were scanned supine with the head positioned to standardized coordinates. All PET scans were done under isoflurane anesthesia and all subjects received a 5–8 min pump infusion of 5.21–5.75 mCI of 18F-flumazenil. The YNPRC veterinarian staff monitored anesthesia, heart rate, blood oxygenation, and respiration throughout the duration of the scanning period. During the experimental period, the YNPRC Field Station acquired a Siemens P4 microPET scanner (Concorde Microsystems, Knoxville, TN, USA) with which the 5 remaining subjects were scanned using identical procedures and staff. Our previously published PET neuroimaging study that was affected by the same change in the YNPRC Imaging Center found instrumentation bias due to differences in the performance of the microPET scanners (Embree et al, 2012). In order to control for this bias we normalized data by calculating the z-distribution for each scanner and converting the mean and variation of the data from the P4 scanner to that of the Focus 220 distribution (Embree et al, 2012). Data from each PET scan were combined into 21 frames and an image reconstructed as previously described (Embree et al, 2012). Both PET images for each subject were summed across frames and then manually rigid-body registered to her structural MRI image using in-house scripts written in IDL (Embree et al, 2012). Regions of interest (ROIs) were manually drawn on an individual subject’s MRI image and then transferred to the PET images (Embree et al, 2012). BPND was calculated by the generation of time-activity curves and Logan analysis (Embree et al, 2012; Logan et al, 1990) using the pons as the reference region similar to methods used in clinical populations (Geuze et al, 2008; Klumpers et al, 2008).
MRI imaging
Each subject received a T1-weighted structural MRI scan to allow PET scan co-registration at the YNPRC Imaging Center. MRI scans were acquired under anesthesia (1–1.5% isoflurane, inhalation to effect) using a 3 T Siemens scanner, an 8-channel phase array knee coil and a T1-weighted MPRAGE sequence (TI/TR/TE = 950/3000/3.49 ms, FOV = 96 mm, eight averages) with a 0.5 × 0.5 × 0.5-mm3 voxel size. MRI images were reconstructed into 3D volumes and rigid-body registered to a rhesus monkey template (Parr et al, 2012) that was aligned to the Wisconsin 112RM-SL rhesus T1-atlas to allow for drawing of the ROIs in the Saleem and Logothetis rhesus macaque brain atlas space (Saleem and Logothetis, 2007).
ROI drawing
ROIs were based on procedures and neuroanatomical definitions previously published in rhesus by our group (Embree et al, 2012; Parr et al, 2012). Rhesus macaque brain atlases (Paxinos et al, 2000; Saleem et al, 2007) were used to guide ROI tracing within structural MRI images in coronal and sagittal views (Embree et al, 2012). The prefrontal cortex was drawn, including the medial and dorsolateral prefrontal cortex, the anterior cingulate cortex, and the orbitofrontal cortex. Structures in the temporal lobe (amygdala and hippocampus), and the hypothalamus were also drawn. All of these regions have been implicated in the regulation of emotional and stress-related behavior, contribute to LHPA axis regulation, and express GABAAR (Herman et al, 2004; Mody et al, 2011; Sarkar et al, 2011; Serra et al, 2000; Skerritt et al, 1981; Skilbeck et al, 2010). The pons was drawn and used as a reference region following previously published protocols (Geuze et al, 2008; Klumpers et al, 2008).
Statistical analysis
The main effects of status (dominant vs. subordinate) and treatment (astressin B vs. saline) and the interaction between these factors on GABAAR BPND in the prefrontal cortex, temporal lobe, and hypothalamus were analyzed by analysis of variance for repeated measures and post-hoc t-tests conducted when necessary. A test result with a p ≤ 0.05 was considered significant. Bivariate correlations were conducted to assess the association between GABAAR BPND binding within each of the three ROIs assessed under the saline condition.
Results
Rate of agonistic behavior
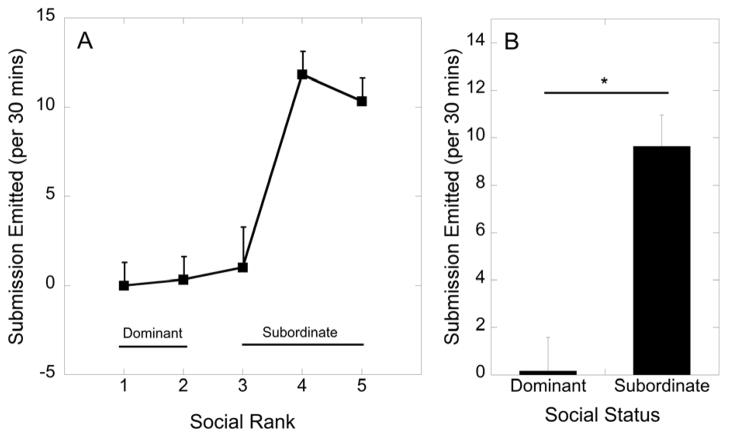
The amount of submission emitted by the animals that participated in the current study was significantly influenced by rank (F 4, 8 = 17.9, p < 0.001; Figure 1A). Categorization of females ranked 1 and 2 as dominant and 3–5 as subordinate yielded a significant effect of social status on the amount of submission emitted, as subordinate females emitted more submission than dominant females (F 1, 11 = 23.9, p < 0.001; Figure 1B).
Figure 1.
(A) Mean ± SEM frequency of submission emitted per 30 minutes by females at each dominance position. (B) Mean ± SEM frequency of submission emitted per 30 minutes by females categorized as dominant and subordinate. Asterisk denotes that subordinate females emit more submission that dominant females (p < 0.001).
GABAAR BPND
GABAAR BPND in the prefrontal cortex was significantly affected by a status x treatment interaction (F 1, 15 = 4.40, p = 0.05). During saline administration, subordinate females exhibited significantly higher levels of GABAAR BPND in the prefrontal cortex than did dominant females (p = 0.033; Figure 2A). Administration of astressin B eliminated this status difference, as GABAAR BPND in the prefrontal cortex following astressin B treatment was not different between dominant and subordinate females (p > 0.05; Figure 2A). GABAAR BPND in the temporal lobe (F 1, 15 = 2.26, p = 0.15; Figure 2B) and in the hypothalamus (F 1, 15 = 0.17, p = 0.68; Figure 2C) was not significantly affected by a status x treatment interaction, or by a main effect of social status or treatment (p > 0.05). GABAAR BPND during the E2, saline treatment condition in the prefrontal cortex was significantly correlated with GABAAR BPND in the temporal lobe (r=0.78; p<0.001) and in the hypothalamus (r=0.97; p<0.001).
Figure 2.
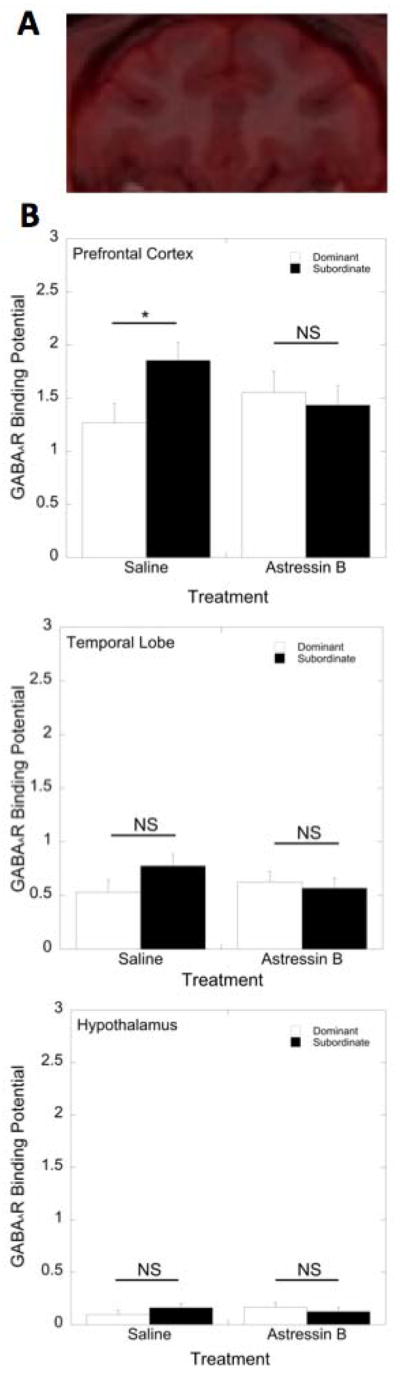
(A) Representative example of GABAAR binding in prefrontal regions: co-registration of PET and MRI images. (B) Mean ± SEM GABAAR BPND between dominant (open bars) and subordinate (closed bars) females in the prefrontal cortex, temporal lobe, and hypothalamus during the saline and astressin B treatment conditions. Asterisk indicates that subordinate females have increased GABAAR BPND in the prefrontal cortex compared to dominant females (p < 0.05).
Discussion
The current data indicate that social subordination in female rhesus monkeys results in increased GABAAR binding potential in the prefrontal cortex, a brain region important for emotional and LHPA axis regulation (Koenigs and Grafman, 2009; McEwen et al, 2012), during E2 replacement. GABAAR binding did not differ in the amygdala, hippocampus, and hypothalamus of E2-replaced dominant and subordinate females, GABAAR binding in these brain regions were significantly positively correlated with GABAAR binding in the prefrontal cortex, suggesting GABAAR expression in these regions are interdependent. Additionally, our data indicate that administration of a CRHR1/2 antagonist (astressin B) reduced GABAAR BPND in the prefrontal cortex of subordinate females to levels observed in dominant females. Thus, these data extend our previous observations of E2-mediated phenotypic differences between dominant and subordinate adult female rhesus monkeys (Asher et al, 2012; Michopoulos et al, 2011b; Reding et al, 2012) and suggest that these differences could be explained in part by stress-induced alterations in E2’s ability to modulate GABAergic tone in the prefrontal cortex.
Exposure to both acute and chronic stressors alters the function of the GABAergic neurotransmitter system, altering the expression of GABA and GAD in the hypothalamus, hippocampus, and BNST (Bowers et al, 1998; Cullinan et al, 2008) and changing GABAAR expression throughout the brain (Mody et al, 2011; Skilbeck et al, 2010). Although stress-related psychopathology in human populations (e.g. PTSD and major depression) is characterized by region-specific decreases in GABAAR binding potential (Geuze et al, 2008; Klumpers et al, 2010), these decreases occur in temporal lobe regions, such as the amygdala, and imply reduced inhibitory tone in these regions are involved in the particular psychopathology. Acute psychological stressors have been shown to both increase and decrease GABAAR levels in rodents. Specifically, an acute swim stressor in mice increases GABAAR in the brain (Skerritt et al, 1981) and the use of a communication box stressor in rats decreases benzodiazepine receptor binding (Fukumitsu et al, 2002). These differences in the directionality of change in GABAAR levels are thought to be due to difference in experimental paradigms used, duration of stressors, sex and species of subjects, and laboratory context (Mody et al, 2011).
Changes in the expression of GABAAR subunits occur following stress exposure (Mody et al, 2011) and E2 administration (Herbison et al, 1995), and are variable throughout the brain, but can influence GABAergic inhibitory tone. Decreases in GABAAR 1, 2, subunit expression and increases in 5 subunit expression in the paraventricular nucleus (PVN) of the hypothalamus (Verkuyl et al, 2004) and increases in subunit expression in the hippocampus are noted in rats following stress exposure (Maguire and Mody, 2007). GABAARs containing subunits are expressed on CRH neurons in the PVN and critical for the regulation of the LHPA axis via tonic extrasynaptic GABAergic function (Sarkar et al, 2011). Thus, the changes in GABAAR binding to flumazenil described in the current study could be a result of altered GABAAR subunit composition in response to E2 in the PFC of subordinate females. Flumazenil is a benzodiazepine antagonist that binds at the interface between the and subunit and has a higher affinity for 4 and 6 subunits (Votey et al, 1991; Wafford et al, 1996). GABAARs containing 4 and a6 subunits are found in low levels in the hippocampus and cortex in rats (Wisden et al, 1992), are insensitive to benzodiazepines, and are modulated by neurosteroids (Wafford et al, 1996). Levels of 4 and a6 subunits are attenuated in chronic stress states in rodents (Serra et al, 2000) and in women (Klatzkin et al, 2006; Uzunova et al, 1998). Taken together, these data suggest that the increase in the GABAAR binding during E2 replacement in response to social subordination described in the current study is due to a reorganization of GABAAR subunit expression rather than a change in absolute levels of GABAAR, similar to what has been shown in mice chronic following psychosocial stress exposure (Poulter et al, 2010). The role of hormones in affecting stress-induced alterations in GABAAR levels is strengthened by the current findings wherein all females received acute E2 administration to assess whether social subordination influenced E2’s ability to modify the GABAergic system.
The administration of a CRH receptor antagonist, astressin B, reduced GABAAR binding in subordinates and reversed the status difference between dominant and subordinate animals in E2-induced GABAAR binding in the PFC. Astressin B is a peptide antagonist of CRHR1/2, indicating that blockage of pituitary CRHR1/2 resulting from peripheral administration resulted in decreases of GABAAR BPND in the PFC of subordinate females treated with E2. Astressin B administration has previously been shown to decrease peripheral cortisol levels (Michopoulos et al, 2010) in subordinate female rhesus macaques that show a dysregulation of LHPA activity (Arce et al, 2010; Collura et al, 2009; Michopoulos et al, 2012b; Wilson et al, 2008). Glucocorticoids are indeed capable of influencing the activity of the GABAergic system, as corticosterone in rats decreases miniature inhibitory postsynaptic currents (Verkuyl and Joels, 2003) and the number of GABAergic synapses on CRH neurons in the PVN (Miklos and Kovacs, 2002). Furthermore, the ability of astressin B to alter E2-induced changes in GABAAR binding in the PFC might be linked to changes in glucocorticoid (GR) and mineralocorticoid (MR) receptors, as both GRs and MRs are highly expressed in the PFC of macaques (Sanchez et al, 2000). Overall, the finding that peripheral administration of astressin B normalized GABAAR binding in response to E2 administration in subordinate females warrants further investigation as to whether astressin B can directly influence GABAAR expression, subunit reorganization, and function, or whether astressin B induces alterations in LHPA physiology, GR and MR levels, or behavior that modify the GABAergic system.
In conclusion, social subordination in ovariectomized female rhesus monkeys given E2 replacement results in region-specific alteration in the central GABAA neurotransmitter system that can be reversed via peripheral administration of a CRHR1/2 antagonist. The ubiquitous nature of GABAAR expression (Laurie et al, 1992a; Laurie et al, 1992b; Wisden et al, 1992) and the small sample size used in the current study could have influenced our ability to detect small and yet biologically significant differences in GABAAR BPND in the other regions of interest. The strong correlation between GABAAR BPND in all regions assessed in the current study could indicate that social subordination results in global alterations of GABAergic function during E2 replacement. However, follow-up studies using radioligands specific for particular GABAAR subunits that are known to be altered with stress exposure, such as the subunit (Mody et al, 2011), are necessary to elucidate how social subordination and E2 influence GABAergic tone in a region-specific manner. These studies are critical for determining how subordination-induced alterations in E2 modulation of GABAAR binding influence the changes in behavioral and physiological sensitivity to E2 characteristic of subordinate status in female macaques (Michopoulos et al, 2009; Michopoulos et al, 2011b; Reding et al, 2012; Wallen, 1990). A limitation of the current study is that animals were not studied under a non-E2 condition, thus limiting us in our interpretation of the data as it relates to E2’s direct effects on the GABAergic system. Additionally, because progesterone, via its metabolite allopregnanolone, can act to alter the activity of both the LHPA axis and the GABAergic system (Mody et al, 2011), it is important that further studies are done to assess how progesterone levels influence psychosocially-induced alterations in GABAAR binding. Finally, the ability for astressin B to have central effects on the GABAergic system lends support to the idea that CRH receptors antagonists could be a viable pharmacologic approach with which to attenuate the adverse effects of psychosocial stress exposure on health in women, including women with stress-induced anovulation that have elevated central levels of cortisol (Brundu et al, 2006). Social subordination in female macaques is a valid ethological approach with which to study these important questions.
Research Highlights.
Social subordination alters GABAARs binding in estradiol-treated female monkeys
Status effect on GABAAR is site specific, only seen in the prefrontal cortex
CRH receptor antagonism reverses status differences in GABAAR binding,
Implicates the stress axis in the dysregulation of GABAAR in subordinate females.
Provides mechanism by which subordination alters the actions of estradiol.
Acknowledgments
The study was conducted with invaluable assistance from Jennifer Whitley, Shannon Bounar, Christine Marstellar, Paul Chen, and Juliet Brown. The current study was supported by NIH HD046501 (MW), MH081816 (DT), DK026741 (JR), and in part OD P51OD011132. JR is the Dr. Frederik Paulsen Chair in Neurosciences Professor. Portions of this work were supported by an investigator-initiated grant to SLB from Pfizer Pharmaceuticals Group to study hormones and brain. The YNPRC is fully accredited by the American for the Assessment and Accreditation of Laboratory Care, International.
Footnotes
Disclosure Statement: The current study was supported by NIH HD046501 (MW), MH081816 (DT), DK026741 (JR), and in part OD P51OD011132. JR is the Dr. Frederik Paulsen Chair in Neurosciences Professor. Portions of this work were supported by an investigator-initiated grant to SLB from Pfizer Pharmaceuticals Group to study hormones and brain. SLB is a member of the University of Virginia Medical Alumni Association Board of Directors; has been on the Agile Therapeutics Medical Advisory Board; is a consultant for the AHC Media, LLC annual business meeting; is a member of the Noven Pharmaceutical Medical Advisory Board, the Watson Pharmaceutical Women’s Health Strategic Advisory Board, the Teva Pharmaceutical Industries, Ltd., Expert Advisory Board, the Pfizer International Menopause Society 2011 Conbriza Symposium, the Shionogi, Inc. Medical Advisory Board; and has provided expert testimony for Leydig, Voit & Mayer, LLC, Faegre Baker Daniels, LLC, and Goodmans LLP; receives royalties from the UpToDate Peer Review Board; has received payment of travel expenses from Agile Therapeutics, AHC Media, LLC, Noven Pharmaceuticals, Watson Pharmaceutical, Teva Pharmaceutical Industries, Ltd., Pfizer, and Shionogi, Inc.; was on the ACOG Editorial Committee, Guidelines for Women’s Health Care, the American Journal of Obstetrics and Gynecology advisory board for subspecialty neuroendocrinology and reproductive neurobiology, the Endocrine Society Endocrine Self-Assessment Program Committee and the Clinical Practice Guideline Task Force on Hypothalamic Amenorrhea, the nominating committee and the editorial board for Endocrinology, the Menopause editorial board, the ISIS CVD Network Member of the Society for Women’s Health Research, and is a past president of the Society for Gynecologic Investigation. All other authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, et al. Are subordinates always stressed? a comparative analysis of rank differences in cortisol levels among primates. Hormones and behavior. 2003;43(1):67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Altemus M, Roca C, Galliven E, Romanos C, Deuster P. Increased Vasopressin and Adrenocorticotropin Responses to Stress in the Midluteal Phase of the Menstrual Cycle. The Journal of clinical endocrinology and metabolism. 2001;86(6):2525–2530. doi: 10.1210/jcem.86.6.7596. [DOI] [PubMed] [Google Scholar]
- Arce M, Michopoulos V, Shepard KN, Ha QC, Wilson ME. Diet choice, cortisol reactivity, and emotional feeding in socially housed rhesus monkeys. Physiology & behavior. 2010;101(4):446–455. doi: 10.1016/j.physbeh.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher J, Michopoulos V, Reding KM, Wilson ME, Toufexis D. Social stress and the polymorphic region of the serotonin reuptake transporter gene modify estradiol-induced changes on central monoamine concentrations in female rhesus monkeys. Journal of neuroendocrinology. 2012 doi: 10.1111/jne.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP. The function of aggression in primate societies. Am Sci. 1974a;62(3):304–311. [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP, Rose RM. Aggression and social controls in rhesus monkey (Macaca mulatta) groups revealed in group formation studies. Folia Primatol (Basel) 1974b;21(2):81–107. doi: 10.1159/000155607. [DOI] [PubMed] [Google Scholar]
- Bodo C, Kudwa AE, Rissman EF. Both estrogen receptor-alpha and -beta are required for sexual differentiation of the anteroventral periventricular area in mice. Endocrinology. 2006;147(1):415–420. doi: 10.1210/en.2005-0834. [DOI] [PubMed] [Google Scholar]
- Bowers G, Cullinan WE, Herman JP. Region-specific regulation of glutamic acid decarboxylase (GAD) mRNA expression in central stress circuits. J Neurosci. 1998;18(15):5938–5947. doi: 10.1523/JNEUROSCI.18-15-05938.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Rivier JE, Rice KC, Woods JH. Corticotropin-releasing hormone antagonists, astressin B and antalarmin: differing profiles of activity in rhesus monkeys. Neuropsychopharmacology. 2004;29(6):1112–1121. doi: 10.1038/sj.npp.1300410. [DOI] [PubMed] [Google Scholar]
- Brundu B, Loucks TL, Adler LJ, Cameron JL, Berga SL. Increased cortisol in the cerebrospinal fluid of women with functional hypothalamic amenorrhea. The Journal of clinical endocrinology and metabolism. 2006;91(4):1561–1565. doi: 10.1210/jc.2005-2422. [DOI] [PubMed] [Google Scholar]
- Cameron OG, Huang GC, Nichols T, Koeppe RA, Minoshima S, Rose D, et al. Reduced gamma-aminobutyric acid(A)-benzodiazepine binding sites in insular cortex of individuals with panic disorder. Archives of general psychiatry. 2007;64(7):793–800. doi: 10.1001/archpsyc.64.7.793. [DOI] [PubMed] [Google Scholar]
- Collura LA, Hoffman JB, Wilson ME. Administration of human leptin differentially affects parameters of cortisol secretion in socially housed female rhesus monkeys. Endocrine. 2009 doi: 10.1007/s12020-009-9250-7. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Ziegler DR, Herman JP. Functional role of local GABAergic influences on the HPA axis. Brain Struct Funct. 2008;213(1–2):63–72. doi: 10.1007/s00429-008-0192-2. [DOI] [PubMed] [Google Scholar]
- Embree M, Michopoulos V, Votaw JR, Voll RJ, Mun J, Stehouwer JS, et al. The relation of developmental changes in brain serotonin transporter (5HTT) and 5HT1A receptor binding to emotional behavior in female rhesus monkeys: Effects of social status and 5HTT genotype. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumitsu N, Tsuchida D, Ogi S, Uchiyama M, Mori Y. 125I-iomazenil-benzodiazepine receptor binding during psychological stress in rats. Ann Nucl Med. 2002;16(3):231–235. doi: 10.1007/BF02996307. [DOI] [PubMed] [Google Scholar]
- Geuze E, van Berckel BN, Lammertsma AA, Boellaard R, de Kloet CS, Vermetten E, et al. Reduced GABAA benzodiazepine receptor binding in veterans with post-traumatic stress disorder. Molecular psychiatry. 2008;13(1):74–83. 73. doi: 10.1038/sj.mp.4002054. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Farber DM, Jenkins SL, Yen A, Winter JA, Tame JD, et al. Opposing effects of androgen and estrogen on pituitary-adrenal function in nonpregnant primates. Biology of reproduction. 2000;62(5):1445–1451. doi: 10.1095/biolreprod62.5.1445. [DOI] [PubMed] [Google Scholar]
- Grant KA, Shively CA, Nader MA, Ehrenkaufer RL, Line SW, Morton TE, et al. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse (New York, NY. 1998;29(1):80–83. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Grunder G, Siessmeier T, Lange-Asschenfeldt C, Vernaleken I, Buchholz HG, Stoeter P, et al. [18F]Fluoroethylflumazenil: a novel tracer for PET imaging of human benzodiazepine receptors. Eur J Nucl Med. 2001;28(10):1463–1470. doi: 10.1007/s002590100594. [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Miranda-Martinez A, Neri-Gomez T, Camacho-Arroyo I. Sex steroids effects on the content of GAD, TH, GABA(A), and glutamate receptors in the olfactory bulb of the male rat. Neurochemical research. 2008;33(8):1568–1573. doi: 10.1007/s11064-008-9665-1. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Fenelon VS. Estrogen regulation of GABAA receptor subunit mRNA expression in preoptic area and bed nucleus of the stria terminalis of female rat brain. J Neurosci. 1995;15(3 Pt 2):2328–2337. doi: 10.1523/JNEUROSCI.15-03-02328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Mueller NK, Figueiredo H. Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Annals of the New York Academy of Sciences. 2004;1018:35–45. doi: 10.1196/annals.1296.004. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiology & behavior. 2008;93(4–5):807–819. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Hormones and behavior. 2006;49(2):197–205. doi: 10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and biobehavioral reviews. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Chen H, Appt SE, Lees CJ, Franke AA, Berga SL, et al. Impairment of ovarian function and associated health-related abnormalities are attributable to low social status in premenopausal monkeys and not mitigated by a high-isoflavone soy diet. Human reproduction (Oxford, England) 2010;25(12):3083–3094. doi: 10.1093/humrep/deq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Status, stress, and atherosclerosis: the role of environment and individual behavior. Annals of the New York Academy of Sciences. 1999;896:145–161. doi: 10.1111/j.1749-6632.1999.tb08112.x. [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Weick RF, Butler WR, Dierschke DJ, Krey LC, Weiss G, et al. Induced LH surges in the rhesus monkey: strength-duration characteristics of the estrogen stimulus. Endocrinology. 1973;92(6):1740–1747. doi: 10.1210/endo-92-6-1740. [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Morrow AL, Light KC, Pedersen CA, Girdler SS. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. 2006;31(10):1208–1219. doi: 10.1016/j.psyneuen.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Klumpers UM, Veltman DJ, Boellaard R, Comans EF, Zuketto C, Yaqub M, et al. Comparison of plasma input and reference tissue models for analysing [(11)C]flumazenil studies. J Cereb Blood Flow Metab. 2008;28(3):579–587. doi: 10.1038/sj.jcbfm.9600554. [DOI] [PubMed] [Google Scholar]
- Klumpers UM, Veltman DJ, Drent ML, Boellaard R, Comans EF, Meynen G, et al. Reduced parahippocampal and lateral temporal GABAA-[11C]flumazenil binding in major depression: preliminary results. Eur J Nucl Med Mol Imaging. 2010;37(3):565–574. doi: 10.1007/s00259-009-1292-9. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15(5):540–548. doi: 10.1177/1073858409333072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992a;12(3):1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992b;12(11):4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10(5):740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- Lunga P, Herbert J. 17beta-oestradiol modulates glucocorticoid, neural and behavioural adaptations to repeated restraint stress in female rats. Journal of neuroendocrinology. 2004;16(9):776–785. doi: 10.1111/j.1365-2826.2004.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27(9):2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Alves SE, Bulloch K, Weiland NG. Ovarian steroids and the brain: implications for cognition and aging. Neurology. 1997;48(Suppl 7):S8–S15. doi: 10.1212/wnl.48.5_suppl_7.8s. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62(1):3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Berga SL, Kaplan JR, Wilson ME. Social subordination and polymorphisms in the gene encoding the serotonin transporter enhance estradiol inhibition of luteinizing hormone secretion in female rhesus monkeys. Biology of reproduction. 2009;81(6):1154–1163. doi: 10.1095/biolreprod.109.079038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Berga SL, Wilson ME. Estradiol and progesterone modify the effects of the serotonin reuptake transporter polymorphism on serotonergic responsivity to citalopram. Exp Clin Psychopharmacol. 2011a;19(6):401–408. doi: 10.1037/a0025008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Checchi M, Sharpe D, Wilson ME. Estradiol effects on behavior and serum oxytocin are modified by social status and polymorphisms in the serotonin transporter gene in female rhesus monkeys. Hormones and behavior. 2011b;59(4):528–535. doi: 10.1016/j.yhbeh.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Higgins M, Toufexis D, Wilson ME. Social subordination produces distinct stress-related phenotypes in female rhesus monkeys. Psychoneuroendocrinology. 2012a;37(7):1071–1085. doi: 10.1016/j.psyneuen.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Loucks T, Berga SL, Rivier J, Wilson ME. Increased ghrelin sensitivity and calorie consumption in subordinate monkeys is affected by short-term astressin B administration. Endocrine. 2010;38(2):227–234. doi: 10.1007/s12020-010-9378-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Reding KM, Wilson ME, Toufexis D. Social subordination impairs hypothalamic-pituitary-adrenal function in female rhesus monkeys. Hormones and behavior. 2012b doi: 10.1016/j.yhbeh.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Toufexis D, Wilson ME. Social stress interacts with diet history to promote emotional feeding in females. Psychoneuroendocrinology. 2012c;37(9):1479–1490. doi: 10.1016/j.psyneuen.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Wilson ME. Body weight decreases induced by estradiol in female rhesus monkeys are dependent upon social status. Physiology & behavior. 2011c;102(3–4):382–388. doi: 10.1016/j.physbeh.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklos IH, Kovacs KJ. GABAergic innervation of corticotropin-releasing hormone (CRH)-secreting parvocellular neurons and its plasticity as demonstrated by quantitative immunoelectron microscopy. Neuroscience. 2002;113(3):581–592. doi: 10.1016/s0306-4522(02)00147-1. [DOI] [PubMed] [Google Scholar]
- Mody I, Maguire J. The reciprocal regulation of stress hormones and GABA(A) receptors. Front Cell Neurosci. 2011;6:4. doi: 10.3389/fncel.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nature neuroscience. 2002;5(2):169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Paiardini M, Hoffman J, Cervasi B, Ortiz AM, Stroud F, Silvestri G, et al. T-cell phenotypic and functional changes associated with social subordination and gene polymorphisms in the serotonin reuptake transporter in female rhesus monkeys. Brain Behav Immun. 2009;23(2):286–293. doi: 10.1016/j.bbi.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, Boudreau M, Hecht E, Winslow JT, Nemeroff CB, Sanchez MM. Early life stress affects cerebral glucose metabolism in adult rhesus monkeys (Macaca mulatta) Dev Cogn Neurosci. 2012;2(1):181–193. doi: 10.1016/j.dcn.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Toga AW. The rhesus monkey brain in stereotaxic coordinates. Academic Press; San Diego: 2000. [Google Scholar]
- Pfaff DW, Vasudevan N, Kia HK, Zhu YS, Chan J, Garey J, et al. Estrogens, brain and behavior: studies in fundamental neurobiology and observations related to women’s health. Journal of Steroid Biochemistry & Molecular Biology. 2000;74(5):365–373. doi: 10.1016/s0960-0760(00)00114-x. [DOI] [PubMed] [Google Scholar]
- Poulter MO, Du L, Zhurov V, Merali Z, Anisman H. Plasticity of the GABA(A) receptor subunit cassette in response to stressors in reactive versus resilient mice. Neuroscience. 2010;165(4):1039–1051. doi: 10.1016/j.neuroscience.2009.11.028. [DOI] [PubMed] [Google Scholar]
- Reding K, Michopoulos V, Wallen K, Sanchez M, Wilson ME, Toufexis D. Social status modifies estradiol activation of sociosexual behavior in female rhesus monkeys. Hormones and behavior. 2012;62(5):612–620. doi: 10.1016/j.yhbeh.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy BN, Reid RL, Van Vugt DA. The effects of estrogen and progesterone on corticotropin-releasing hormone and arginine vasopressin messenger ribonucleic acid levels in the paraventricular nucleus and supraoptic nucleus of the rhesus monkey. Endocrinology. 1999;140(5):2191–2198. doi: 10.1210/endo.140.5.6684. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Logothetis N. A combined MRI and histology atlas of the rhesus monkey brain in stereotaxic coordinates. Academic Press; London; Burlington, MA: 2007. [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci. 2000;20(12):4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar J, Wakefield S, MacKenzie G, Moss SJ, Maguire J. Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J Neurosci. 2011;31(50):18198–18210. doi: 10.1523/JNEUROSCI.2560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, et al. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABA(A) receptor function in rat brain. Journal of neurochemistry. 2000;75(2):732–740. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- Shively C, Kaplan J. Effects of social factors on adrenal weight and related physiology of Macaca fascicularis. Physiology & behavior. 1984;33(5):777–782. doi: 10.1016/0031-9384(84)90047-7. [DOI] [PubMed] [Google Scholar]
- Shively CA. Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biological psychiatry. 1998;44(9):882–891. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- Skerritt JH, Trisdikoon P, Johnston GA. Increased GABA binding in mouse brain following acute swim stress. Brain research. 1981;215(1–2):398–403. doi: 10.1016/0006-8993(81)90524-2. [DOI] [PubMed] [Google Scholar]
- Skilbeck KJ, Johnston GA, Hinton T. Stress and GABA receptors. Journal of neurochemistry. 2010;112(5):1115–1130. doi: 10.1111/j.1471-4159.2009.06539.x. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002;27(1–2):99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- Tan XJ, Dai YB, Wu WF, Kim HJ, Barros RP, Richardson TI, et al. Reduction of dendritic spines and elevation of GABAergic signaling in the brains of mice treated with an estrogen receptor beta ligand. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(5):1708–1712. doi: 10.1073/pnas.1121162109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J, Barreiro LB, Johnson ZP, Hansen KD, Michopoulos V, Toufexis D, et al. Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(17):6490–6495. doi: 10.1073/pnas.1202734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uphouse L, Selvamani A, Lincoln C, Morales L, Comeaux D. Mild restraint reduces the time hormonally primed rats spend with sexually active males. Behavioural brain research. 2005;157(2):343–350. doi: 10.1016/j.bbr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, et al. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(6):3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkuyl JM, Hemby SE, Joels M. Chronic stress attenuates GABAergic inhibition and alters gene expression of parvocellular neurons in rat hypothalamus. The European journal of neuroscience. 2004;20(6):1665–1673. doi: 10.1111/j.1460-9568.2004.03568.x. [DOI] [PubMed] [Google Scholar]
- Verkuyl JM, Joels M. Effect of adrenalectomy on miniature inhibitory postsynaptic currents in the paraventricular nucleus of the hypothalamus. Journal of neurophysiology. 2003;89(1):237–245. doi: 10.1152/jn.00401.2002. [DOI] [PubMed] [Google Scholar]
- Votey SR, Bosse GM, Bayer MJ, Hoffman JR. Flumazenil: a new benzodiazepine antagonist. Ann Emerg Med. 1991;20(2):181–188. doi: 10.1016/s0196-0644(05)81219-3. [DOI] [PubMed] [Google Scholar]
- Vulliemoz NR, Xiao E, Xia-Zhang L, Rivier J, Ferin M. Astressin B, a nonselective corticotropin-releasing hormone receptor antagonist, prevents the inhibitory effect of ghrelin on luteinizing hormone pulse frequency in the ovariectomized rhesus monkey. Endocrinology. 2008;149(3):869–874. doi: 10.1210/en.2007-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human gamma-aminobutyric acidA receptors containing the alpha 4 subunit. Molecular pharmacology. 1996;50(3):670–678. [PubMed] [Google Scholar]
- Wallen K. Desire and ability: hormones and the regulation of female sexual behavior. Neuroscience and biobehavioral reviews. 1990;14(2):233–241. doi: 10.1016/s0149-7634(05)80223-4. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Olfson M. Depression in women: implications for health care research. Science (New York, NY. 1995;269(5225):799–801. doi: 10.1126/science.7638596. [DOI] [PubMed] [Google Scholar]
- White S, Uphouse L. Estrogen and progesterone dose-dependently reduce disruptive effects of restraint on lordosis behavior. Hormones and behavior. 2004;45(3):201–208. doi: 10.1016/j.yhbeh.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ. Quantifying food intake in socially housed monkeys: social status effects on caloric consumption. Physiology & behavior. 2008;94(4):586–594. doi: 10.1016/j.physbeh.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Pazol K, Legendre A, Fisher J, Chikazawa K. Gonadal steroid modulation of the limbic - hypothalamic - pituitary - adrenal (LHPA) axis is influenced by social status in female rhesus monkeys. Endocrine. 2005;26(2) doi: 10.1385/ENDO:26:2:089. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12(3):1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]