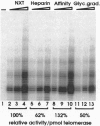
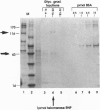
Abstract
Telomerase is a ribonucleoprotein enzyme that uses its internal RNA moiety as a template for synthesis of telomeric repeats at chromosome ends. Here we report the purification of telomerase from Euplotes aediculatus by affinity chromatography with antisense 2'-O-methyl oligonucleotides, a method that was developed for small nuclear ribonucleoprotein particles (snRNPs). Elution of bound ribonucleoprotein from the antisense oligonucleotide under nondenaturing conditions was achieved by a novel approach, using a displacement oligonucleotide. Polypeptides of 120 kDa and 43 kDa (a doublet) copurify with the active telomerase and appear stoichiometric with telomerase RNA. A simple model for DNA end replication predicts that after semiconservative DNA replication, telomerase will extend the newly synthesized, blunt-ended leading strand. We show that purified Euplotes telomerase has no activity with blunt-ended primers. Instead, efficient extension requires 4 to 6 single-stranded nucleotides at the 3' end. Therefore, this model predicts the existence of other activities such as helicases or nucleases that generate a single-stranded 3' end from a blunt end, thus activating the end for telomerase extension.
Full text
PDF

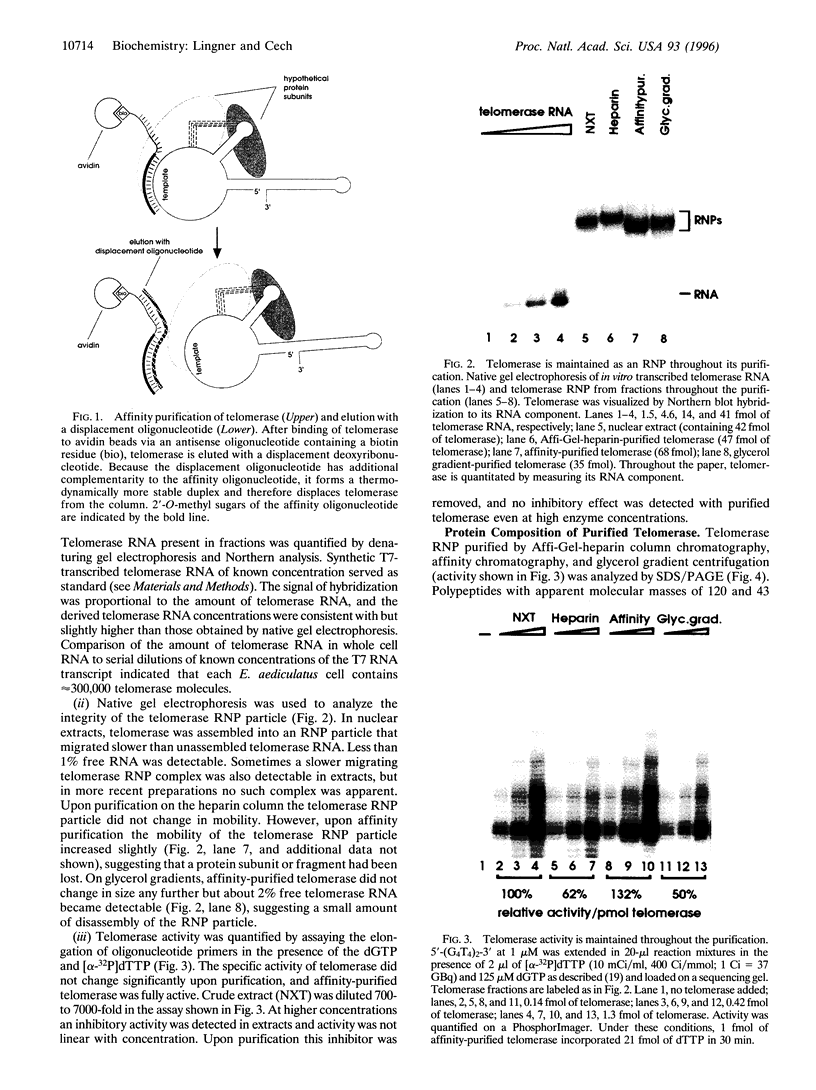
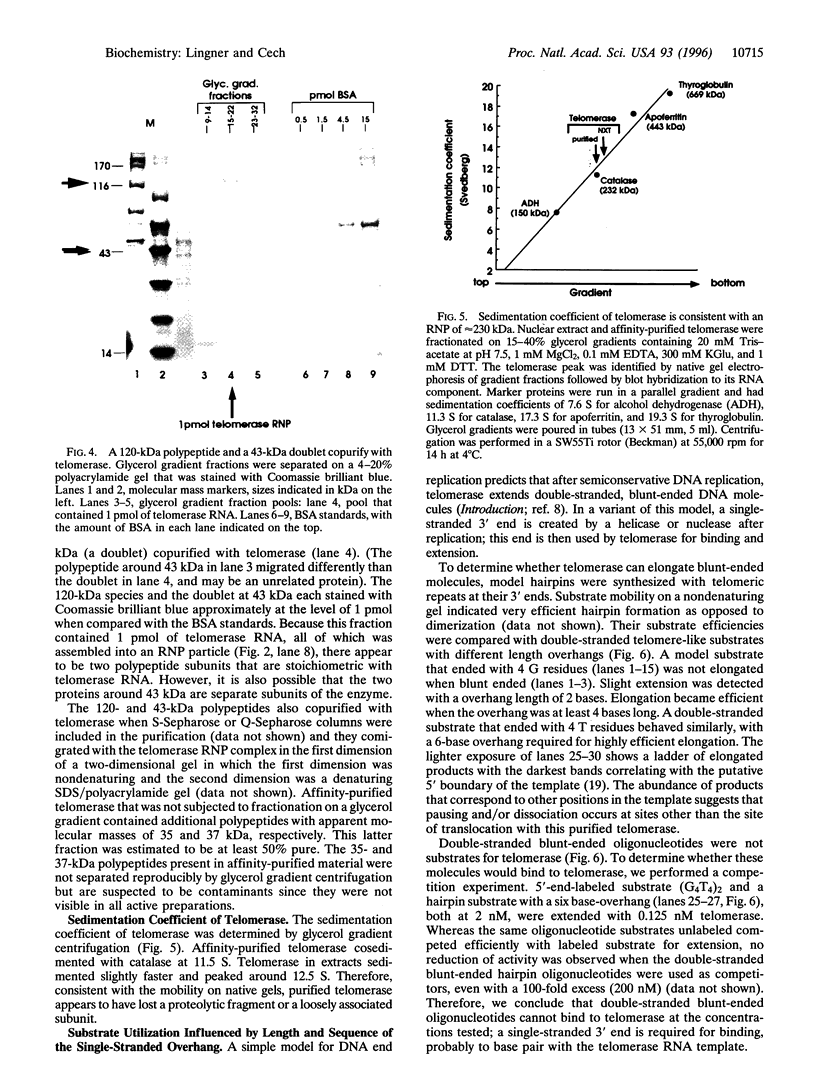

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autexier C., Greider C. W. Functional reconstitution of wild-type and mutant Tetrahymena telomerase. Genes Dev. 1994 Mar 1;8(5):563–575. doi: 10.1101/gad.8.5.563. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Telomerases. Annu Rev Biochem. 1992;61:113–129. doi: 10.1146/annurev.bi.61.070192.000553. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Collins K., Kobayashi R., Greider C. W. Purification of Tetrahymena telomerase and cloning of genes encoding the two protein components of the enzyme. Cell. 1995 Jun 2;81(5):677–686. doi: 10.1016/0092-8674(95)90529-4. [DOI] [PubMed] [Google Scholar]
- Fang G., Cech T. R. Telomerase RNA localized in the replication band and spherical subnuclear organelles in hypotrichous ciliates. J Cell Biol. 1995 Jul;130(2):243–253. doi: 10.1083/jcb.130.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley D., Lee M. S., Blackburn E. H. Altering specific telomerase RNA template residues affects active site function. Genes Dev. 1995 Sep 15;9(18):2214–2226. doi: 10.1101/gad.9.18.2214. [DOI] [PubMed] [Google Scholar]
- Greenwood S. J., Sogin M. L., Lynn D. H. Phylogenetic relationships within the class Oligohymenophorea, phylum Ciliophora, inferred from the complete small subunit rRNA gene sequences of Colpidium campylum, Glaucoma chattoni, and Opisthonecta henneguyi. J Mol Evol. 1991 Aug;33(2):163–174. doi: 10.1007/BF02193631. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985 Dec;43(2 Pt 1):405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Greider C. W. Telomere length regulation. Annu Rev Biochem. 1996;65:337–365. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- Henderson E. R., Blackburn E. H. An overhanging 3' terminus is a conserved feature of telomeres. Mol Cell Biol. 1989 Jan;9(1):345–348. doi: 10.1128/mcb.9.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobutcher L. A., Swanton M. T., Donini P., Prescott D. M. All gene-sized DNA molecules in four species of hypotrichs have the same terminal sequence and an unusual 3' terminus. Proc Natl Acad Sci U S A. 1981 May;78(5):3015–3019. doi: 10.1073/pnas.78.5.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamond A. I., Sproat B., Ryder U., Hamm J. Probing the structure and function of U2 snRNP with antisense oligonucleotides made of 2'-OMe RNA. Cell. 1989 Jul 28;58(2):383–390. doi: 10.1016/0092-8674(89)90852-0. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Gallagher R. C., Bradley J., Blackburn E. H. In vivo and in vitro studies of telomeres and telomerase. Cold Spring Harb Symp Quant Biol. 1993;58:707–718. doi: 10.1101/sqb.1993.058.01.078. [DOI] [PubMed] [Google Scholar]
- Lingner J., Cooper J. P., Cech T. R. Telomerase and DNA end replication: no longer a lagging strand problem? Science. 1995 Sep 15;269(5230):1533–1534. doi: 10.1126/science.7545310. [DOI] [PubMed] [Google Scholar]
- Lingner J., Hendrick L. L., Cech T. R. Telomerase RNAs of different ciliates have a common secondary structure and a permuted template. Genes Dev. 1994 Aug 15;8(16):1984–1998. doi: 10.1101/gad.8.16.1984. [DOI] [PubMed] [Google Scholar]
- McEachern M. J., Blackburn E. H. Runaway telomere elongation caused by telomerase RNA gene mutations. Nature. 1995 Aug 3;376(6539):403–409. doi: 10.1038/376403a0. [DOI] [PubMed] [Google Scholar]
- Olovnikov A. M. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973 Sep 14;41(1):181–190. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- Prescott D. M. The DNA of ciliated protozoa. Microbiol Rev. 1994 Jun;58(2):233–267. doi: 10.1128/mr.58.2.233-267.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero D. P., Blackburn E. H. A conserved secondary structure for telomerase RNA. Cell. 1991 Oct 18;67(2):343–353. doi: 10.1016/0092-8674(91)90186-3. [DOI] [PubMed] [Google Scholar]
- Singer M. S., Gottschling D. E. TLC1: template RNA component of Saccharomyces cerevisiae telomerase. Science. 1994 Oct 21;266(5184):404–409. doi: 10.1126/science.7545955. [DOI] [PubMed] [Google Scholar]
- Swanton M. T., Greslin A. F., Prescott D. M. Arrangement of coding and non-coding sequences in the DNA molecules coding for rRNAs in Oxytricha sp. DNA of ciliated protozoa. VII. Chromosoma. 1980;77(2):203–215. doi: 10.1007/BF00329545. [DOI] [PubMed] [Google Scholar]
- Wellinger R. J., Ethier K., Labrecque P., Zakian V. A. Evidence for a new step in telomere maintenance. Cell. 1996 May 3;85(3):423–433. doi: 10.1016/s0092-8674(00)81120-4. [DOI] [PubMed] [Google Scholar]
- Wellinger R. J., Wolf A. J., Zakian V. A. Saccharomyces telomeres acquire single-strand TG1-3 tails late in S phase. Cell. 1993 Jan 15;72(1):51–60. doi: 10.1016/0092-8674(93)90049-v. [DOI] [PubMed] [Google Scholar]
- Yu G. L., Bradley J. D., Attardi L. D., Blackburn E. H. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature. 1990 Mar 8;344(6262):126–132. doi: 10.1038/344126a0. [DOI] [PubMed] [Google Scholar]
- Yudkin J. Sugar and disease. Nature. 1972 Sep 22;239(5369):197–199. doi: 10.1038/239197a0. [DOI] [PubMed] [Google Scholar]
- Zahler A. M., Prescott D. M. Telomere terminal transferase activity in the hypotrichous ciliate Oxytricha nova and a model for replication of the ends of linear DNA molecules. Nucleic Acids Res. 1988 Jul 25;16(14B):6953–6972. doi: 10.1093/nar/16.14.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakian V. A. Telomeres: beginning to understand the end. Science. 1995 Dec 8;270(5242):1601–1607. doi: 10.1126/science.270.5242.1601. [DOI] [PubMed] [Google Scholar]
- Zaug A. J., Dávila-Aponte J. A., Cech T. R. Catalysis of RNA cleavage by a ribozyme derived from the group I intron of Anabaena pre-tRNA(Leu). Biochemistry. 1994 Dec 13;33(49):14935–14947. doi: 10.1021/bi00253a033. [DOI] [PubMed] [Google Scholar]