Abstract
The analysis of airway fluid, as sampled by bronchoalveolar lavage (BAL), provides a minimally invasive route to interrogate lung biology in health and disease. Here, we used immunodepletion, coupled with gel- and label-free LC-MS/MS, for quantitation of the BAL fluid (BALF) proteome in samples recovered from human subjects following bronchoscopic instillation of saline, lipopolysaccharide (LPS) or house dust mite antigen into three distinct lung subsegments. Among more than 200 unique proteins quantified across nine samples, neutrophil granule-derived and acute phase proteins were most highly enriched in the LPS-exposed lobes. Of these, peptidoglycan response protein 1 was validated and confirmed as a novel marker of neutrophilic inflammation. Compared to a prior transcriptomic analysis of airway cells in this same cohort, the BALF proteome revealed a novel set of response factors. Independent of exposure, the enrichment of tracheal-expressed proteins in right lower lung lobes suggests a potential for constitutive intralobar variability in the BALF proteome; sampling of multiple lung subsegments also appears to aid in the identification of protein signatures that differentiate individuals at baseline. Collectively, this proof-of-concept study validates a robust workflow for BALF proteomics and demonstrates the complementary nature of proteomic and genomic techniques for investigating airway (patho)physiology.
Keywords: Bronchoalveolar lavage, proteomics, mass spectrometry
INTRODUCTION
Bronchoalveolar lavage (BAL), as sampled by fiber-optic bronchoscopy, remains an important mechanism for collection and analysis of the airway cells, proteins and metabolites. The soluble protein component of BAL fluid (BALF) is dominated by plasma-derived proteins, as well as resident proteins that are secreted from the airway epithelium and immune responsive cells. Analysis of BALF proteins using immunoassays that target known cytokines, chemokines or growth factors has enabled phenotyping of numerous pulmonary diseases. Proteomic analysis of BALF has traditionally been viewed as a complementary approach for discovery-based analysis of airway lining fluid in health and disease1-4, but its promise has not been fully realized.
Despite a large number of prior studies, there is no consensus as to the best analytical approach for quantitation of the BALF proteome. The large dynamic range of protein abundance in BALF, and the high concentrations of plasma proteins (e.g. albumin and immunoglobulins), suggests that immunodepletion of abundant plasma proteins should be a requisite step in such analysis; however, this technique is still only occasionally employed as a means of improving depth-of-coverage5-7. Historically, two-dimensional gel electrophoresis (2D-GE) has been most often employed to resolve and visualize the BALF proteome6, 8-11, and it has shown utility for the unbiased identification of disease-associated changes in the BALF proteome12-18. More recently, the identification and quantitation of BALF proteins has been coupled to shotgun proteomics with or without separation of proteins by SDS-PAGE19-26. Increasingly, these approaches have been combined with label-free quantitation for the comparison of BALF proteome changes in environmental exposure and in disease19, 23, 27, 28.
Here, using a methodology recently validated for serum proteomics29, we sought to better explore the capabilities of gel- and label-free LC-MS/MS for proteome profiling of human BALF. As proof-of-concept, we chose a subset of BALF samples derived from a well-defined cohort of individuals exposed to saline, E. coli lipopolysaccharide (LPS) or house dust mite antigen (HDM) in three distinct lung subsegments30. Importantly, the transcriptomes of BAL cells and airway epithelia had been previously determined in these subjects, allowing us to investigate whether gene expression changes in airway cells might correlate with protein level changes in BALF and to determine whether proteomic analysis might yield any new information with regard to the response of the airways to inflammatory insults.
MATERIALS AND METHODS
Human samples
Exposure studies were previously performed under an approved institutional review board (IRB) protocol30. Three randomly selected normal, non-atopic, non-asthmatic subjects were selected for proteomic analysis. Briefly, in the following order: 10 ml of normal saline (SAL) was instilled into the right lower lobe (RLL) subsegmental bronchus; 10 ml of LPS (40 EU/kg) was instilled into a right middle lobe (RML) subsegmental bronchus; and 10 ml of a solution of D. farinae house dust mite antigen containing was instilled into a subsegmental bronchus of the lingula lobe. Repeat bronchoscopy was performed 4 h following the initial instillation, and BAL of the RLL, RML and lingula, subsegmental bronchi was with 6 sequential instillations of 20 ml of saline. The first aliquot was discarded to maximize alveolar sampling and the remaining aliquots were pooled. Cell-free supernatants were stored at −80°C. Cell counts and cytokine measurements were previously performed on these samples (Table S1).
BALF processing
Approximately 12 ml of BALF per sample was thawed, and 100 μl of protease inhibitor cocktail (Sigma P8340) was added. Samples were concentrated to ~100 μl with a 10 kDa cutoff Amicon Ultra-4 centrifugal filter (Millipore). Bradford assays were performed, and samples were diluted to 525 μl with Buffer A (Agilent Technologies) and filtered using a 0.2 μm spin filter. Samples were immunodepleted using a MARS14 LC column (Agilent) and Agilent 1100 HPLC. The unbound fraction (i.e. flow-through) was concentrated and exchanged against 50 mM ammonium bicarbonate, pH 8.0 (AMBIC). 5-10 μg of protein was reduced with 10 mM DTT in 0.1 % w/v RapiGest (Waters) at 80 °C for 10 min followed by alkylation with 20 mM iodoacetamide in the dark for 30 min. Sequencing grade trypsin was added (1:50 w/w) and protein was digested overnight at 37 °C with mixing. Following digestion, samples were adjusted to 1% v/v trifluoroacetic acid and 2% v/v acetonitrile and incubated at 60 °C for 2 h. Following centrifugation at 20,000 xg for 5 min, samples were transferred to Maximum Recovery LC vials (Waters), and 50 fmol of MassPREP ADH digestion standard (Waters) was added per μg of BALF protein.
LC-MS/MS analysis
Peptide digests were analyzed using a nanoAcquity UPLC system coupled to a Synapt G1 HDMS mass spectrometer (Waters). 1 μg digest was trapped on a 20 μm × 180 mm Symmetry C18 column (Waters) at 20 μl/min for 2 min in water containing 0.1% formic acid (FA), and further separated on a 75 μm × 250 mm column with 1.7 μm C18 BEH particles (Waters) using a gradient of 5 to 40% ACN/0.1% FA over 90 min at a flow rate of 0.3 μl/min and a column temp of 45 °C. Samples were first analyzed once each in data-dependent (DDA) mode and twice in data-independent (MSE) mode (run order given in column headings, Table S2). DDA analyses used a 0.9 s precursor scan followed by MS/MS product ion scans on the top 3 most intense ions using a dynamic exclusion window of 120 s. MSE analyses used 0.9 s cycle time alternating between low collision energy (6 V) and high collision energy ramp (15 to 40 V).
Label-free quantitation
Data was processed using Rosetta Elucidator v3.3 (Rosetta Biosoftware). Briefly, LC-MS runs were aligned by accurate-mass and retention time as described previously29. Feature intensities for each injection were subjected to robust median scaling (top and bottom 10% excluded) to generate a single intensity measurement for each feature (accurate mass and retention time pair) in each sample. Peptide/protein identifications were made with Mascot v2.2 (Matrix Sciences) or Protein Lynx Global Server v 2.5 (PLGS; Waters) for data-dependent and MSE acquisitions, respectively. Searches were performed against the Swiss-Prot database v57.1, with human taxonomy and containing a reverse decoy database. Mascot search parameters were 20 ppm precursor and 0.04 Da product ion tolerance, with oxidation (M), deamidation (NQ) and hydroxylation (P). Peptide identifications were accepted if they had a <1% FDR as determined by decoy database searching and the PeptideTeller algorithm in Elucidator. After filtering for high quality features based on chromatographic peak-shape (peak time score >0.75), 2777 peptides were quantified (Table S2). Finally, peptide intensities for each protein were summed on a per-sample basis to generate aggregate protein intensities for 441 unique proteins (Table S3). Fold-changes were calculated based on mean intragroup protein intensities and p-values for binary comparisons were calculated by an unpaired t-test with Benjamini Hochberg FDR correction using log2-transformed data within the Rosetta Elucidator package.
Antibody-based assays
Enzyme-linked immunoassay (ELISA) for peptidoglycan recognition protein (PGRP1) was assayed using the Human PGRP-S DuoSet (Ramp;D Systems; DY2590) according to the manufacturer’s instructions. BALF was diluted up to 40-fold with 1% bovine serum albumin in PBS prior to incubation with capture antibody. Bactericidal/permeability-increasing protein-like 1 (BPIL1) was measured in concentrated BALF using 1:1000 rabbit anti-BPIL1 antibody (Proteintech; 13461-2-AP).
RESULTS
Human BALF samples
We randomly selected matched BALF samples that had been collected from three non-atopic, non-asthmatic subjects after bronchoscopic instillation of normal saline (SAL) in the right lower lobe (RLL), E. coli LPS in the right middle lobe (RML) and house dust mite antigen in the lingula (i.e. nine total samples). Data had been previously collected on these subjects as part of an airway transcriptome (mRNA) analysis of allergic asthma30, and the cell free BALF supernatants were subsequently stored at −80 °C. Among these subjects, the volume of fluid recovered from a 100 ml lavage averaged only ~34 ml from the RLL but 62 and 74 mls, respectively, from the RML and lingula (Table 1 and Table S1). The smaller return from BAL of the lower lobes versus other subsegments is well known31. The percentage of polymorphonuclear BAL cells (%PMNs) was highest in the LPS-instilled RML but was also elevated in the RLL from two of the three subjects (Table 1). In addition, levels of the inflammatory cytokine interleukin-6 (IL-6) were markedly elevated in LPS-instilled lobes in each of the three subjects (Table 1) compared with other assayed cytokines (Table S1).
| Subject | Subsegment | Exposure | Lavage volume (ml) |
%PMN | IL-6 (pg/ml) |
|---|---|---|---|---|---|
| 1 | RLL | SAL | 32 | 43 | 1627 |
| lingula inferior | HDM | 65 | 4 | 1763 | |
| RML medial | LPS | 60 | 79 | 32000 | |
| 2 | RLL | SAL | 36 | 15 | 576 |
| lingula inferior | HDM | 77 | 4 | 1819 | |
| RML medial | LPS | 62 | 82 | 13406 | |
| 3 | RLL | SAL | 35 | 34 | 3376 |
| lingula inferior | HDM | 80 | 9 | 387 | |
| RML medial | LPS | 65 | 77 | 20183 |
Immunodepletion of BALF
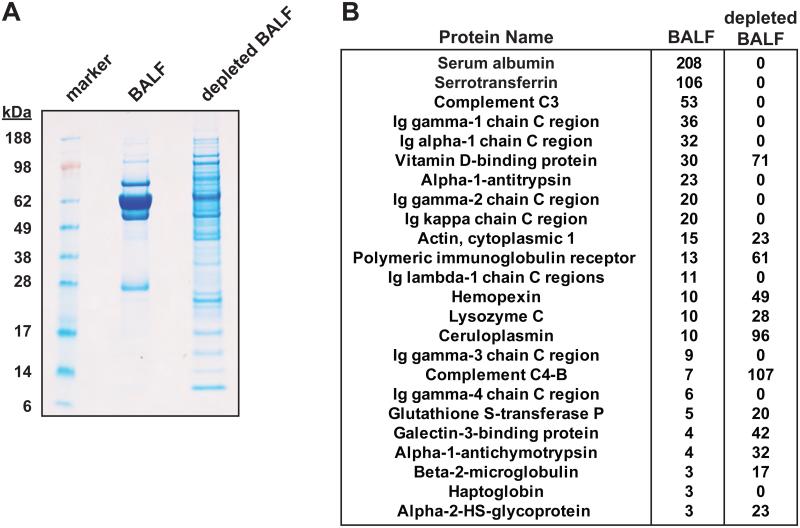
Immunodepletion of abundant plasma proteins has been utilized to increase depth of proteome coverage in plasma and other extracellular fluids32, 33 but only occasionally in human BALF proteomics6, 19. In a pilot study of a single sample, we examined the efficacy of immunodepletion using a liquid chromatography-based multiple affinity removal system which targets the top 14 most abundant human plasma proteins (Agilent MARS 14). Many more proteins were visualized by SDS-PAGE post-depletion (Fig. 1A), and a triplicate LC-MS/MS analysis of each sample yielded a 3-fold increase in identified proteins post- versus pre-depletion (100 versus 33 proteins, 3 or more identified spectra per protein; Supporting Information). Numerous plasma-derived proteins—including albumin, serotransferrin, complement C3 and immunoglobulins—were undetectable following immunodepletion, (Fig. 1B). At the same time, the spectral counts of other highly abundant airway proteins increased up to >10-fold (Fig. 1B). There did not appear to be any adventitious loss of resident airway proteins due to immunopletion.
Figure 1.
Immunodepletion of human bronchoalveolar lavage fluid. A single BALF sample was concentrated by centrifugal ultracentrifugation (10 kDa cutoff) and immunodepleted using a MARS-14 column. (A) 10 μg of undepleted and depleted BALF were separated on a 4-12% Bis-Tris gel (NuPage; MES-SDS running buffer) followed by Colloidal Blue staining. (B) 1 μg of undepleted and depleted BALF were analyzed in triplicate by 1D-LC-MS/MS (Supporting Information). Twenty-four proteins were identified by three or more spectra in undepleted BALF, and the total number of spectra identified (i.e. spectral counts) between the undepleted and depleted BALF are shown.
Within our study-set, BALF protein yields after centrifugal concentration from 12 ml total volume per sample ranged from ~350-1450 μg total protein (Table 1). Rather than normalize samples prior to immunodepletion, we depleted the entirety of each of these samples using a single 500 μl injection onto the MARS 14 column. This strategy avoided any potential pitfalls associated with various estimations of undiluted epithelial lining fluid volume34, 35.
Quantitative gel-free, label-free LC-MS/MS
Following immunodepletion, samples were normalized to protein amount, trypsinized, and analyzed by gel-free, label-free 1D-LC-MS/MS29, 36, 37. Prior to analysis, LC column conditioning was performed with two 1 μg injections of a pooled BALF sample. Next, 1 μg of each of the nine samples was analyzed in triplicate for quantitation, twice by data-independent-acquisition (MSE) and once by data-dependent acquisition, to take advantage of the complimentary nature of these approaches for peptide identification. Data processing in Rosetta Elucidator included accurate mass and retention time alignment across all 27 LC-MS/MS analyses and quantitation of identified features based on area under the curve (see Methods). Database searching and feature annotation at a 1% peptide false-discovery rate resulted in the relative quantitation of 2777 peptides belonging to 441 proteins across all samples, with 219 proteins being quantified with 2 or more peptides (Table S2-S3). Relative protein expression values for each sample were determined by summing the intensities of all associated peptides to a given protein38. A few targets of immunopletion, including albumin and complement C3, were still at quantifiable levels across the study, and, their expression values were flagged as unreliable (Table S3).
Technical reproducibility of our method was assessed by percent coefficient of variation (%CV) for triplicate analyses of each sample. The mean %CV was ~20% for proteins quantified by a single peptide (“single-hit” proteins) and ~7% for proteins quantified based on the aggregate of two or more peptides (Table S3). Similarly, >80% of proteins quantified by two or more peptides had a mean CV of less than 10%, and ~95% had a mean CV of less than 15% (Fig. 2A), whereas the single-hit proteins had a much wider distribution (Fig. 2A). Although principal component analysis on all quantified proteins did not segregate the samples by treatment group, this analysis again demonstrated the high technical reproducibility of replicate analyses (Fig. 2B). Collectively, these data demonstrate the high degree of analytical reproducibility obtained using label-free quantitation.
Figure 2.
Technical reproducibility metrics with the quantitative dataset. (A) Within each LC-MS/MS analysis, percent coefficient of variation (%CV) was calculated for each of the 441 quantified proteins. These values were averaged across the 9 samples to obtain a single %CV per protein (Table S2). Proteins quantified based on a single peptide (grey) and two or more peptides (black) were grouped into %CV bins as indicated. (B) A principal component analysis was performed in Rosetta Elucidator using all protein expression data for each of the 27 LC-MS/MS analyses, and principal components 1-3 (PC1-3) were plotted for each sample.
Changes in BALF protein levels in response to subsegmental exposure
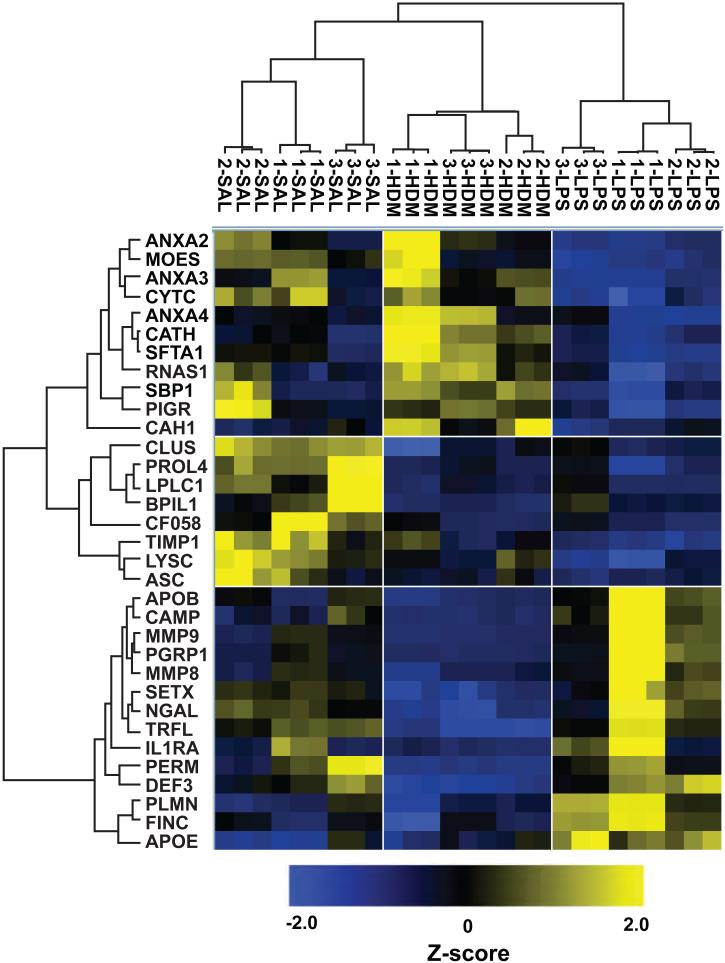
We used two separate methods to compare differences in BALF protein levels between the three treatment groups. First, we calculated fold changes for relevant comparisons (LPS versus HDM, LPS versus SAL, and HDM versus SAL; Tables 2-3 and S3) using average intra-treatment group protein expression values (3 samples per group x 3 technical replicates per sample). For visualization purposes, we also performed hierarchical clustering of all 27 individual LC-MS/MS analyses using the Z-score normalized intensities for proteins that were quantified by 2 or more peptides and had an ANOVA p-value of <0.0025 (Note: this p-value was chosen because it clustered individual LC-MS/MS analyses by sample number and treatment group while returning a small subset of the total quantified proteome). These criteria resulted in a cluster of 33 proteins that tightly segregated each of the three treatment groups as well as technical replicates (Fig. 3).
Table 2.
Differentially expressed proteins in LPS versus HDM and SAL lobesa
| Primary Protein Name |
Protein Description | Peptide Count |
Fold Change LPS vs. HDM |
P-value (LPS vs. HDM) |
Fold Change LPS vs. SAL |
P-value (LPS vs. SAL) |
|---|---|---|---|---|---|---|
| CAMP_HUMAN | Cathelicidin antimicrobial peptideb | 2 | 11.8 | 1.16E-06 | 2.7 | 0.018 |
| MMP9_HUMAN | Matrix metalloproteinase-9b | 33 | 9.5 | 1.51E-05 | 2.4 | 0.025 |
| PGRP1_HUMAN | Peptidoglycan recognition protein 1b | 4 | 9.4 | 1.50E-04 | 2.6 | 0.022 |
| IL1RA_HUMAN | Interleukin-1 receptor antagonist protein | 2 | 5.2 | 0.001 | 1.7 | n.s |
| APOB_HUMAN | Apolipoprotein B-100 | 20 | 5.0 | 1.18E-05 | 2.0 | 0.021 |
| MMP8_HUMAN | Neutrophil collagenaseb | 12 | 4.5 | 0.001 | 2.0 | 0.039 |
| CO5_HUMAN | Complement C5 | 3 | 4.3 | 9.42E-04 | 1.7 | 0.042 |
| ITIH2_HUMAN | Inter-alpha-trypsin inhibitor heavy chain H2 | 31 | 3.5 | 5.72E-05 | 2.0 | 0.042 |
| DEF3_HUMAN | Neutrophil defensin 3b | 2 | 3.4 | 1.61E-06 | 1.2 | n.s |
| ITIH1_HUMAN | Inter-alpha-trypsin inhibitor heavy chain H1 | 23 | 3.4 | 6.47E-05 | 2.0 | 0.036 |
| PERM_HUMAN | Myeloperoxidaseb | 9 | 3.3 | 4.28E-05 | −1.1 | n.s |
| TRFL_HUMAN | Lactotransferrinb | 61 | 2.9 | 3.91E-06 | 1.1 | n.s |
| BPIL1_HUMAN | Bactericidal/permeability-increasing protein-like 1 |
4 | 2.8 | 4.25E-04 | −2.4 | 0.023 |
| PLMN_HUMAN | Plasminogen | 47 | 2.6 | 1.74E-04 | 2.0 | 0.002 |
| COF2_HUMAN | Cofilin-2 | 2 | 2.5 | 0.005 | 1.4 | n.s |
| FINC_HUMAN | Fibronectin | 49 | 2.5 | 0.002 | 1.9 | 1.17E-04 |
| HEP2_HUMAN | Heparin cofactor 2 | 9 | 2.4 | 1.10E-04 | 1.9 | 0.026 |
| APOA4_HUMAN | Apolipoprotein A-IV | 30 | 2.4 | 7.02E-04 | 2.2 | 0.004 |
| FCN3_HUMAN | Ficolin-3 | 2 | 2.3 | 1.92E-04 | 2.7 | 0.003 |
| NGAL_HUMAN | Neutrophil gelatinase-associated lipocalinb | 11 | 2.3 | 1.74E-04 | 1.1 | n.s |
| AMBP_HUMAN | Protein AMBP | 16 | 2.3 | 3.34E-04 | 1.7 | 0.023 |
| SBP1_HUMAN | Selenium-binding protein 1 | 21 | −2.3 | 7.80E-05 | −1.7 | 0.037 |
| TPIS_HUMAN | Triosephosphate isomerase | 19 | −2.4 | 8.73E-04 | −2.1 | 0.007 |
| S10A6_HUMAN | Protein S100-A6 | 6 | −2.4 | 1.74E-04 | −2.1 | 0.023 |
| PEBP1_HUMAN | Phosphatidylethanolamine-binding protein 1 |
13 | −2.4 | 3.85E-04 | −2.0 | 0.016 |
| SH3L1_HUMAN | SH3 domain-binding glutamic acid-richlike protein | 3 | −2.5 | 5.24E-04 | −2.7 | 0.014 |
| GSTA5_HUMAN | Glutathione S-transferase A5 | 2 | −2.6 | 1.74E-04 | −2.3 | 0.007 |
| CATH_HUMAN | Cathepsin H | 8 | −2.6 | 3.91E-06 | −1.3 | n.s |
| RNAS1_HUMAN | Ribonuclease pancreatic | 3 | −2.7 | 7.57E-04 | −1.7 | 0.035 |
| FABP5_HUMAN | Fatty acid-binding protein, epidermal | 2 | −2.8 | 3.34E-04 | −1.9 | n.s |
| ANXA5_HUMAN | Annexin A5 | 20 | −2.8 | 1.24E-04 | −2.6 | 0.008 |
| HEBP2_HUMAN | Heme-binding protein 2 | 3 | −2.8 | 1.92E-04 | −2.8 | 0.018 |
| SODC_HUMAN | Superoxide dismutase [Cu-Zn] | 6 | −2.9 | 9.88E-04 | −2.7 | 0.017 |
| CAH1_HUMAN | Carbonic anhydrase 1 | 2 | −3.0 | 3.85E-04 | −1.3 | n.s |
| ANXA3_HUMAN | Annexin A3 | 14 | −3.2 | 4.62E-06 | −2.4 | 0.001 |
| SFTA1_HUMAN | Pulmonary surfactant-associated protein A1 | 4 | −3.4 | 4.62E-06 | −1.9 | 0.004 |
| ANXA2_HUMAN | Annexin A2 | 26 | −3.6 | 1.10E-04 | −2.7 | 0.001 |
Table includes proteins w/2+ peptides and ≥2.3-fold or ≤−2.3-fold, (p<0.05, unpaired t-test w/ Benjamini Hochberg FDR correction) in LPS versus HDM lobes. Corresponding fold-changes and p-values are shown for the proteins in LPS versus SAL lobes.
Putative neutrophil granule secreted proteins.
Table 3.
Differentially expressed proteins in HDM versus SAL and LPS lobesa
| Primary Protein Name |
Protein Description | Peptide Count |
Fold Change HDM vs. SAL |
P-value (HDM vs. SAL) |
Fold change HDM vs. LPS |
P-value (HDM vs. LPS) |
|---|---|---|---|---|---|---|
| CAH1 HUMAN | Carbonic anhydrase 1 | 2 | 2.2 | 0.007 | 3.0 | 3.85E-04 |
| CATH_HUMAN | Cathepsin H | 8 | 2.0 | 2.60E-04 | 2.6 | 3.91E-06 |
| CRAC1 HUMAN | Cartilage acidic protein 1 | 17 | 1.8 | 0.023 | 2.0 | 3.85E-04 |
| SFTA1_HUMAN | Pulmonary surfactant-associated protein A1 |
4 | 1.8 | 0.008 | 3.4 | 4.62E-06 |
| RNAS1 HUMAN | Ribonuclease pancreatic | 3 | 1.6 | 0.02 | 2.7 | 7.57E-04 |
| C163A_HUMAN | Scavenger receptor cysteine-rich type 1 protein M130 |
3 | 1.5 | 0.043 | 1.4 | 0.009 |
| ATRN_HUMAN | Attractin | 3 | 1.5 | 0.036 | 1.4 | 0.053 |
| LYSC_HUMAN | Lysozyme C | 19 | −1.5 | 8.00E-03 | 1.8 | 0.003 |
| S10A9 HUMAN | Protein S100-A9 | 9 | −1.8 | 0.008 | 1.1 | 0.408 |
| SETX_HUMAN | Probable helicase senataxin | 2 | −1.9 | 2.97E-05 | −2.2 | 1.74E-04 |
| TKT_HUMAN | Transketolase | 6 | −2.0 | 0.005 | −1.5 | 0.18 |
| NGAL_HUMAN | Neutrophil gelatinase-associated lipocalin |
11 | −2.0 | 3.80E-06 | −2.3 | 1.74E-04 |
| LEG3 HUMAN | Galectin-3 | 4 | −2.2 | 0.05 | 1.1 | 0.348 |
| MMP8_HUMAN | Neutrophil collagenase | 12 | −2.3 | 0.049 | −4.5 | 0.001 |
| ASC_HUMAN | Apoptosis-associated speck-like protein containing a CARD |
2 | −2.4 | 0.049 | 1.7 | 0.009 |
| APOB_HUMAN | Apolipoprotein B-100 | 20 | −2.5 | 0.008 | −5.0 | 1.18E-05 |
| PROL4_HUMAN | Proline-rich protein 4 | 6 | −2.6 | 1.10E-06 | 1.2 | 0.232 |
| TRFL_HUMAN | Lactotransferrin | 61 | −2.7 | 5.72E-07 | −2.9 | 3.91E-06 |
| PGK1 HUMAN | Phosphoglycerate kinase 1 | 2 | −2.8 | 0.008 | −1.7 | 0.003 |
| DMBT1_HUMAN | Deleted in malignant brain tumors 1 protein |
6 | −2.9 | 0.023 | 1.5 | 0.348 |
| DEF3_HUMAN | Neutrophil defensin 3 | 2 | −2.9 | 4.13E-06 | −3.4 | 1.61E-06 |
| IL1RA_HUMAN | Interleukin-1 receptor antagonist protein |
2 | −3.0 | 0.049 | −5.2 | 0.001 |
| CO3_HUMAN | Complement C3 | 5 | −3.1 | 0.006 | −2.2 | 0.008 |
| CF058 HUMAN | Uncharacterized protein C6orf58 | 7 | −3.3 | 1.00E-03 | 1.1 | 0.856 |
| LPLC1_HUMAN | Long palate, lung and nasal epithelium carcinoma-associated protein 1 |
2 | −3.6 | 2.60E-04 | −1.1 | 0.97 |
| PGRP1_HUMAN | Peptidoglycan recognition protein 1 |
4 | −3.6 | 0.001 | −9.4 | 1.50E-04 |
| PERM HUMAN | Myeloperoxidase | 9 | −3.7 | 0.002 | −3.3 | 4.28E-05 |
| ZG16B_HUMAN | Zymogen granule protein 16 homolog B |
4 | −4.0 | 5.18E-04 | −3.5 | 0.658 |
| MMP9_HUMAN | Matrix metalloproteinase-9 | 33 | −4.0 | 0.002 | −9.5 | 1.51E-05 |
| CAMP HUMAN | Cathelicidin antimicrobial peptide | 2 | −4.5 | 0.021 | −11.8 | 1.16E-06 |
| BPIL1 HUMAN | Bactericidal/permeability- increasing protein-like 1 |
4 | −6.7 | 2.13E-04 | −2.8 | 4.25E-04 |
Table includes proteins w/2+ peptides and ≥1.5-fold or ≤-1.5-fold, (p<0.05, unpaired t-test w/ Benjamini Hochberg FDR correction) in HDM versus SAL lobes. Corresponding fold-changes and p-values are shown for the proteins in LPS versus SAL lobes.
Putative tracheal-expressed proteins.
Figure 3.
Hierarchical clustering of proteins as a function of subsegmental exposure. Data was grouped by treatment (triplicate analyses of three samples per group) followed by log2 transformation and error-weighted ANOVA with Benjamini Hochberg FDR correction. Data was filtered on an ANOVA p-value of ≤0.0025 and proteins identified by 2+ peptides prior to cluster analysis. Lines that define the six quadrants separate major branches on treatment group and protein axes.
As expected, neutrophil-derived proteins were highly induced in the LPS- compared to the saline- or HDM-treated lobes (Table 2) and were prominent in a main branch of the hierarchical cluster tree (Fig. 3). These included cathelicidin antimicrobial peptide, matrix metalloproteinase 9 (MMP9), myeloperoxidase, neutrophil defensin 3, peptidoglycan recognition protein 1 (PGRP1). The nearly identical expression patterns of MMP9 and PGRP1 (Fig. S1), are consistent with their co-localization and secretion from neutrophil tertiary granules39. Interestingly, the contralateral HDM-exposed lobes had fewer %PMNs and neutrophil-derived proteins than the saline-treated RLL, possibly representing spill-over from the anatomically proximal LPS-treated RML (Table 1). PGRP1 has not been previously explored as a BALF marker of neutrophilic inflammation. As a validation, total protein and PGRP1 levels were further quantified in undepleted BALF across eleven additional non-atopic, non-asthmatic controls (Fig. 4). While protein levels in these subjects were only slightly elevated in LPS lobes (Fig. 4A), PGRP1 was markedly increased when normalized either to BALF volume or total BALF protein, suggesting that it is a sensitive marker of LPS-induced inflammation (Fig. 4B-C).
Figure 4.
Validation of peptidoglycan recognition protein (PGRP1) as a marker of subsegmental LPS exposure. BALF was analyzed from non-atopic, non-asthmatic individuals (n=11) who were identically exposed to SAL, LPS and HDM. Due to low recovery from RLL, BALF from n=9 SAL lobes was analyzed. (A) BALF protein was quantified by protein assay. ELISA was performed on BALF (diluted 1- to 40-fold) and ng PGRP1 was normalized for (B) BALF volume or (C) BALF protein. Mean ± s.e.m. (n=9-11) are indicated by horizontal line and error bars. *p<0.05 versus SAL or HDM or **p<0.01 versus SAL or HDM (ANOVA).
Other exemplary LPS-induced proteins included complement protein C5 and fibronectin (Table 2). In humans, C5 has been previously shown to be induced in BALF after LPS inhalation 40, and the protein is a precursor of the neutrophil chemoattractant, C5a. Fibronectin is an abundant plasma protein that is also produced by resident lung cells in the formation of extracellular matrix, and this increase may be consistent with the early fibroproliferative phase of acute lung injury41. Among exclusively plasma-derived proteins, four isoforms of the inter-alpha trypsin inhibitor (IaI) heavy chain (ITIH1-ITIH4), which are primarily expressed in liver, were all induced >1.5-fold in BALF from LPS versus HDM lobes (Table 2 and S3). Of these, ITIH1 and ITIH2 were induced >3-fold in LPS versus HDM lobes. Their expression patterns were nearly identical (Fig. S1), which is consistent with the covalent linkage of these isoforms to the IaI light chain, bikunin, via a chondroitin sulfate chain42. Alpha-1-microglobin/bikunin precursor protein (AMBP), from which bikunin is derived, also had a similar expression pattern to ITIH2/3 (Fig. S1). Both IaI and bikunin have been shown to inhibit cytokine production by macrophages and neutrophils after LPS stimulation in vitro43, 44, suggesting that their increased expression in the airway may be to modulate the immune response45.
Surfactant protein A (SP-A), which is primarily expressed by alveolar type II pneumocytes and is a modulator of allergic inflammation46, was prominent in a second branch of the cluster tree (Fig. 3). It should be noted that humans express two SP-A isoforms (SFTPA1 and SFTPA2), and the identified peptides are shared between these isoforms (although they were assigned to SFTPA1). SP-A was increased in the HDM, and decreased in the LPS, relative to SAL lobes (Fig. 3 and S1; Table 3). Cathepsin H, a protease important for processing of surfactant47, 48, was most proximal to SP-A on the cluster tree (Fig. 3), and appropriately, it exhibited a very similar expression pattern (Fig. S1). Interestingly, while these two proteins were significantly higher in the HDM lobes (Table 3), they were ~2-fold lower in LPS versus SAL lobes (Table 2). A decrease in SP-A expression following LPS instillation, which was previously noted in a similar study49, is suggestive of LPS modulation of Th2 inflammation. A decrease in SP-A has also been observed in cystic fibrosis, a disease characterized by airway neutrophilic inflammation28. Annexins 2-4 (ANXA2, ANXA3, ANXA4) also clustered in the same major branch; further, the relative expressions of ANXA2, ANXA3 and ANXA5 were all >2.4-fold lower in LPS versus SAL lobes (Table 2 and Fig. S1). It is possible that the concomitant reduction in SP-A and annexins reflect a common mechanism, as these proteins interact and may also be coordinately secreted with SP-A from alveolar type II cells via lamellar body exocytosis50, 51.
Interlobar and subject-dependent variability in BALF proteomes
Hierarchical clustering of the three treatment groups also identified a third major branch enriched in proteins that were most highly expressed in saline-treated lobes (Fig. 3). These included bactericidal/permeability-increasing protein-like 1 (BPIL1), which was ~7-fold higher in the SAL versus HDM lobes (RLL versus lingula) and was ~3-fold higher in the SAL versus LPS lobes (RLL versus RML; Table 2-3 and Fig. 5A). BPIL1 has high tissue expression in the upper airways but not in the distal lung, and its tissue expression correlates most highly with C6orf5852, its nearest neighbor on the cluster tree (Fig. 3 and S1). Other proteins that were likely derived from upper airway or saliva proteomes53, 54 and were most highly expressed in SAL lobes include long palate, lung, and nasal epithelium clone 1 (LPLC1; Fig. S1), deleted in malignant brain tumors 1 (DMBT1), zymogen granule protein 16 homolog B and proline-rich protein 4. With the exception of ZG16B, all of these proteins also had 2.5-fold or greater expression in SAL compared to the HDM or LPS lobes (Table S3). The enrichment of BPIL1 in the SAL versus HDM lobe of subjects 1 and 3 was confirmed by western blotting of immunodepleted BALF (Fig. 5B). However, when pairs of undepleted BALF from SAL- versus HDM-exposed lobes were examined from 8 other individuals, the trend was not as pronounced (Fig. 5C).
Figure 5.
Intralobar variability of BPIL1 expression. (A) Aggregate protein intensity of BPIL1 across all nine analyzed lobes. (B) 5 μg of immunodepleted BALF from subjects 1-3 was analyzed by western blotting with anti-BPIL1 antibody. (C) Undepleted BALF from SAL- versus HDM-treated lobes was analyzed from eight non-atopic, non-asthmatic controls by western blotting as in (B). Data in (A) is mean ± s.e.m. (n=3 technical replicates per sample).
We also wondered whether our data might reveal variable airway protein expression between the three individuals. We grouped the data by individual, followed by ANOVA and hierarchical clustering. A p-value cut-off of <0.01 generated a tree containing 43 proteins which segregated each of the individuals (Fig. 6). Three major protein branches were identified, each clustering proteins with high expression in one of the three individuals (Fig. 6). Exemplary proteins included: aldehyde dehydrogenase (ALDH3A1), which was at least ~3-fold higher in each of three lobes of subject 1 as compared to subjects 2 and 3 (Fig. S1); phosphatidylethanolamine-binding protein 4 (PEBP4), which was increased ~2-fold in each lobe of subject 2 relative to subjects 1 and 3 (Fig. S1); and two proteins of the cystatin superfamily, fetuin B and histidine-rich glycoprotein (HRG), which were markedly higher in all lobes from subject 3 (Fig. S1). This analysis highlights the potential utility of sampling multiple lobes in proteomic profiling of BALF, as it is unlikely that these differences would have confidently been identified if protein expression were analyzed in only a single lobe.
Figure 6.
Hierarchical clustering of proteins as a function of individual. Data was grouped by individual (9 analyses per subject) followed by log2 transformation and error-weighted ANOVA with Benjamini Hochberg FDR correction. Data was filtered on an ANOVA p-value of <0.01 and proteins identified by 2+ peptides prior to cluster analysis. Lines which define the six quadrants separate major branches on subject # and protein axes.
DISCUSSION
Airway fluid proteins arise from a variety of cell sources, including respiratory epithelium and resident inflammatory cells, and plasma that diffuses across the pulmonary capillary bed. As such, the airway proteome should be an attractive milieu for global profiling of the response of the lung to environmental stimuli. Despite the potential of proteomics for the study of lung (patho)physiology, there has been little progress in standardizing methods of BALF processing prior to MS analysis, and the overriding belief has been that insufficient depth of coverage, poor quantitative reproducibility and lack of sufficient throughput limits the utility of airway proteomics compared to genomics. In the present study, we show that immunodepletion can be combined with gel-free LC-MS/MS and label-free quantitation for robust interrogation of the BALF proteome. Similar methods have been used to analyze dozens of human plasma samples29, 36, and we posit that this approach should be readily employed for the large-scale analysis of BALF, including existing banked repositories.
As a means to evaluate response to environmental stimuli, our analysis shows a correlation between airway neutrophil influx and the relative levels of neutrophil-derived proteins in LPS-treated lung lobes. Indeed, we identified six proteins which are localized to multiple neutrophil granule subtypes55-58 among the ten most highly-induced proteins in LPS-treated lobes. Since our ability to detect these species depends on their relative abundance, this suggests that these proteins are among the most abundant products of neutrophil degranulation. In a similar study of airway inflammation in response to LPS instillation, BALF neutrophils, gelatinase activity and acute-phase cytokines (e.g. IL-6 and TNFα) peaked 6 h-post LPS and were reduced to baseline by approximately 24 h post-LPS, whereas lactotransferrin and myeloperoxidase remained high even at 48 h59. In the present analysis, we have identified PGRP1 as a novel, early marker of acute airway inflammation, and it is tempting to speculate that it may also be a useful biomarker of resolving neutrophilic inflammation at later time points post-injury.
There are substantial differences in the results of our study compared to two previous analyses of the human BALF proteome following LPS instillation49, 60. These studies retrospectively analyzed samples collected either 6 h or 16 h after a randomized instillation of 4 ng/kg LPS or saline in the RML versus lingula59, 61. As in our cohort, the induction of IL-6 was a reliable indicator of LPS exposure with concomitant neutrophil influx59, 61. However, the depth of coverage was limited in these studies, due to the use of 2D-GE without prior immunodepletion of serum proteins. Bowler et al. for example49, identified only a single LPS-inducible protein, haptoglobin. Although this protein can be released by neutrophil granules62, plasma is the likely source of BALF haptoglobin and thus was removed by immunodepletion in our study. Similarly, de Torre et al. also noted changes in many plasma-derived proteins (e.g. APOA1, transthyretin, haptoglobin) that we removed by immunodepletion60. In this latter study, increases in the inflammatory markers calgranulin A and B (S100A8/A9) were also identified and validated as markers of airway inflammation in BALF from normal subjects versus patients with ARDS60. Although we quantified S100A8/A9, it was not increased after LPS treatment, which could reflect differences in the airway inflammatory response compared to ARDS. Overall, we posit that immunodepletion was the greatest contributor to our improved depth of coverage of neutrophil-derived and other acute phase proteins.
The relatively large number of proteins quantified in our dataset allows us to correlate exposure-induced changes in the BALF proteome compared to airway cell transcriptomes in the same cohort, of which both BAL cells (predominantly macrophages in SAL and HDM lobes with a much higher proportion of neutrophils in LPS lobes) and bronchial epithelial cells (obtained by bronchoscopic brushing) were both analyzed30. Across the entire cohort of 39 subjects from which our samples were collected, it is surprising that genes encoding the enriched neutrophil-derived proteins were not strongly upregulated in BAL cells collected from the endotoxin-exposed lobes, despite the pronounced influx of neutrophils into the airway. A large number of LPS-inducible genes were associated with inflammation and immune response, and included targets of nuclear factor kappa b and interferon-inducible transcription factors. Although secreted cytokines and chemokines were prominent among LPS-inducible genes, IL1RA, a product of immune cells and airway epithelia, was the only protein that was increased in >2-fold in the BALF proteome and corresponding epithelial and BAL cell transcriptomes following LPS exposure30. None of the neutrophil-derived proteins that were so markedly upregulated in BALF were identified by this transcriptome analysis.
In a separate transcriptome analysis of neutrophils from humans whose airways were exposed to LPS, Coldren et al. found that neutrophils isolated from human blood at 16 h post-LPS instillation had much higher levels of CAMP, MMP9 and PGRP1 than were found in neutrophils isolated from lung alveolae63. This data also suggest that the influx of specific neutrophil granule proteins into the airway cannot be predicted by transcriptional analysis of BAL cells. However, consistent with our data, IL1RA mRNA was also shown in the same study to be highly induced in alveolar versus circulating neutrophils post-LPS63. Serum-derived acute phase proteins represent an additional class of LPS-responsive proteins that are not captured by transcriptomic profiling of airway cells but than can be readily quantified in BALF proteomic analysis. Collectively, these data show a marked discordance between BALF protein and mRNA levels following LPS exposure and highlight the importance of unbiased proteomic analysis in furthering our understanding of airway (patho)physiology.
Significant intralobar airway protein variability has not been revealed in prior analyses. Unexpectedly, our study found an overabundance of tracheal-derived proteins in the RLL,. While we have not ruled out the observed enrichment of tracheal-derived proteins to be an artifact of immunodepletion, the bronchoscopic lavage method, or lung anatomy, might provide explanations for this phenomenon. The RLL was the first to undergo instillation (with SAL) and subsequently lavaged, and it is possible that these upper airway-derived proteins represent contamination from the bronchoscope. While fluid recovered from the first 20 ml lavage was discarded to exclude tracheobronchial components from the lavage fluid64, 65, low BALF return from the RLL, in part a function of airway orientation, might still bias towards a greater proportion of tracheobronchial proteins in this lobe. The relative abundance of tracheal-derived proteins in the RLL BALF might also be a function of normal anatomy as the right mainstem bronchus is more vertically aligned to the trachea as evidenced by radiography and aspiration studies66, 67. Fluid distribution also appears to be influenced by the subject’s position (upright versus reclined) as supported by imaging of fluid dynamics in an in vivo rabbit model68. Although the argument can be made that proteins derived from the tracheal epithelium preferentially pool in the RLL, further study is needed to determine whether this is a common occurrence and whether such contamination of BALF by upper airway-derived proteins during bronchoscopy can be avoided.
Although intralobar variability may affect the expression of only a relatively small number of proteins, it raises concerns for interpretation of lobe-specific data. Thus, while it is intriguing that SP-A and annexin isoforms are upregulated in the HDM-treated lingula, or downregulated in LPS-treated RML, without any independent functional correlates it is difficult to speculate on the significance of these findings in our analysis. Thus, it is imperative that future proteomic studies account for the potential of intralobar variability. One way to circumvent this phenomenon is by randomization of treatments to contralateral lobes (e.g. RML and lingula), as was done in the other studies of endotoxin instillation59, 61. Alternatively, a randomized crossover design71, where BALF is recovered from the same lobe following a lengthy (e.g. 14-day) washout, would also allow the same lobe(s) to be sampled under “test” and “control” conditions and eliminate any potential contribution of intralobar protein variability.
In addition to studying the effects of environmental or other exposures on the composition of the lung’s extracellular lining fluid, modern proteomics approaches should prove useful for phenotyping lung diseases. As seen here and elsewhere29, 72, protein-level hierarchical clustering and peptide-level metaprotein modeling of gel- and label-free proteomic data have demonstrated the capacity of this approach for identifying potential disease-specific signatures that are likely to be distinct from but complement those that can be profiled using genomic technologies. Our data also suggests that sampling and analysis of multiple lung lobes might improve statistical powering for identifying markers of diffuse disease, or for better determining the protein(s) that are regionally versus systemically affected in focal diseases. However, this would need to be weighed against the progressive risk to the patient with increasing number of lavaged lobes. Until now, little consideration has been made to the intralobar variations in the BALF proteome that may exist under normal physiological conditions, nor have BALF proteomes been rigorously explored to define the regional effects of disease. Collectively, our observations should help to guide future efforts to explore the BALF proteome.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by National Institutes of Health grants HL106121 and ES012496 and a CTSA award to Duke University (UL1RR024128).
Footnotes
SUPPORTING INFORMATION
Supporting Information includes: an Excel file, “Foster_JPR_Supporting_Information.xlsx” contains: Table S1) BAL cell and cytokine data from subjects 1-3; Table S2) Expression data for >2700 quantified peptides. Sample run orders for LC-MS/MS analyses are shown in column headings for individual runs; and Table S3) Aggregate expression data for all quantified proteins. Fold changes were calculated by ratioing average expression (n=9 per group) and reported ANOVA p-values were calculated from log2-transformed data using an unpaired t-test w/ Benjamini Hochberg FDR correction. In addition, Fig. S1 includes aggregate protein expression for exemplary proteins that were found to be significantly different as a function of treatment group, lobe or individual.
Scaffold files containing MS/MS data for comparison of depleted and un-depleted BALF “HuBALF_depletion.sf3” and all assigned MS/MS spectra from quantitative analysis “HuBALF_MSMS.sf3” are available for download from https://discovery.genome.duke.edu/express/resources/2402/HuBALF_depletion.sf3 and https://discovery.genome.duke.edu/express/resources/2402/HuBALF_MSMS.sf3. The free Scaffold viewer is available for download at http://www.proteomesoftware.com.
REFERENCES
- 1.Sepper R, Prikk K. Proteomics: is it an approach to understand the progression of chronic lung disorders. J Proteome Res. 2004;3(2):277–81. doi: 10.1021/pr049955x. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch J, Hansen KC, Burlingame AL, Matthay MA. Proteomics: current techniques and potential applications to lung disease. Am J Physiol Lung Cell Mol Physiol. 2004,;287(1):L1–23. doi: 10.1152/ajplung.00301.2003. [DOI] [PubMed] [Google Scholar]
- 3.Magi B, Bargagli E, Bini L, Rottoli P. Proteome analysis of bronchoalveolar lavage in lung diseases. Proteomics. 2006,;6(23):6354–69. doi: 10.1002/pmic.200600303. [DOI] [PubMed] [Google Scholar]
- 4.Govender P, Dunn MJ, Donnelly SC. Proteomics and the lung: Analysis of bronchoalveolar lavage fluid. Proteomics Clin Appl. 2009;3(9):1044–51. doi: 10.1002/prca.200900032. [DOI] [PubMed] [Google Scholar]
- 5.Plymoth A, Lofdahl CG, Ekberg-Jansson A, Dahlback M, Lindberg H, Fehniger TE, Marko-Varga G. Human bronchoalveolar lavage: biofluid analysis with special emphasis on sample preparation. Proteomics. 2003,;3(6):962–72. doi: 10.1002/pmic.200300387. [DOI] [PubMed] [Google Scholar]
- 6.Chang DW, Hayashi S, Gharib SA, Vaisar T, King ST, Tsuchiya M, Ruzinski JT, Park DR, Matute-Bello G, Wurfel MM, Bumgarner R, Heinecke JW, Martin TR. Proteomic and computational analysis of bronchoalveolar proteins during the course of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2008;178(7):701–9. doi: 10.1164/rccm.200712-1895OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haque R, Umstead TM, Freeman WM, Floros J, Phelps DS. The impact of surfactant protein-A on ozone-induced changes in the mouse bronchoalveolar lavage proteome. Proteome Sci. 2009;7(12) doi: 10.1186/1477-5956-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenz AG, Meyer B, Costabel U, Maier K. Bronchoalveolar lavage fluid proteins in human lung disease: analysis by two-dimensional electrophoresis. Electrophoresis. 1993;14(3):242–4. doi: 10.1002/elps.1150140141. [DOI] [PubMed] [Google Scholar]
- 9.Wattiez R, Hermans C, Cruyt C, Bernard A, Falmagne P. Human bronchoalveolar lavage fluid protein two-dimensional database: study of interstitial lung diseases. Electrophoresis. 2000;21(13):2703–12. doi: 10.1002/1522-2683(20000701)21:13<2703::AID-ELPS2703>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 10.Noel-Georis I, Bernard A, Falmagne P, Wattiez R. Database of bronchoalveolar lavage fluid proteins. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;771(1-2):221–36. doi: 10.1016/s1570-0232(02)00114-9. [DOI] [PubMed] [Google Scholar]
- 11.Wattiez R, Falmagne P. Proteomics of bronchoalveolar lavage fluid. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;815(1-2):169–78. doi: 10.1016/j.jchromb.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Sabounchi-Schutt F, Astrom J, Hellman U, Eklund A, Grunewald J. Changes in bronchoalveolar lavage fluid proteins in sarcoidosis: a proteomics approach. Eur Respir J. 2003;21(3):414–20. doi: 10.1183/09031936.03.00060902. [DOI] [PubMed] [Google Scholar]
- 13.Plymoth A, Lofdahl CG, Ekberg-Jansson A, Dahlback M, Broberg P, Foster M, Fehniger TE, Marko-Varga G. Protein expression patterns associated with progression of chronic obstructive pulmonary disease in bronchoalveolar lavage of smokers. Clin Chem. 2007;53(4):636–44. doi: 10.1373/clinchem.2006.076075. [DOI] [PubMed] [Google Scholar]
- 14.Silva E, Bourin S, Sabounchi-Schutt F, Laurin Y, Barker E, Newman L, Eriksson H, Eklund A, Grunewald J. A quantitative proteomic analysis of soluble bronchoalveolar fluid proteins from patients with sarcoidosis and chronic beryllium disease. Sarcoidosis Vasc Diffuse Lung Dis. 2007;24(1):24–32. [PubMed] [Google Scholar]
- 15.Passadore I, Iadarola P, Di Poto C, Giuliano S, Montecucco C, Cavagna L, Bonino C, Meloni F, Fietta AM, Lisa A, Salvini R, Bardoni AM. 2-DE and LC-MS/MS for a comparative proteomic analysis of BALf from subjects with different subsets of inflammatory myopathies. J Proteome Res. 2009;8(5):2331–40. doi: 10.1021/pr800943t. [DOI] [PubMed] [Google Scholar]
- 16.Mehrani H, Ghanei M, Aslani J, Golmanesh L. Bronchoalveolar lavage fluid proteomic patterns of sulfur mustard-exposed patients. Proteomics Clin Appl. 2009;3(10):1191–200. doi: 10.1002/prca.200900001. [DOI] [PubMed] [Google Scholar]
- 17.Kim TH, Lee YH, Kim KH, Lee SH, Cha JY, Shin EK, Jung S, Jang AS, Park SW, Uh ST, Kim YH, Park JS, Sin HG, Youm W, Koh ES, Cho SY, Paik YK, Rhim TY, Park CS. Role of lung apolipoprotein A-I in idiopathic pulmonary fibrosis: antiinflammatory and antifibrotic effect on experimental lung injury and fibrosis. Am J Respir Crit Care Med. 2010;182(5):633–42. doi: 10.1164/rccm.200905-0659OC. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto T, Miyazaki Y, Shirahama R, Tamaoka M, Inase N. Proteome analysis of bronchoalveolar lavage fluid in chronic hypersensitivity pneumonitis. Allergol Int. 2012;61(1):83–92. doi: 10.2332/allergolint.11-OA-0315. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Kobayashi M, Sousa EA, Liu W, Cai J, Goldman SJ, Dorner AJ, Projan SJ, Kavuru MS, Qiu Y, Thomassen MJ. Differential proteomic analysis of bronchoalveolar lavage fluid in asthmatics following segmental antigen challenge. Mol Cell Proteomics. 2005;4(9):1251–64. doi: 10.1074/mcp.M500041-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Guo Y, Ma SF, Grigoryev D, Van Eyk J, Garcia JG. 1-DE MS and 2-D LC-MS analysis of the mouse bronchoalveolar lavage proteome. Proteomics. 2005;5(17):4608–24. doi: 10.1002/pmic.200500052. [DOI] [PubMed] [Google Scholar]
- 21.Chang CC, Chen SH, Ho SH, Yang CY, Wang HD, Tsai ML. Proteomic analysis of proteins from bronchoalveolar lavage fluid reveals the action mechanism of ultrafine carbon black-induced lung injury in mice. Proteomics. 2007;7(23):4388–97. doi: 10.1002/pmic.200700164. [DOI] [PubMed] [Google Scholar]
- 22.Fu X, Gharib SA, Green PS, Aitken ML, Frazer DA, Park DR, Vaisar T, Heinecke JW. Spectral index for assessment of differential protein expression in shotgun proteomics. J Proteome Res. 2008;7(3):845–54. doi: 10.1021/pr070271+. [DOI] [PubMed] [Google Scholar]
- 23.Chiu KH, Lee WL, Chang CC, Chen SC, Chang YC, Ho MN, Hsu JF, Liao PC. A label-free differential proteomic analysis of mouse bronchoalveolar lavage fluid exposed to ultrafine carbon black. Anal Chim Acta. 2010;673(2):160–6. doi: 10.1016/j.aca.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 24.Gharib SA, Nguyen E, Altemeier WA, Shaffer SA, Doneanu CE, Goodlett DR, Schnapp LM. Of mice and men: comparative proteomics of bronchoalveolar fluid. Eur Respir J. 2010;35(6):1388–95. doi: 10.1183/09031936.00089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosanam H, Sato M, Batruch I, Smith C, Keshavjee S, Liu M, Diamandis EP. Differential proteomic analysis of bronchoalveolar lavage fluid from lung transplant patients with and without chronic graft dysfunction. Clin Biochem. 2012;45(3):223–30. doi: 10.1016/j.clinbiochem.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Varnum SM, Webb-Robertson BJ, Pounds JG, Moore RJ, Smith RD, Frevert CW, Skerrett SJ, Wunschel D. Proteomic analysis of bronchoalveolar lavage fluid proteins from mice infected with Francisella tularensis ssp. novicida. J Proteome Res. 2012;11(7):3690–703. doi: 10.1021/pr3001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plymoth A, Yang Z, Lofdahl CG, Ekberg-Jansson A, Dahlback M, Fehniger TE, Marko-Varga G, Hancock WS. Rapid proteome analysis of bronchoalveolar lavage samples of lifelong smokers and never-smokers by micro-scale liquid chromatography and mass spectrometry. Clin Chem. 2006;52(4):671–9. doi: 10.1373/clinchem.2005.060715. [DOI] [PubMed] [Google Scholar]
- 28.Gharib SA, Vaisar T, Aitken ML, Park DR, Heinecke JW, Fu X. Mapping the lung proteome in cystic fibrosis. J Proteome Res. 2009;8(6):3020–8. doi: 10.1021/pr900093j. [DOI] [PubMed] [Google Scholar]
- 29.Patel K, Lucas JE, Thompson JW, Dubois LG, Tillmann HL, Thompson AJ, Uzarski D, Califf RM, Moseley MA, Ginsburg GS, McHutchison JG, McCarthy JJ. High predictive accuracy of an unbiased proteomic profile for sustained virologic response in chronic hepatitis C patients. Hepatology. 2011;53(6):1809–18. doi: 10.1002/hep.24284. [DOI] [PubMed] [Google Scholar]
- 30.Yang IV, Tomfohr J, Singh J, Foss CM, Marshall HE, Que LG, McElvania-Tekippe E, Florence S, Sundy JS, Schwartz DA. The clinical and environmental determinants of airway transcriptional profiles in allergic asthma. Am J Respir Crit Care Med. 2012;185(6):620–7. doi: 10.1164/rccm.201108-1503OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Limper AH, Specks U, Brutinel WM, Martin WJ, 2nd, Rohrbach MS. Interlobar variation in the recovery of bronchoalveolar lavage fluid, cell populations, and angiotensin-converting enzyme in normal volunteers. J Lab Clin Med. 1993;121(6):785–91. [PubMed] [Google Scholar]
- 32.Sanderson CJ, de Souza W. A morphological study of the interaction between Trypanosoma cruzi and rat eosinophils, neutrophils and macrophages in vitro. J Cell Sci. 1979;37:275–86. doi: 10.1242/jcs.37.1.275. [DOI] [PubMed] [Google Scholar]
- 33.Fletcher PL, Jr., Fletcher MD, Weninger K, Anderson TE, Martin BM. Vesicle-associated membrane protein (VAMP) cleavage by a new metalloprotease from the Brazilian scorpion Tityus serrulatus. J Biol Chem. 2010;285(10):7405–16. doi: 10.1074/jbc.M109.028365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal RG. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol. 1986;60(2):532–8. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 35.Marcy TW, Merrill WW, Rankin JA, Reynolds HY. Limitations of using urea to quantify epithelial lining fluid recovered by bronchoalveolar lavage. Am Rev Respir Dis. 1987;135(6):1276–80. doi: 10.1164/arrd.1987.135.6.1276. [DOI] [PubMed] [Google Scholar]
- 36.Cyr DD, Lucas JE, Thompson JW, Patel K, Clark PJ, Thompson A, Tillmann HL, McHutchison JG, Moseley MA, McCarthy JJ. Characterization of serum proteins associated with IL28B genotype among patients with chronic hepatitis C. PLoS One. 2011;6(7):e21854. doi: 10.1371/journal.pone.0021854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soderblom EJ, Philipp M, Thompson JW, Caron MG, Moseley MA. Quantitative label-free phosphoproteomics strategy for multifaceted experimental designs. Anal Chem. 2011;83(10):3758–64. doi: 10.1021/ac200213b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reidel B, Thompson JW, Farsiu S, Moseley MA, Skiba NP, Arshavsky VY. Proteomic profiling of a layered tissue reveals unique glycolytic specializations of photoreceptor cells. Mol Cell Proteomics. 2011;10(3):M110 002469. doi: 10.1074/mcp.M110.002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dziarski R, Platt KA, Gelius E, Steiner H, Gupta D. Defect in neutrophil killing and increased susceptibility to infection with nonpathogenic gram-positive bacteria in peptidoglycan recognition protein-S (PGRP-S)-deficient mice. Blood. 2003;102(2):689–97. doi: 10.1182/blood-2002-12-3853. [DOI] [PubMed] [Google Scholar]
- 40.Bolger MS, Ross DS, Jiang H, Frank MM, Ghio AJ, Schwartz DA, Wright JR. Complement levels and activity in the normal and LPS-injured lung. Am J Physiol Lung Cell Mol Physiol. 2007;292(3):L748–59. doi: 10.1152/ajplung.00127.2006. [DOI] [PubMed] [Google Scholar]
- 41.Marshall RP, Bellingan G, Webb S, Puddicombe A, Goldsack N, McAnulty RJ, Laurent GJ. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am J Respir Crit Care Med. 2000;162(5):1783–8. doi: 10.1164/ajrccm.162.5.2001061. [DOI] [PubMed] [Google Scholar]
- 42.Zhuo L, Hascall VC, Kimata K. Inter-alpha-trypsin inhibitor, a covalent protein-glycosaminoglycan-protein complex. J Biol Chem. 2004;279(37):38079–82. doi: 10.1074/jbc.R300039200. [DOI] [PubMed] [Google Scholar]
- 43.Matsuzaki H, Kobayashi H, Yagyu T, Wakahara K, Kondo T, Kurita N, Sekino H, Inagaki K, Suzuki M, Kanayama N, Terao T. Bikunin inhibits lipopolysaccharide-induced tumor necrosis factor alpha induction in macrophages. Clin Diagn Lab Immunol. 2004;11(6):1140–7. doi: 10.1128/CDLI.11.6.1140-1147.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanayama S, Yamada Y, Onogi A, Shigetomi H, Ueda S, Tsuji Y, Haruta S, Kawaguchi R, Yoshida S, Sakata M, Sado T, Kitanaka T, Oi H, Yagyu T, Kobayashi H. Bikunin suppresses expression of pro-inflammatory cytokines induced by lipopolysaccharide in neutrophils. J Endotoxin Res. 2007;13(6):369–76. doi: 10.1177/0968051907086464. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi H. Endogenous anti-inflammatory substances, inter-alpha-inhibitor and bikunin. Biol Chem. 2006;387(12):1545–9. doi: 10.1515/BC.2006.192. [DOI] [PubMed] [Google Scholar]
- 46.Lauredo IT, Forteza RM, Botvinnikova Y, Abraham WM. Leukocytic cell sources of airway tissue kallikrein. Am J Physiol Lung Cell Mol Physiol. 2004;286(4):L734–40. doi: 10.1152/ajplung.00129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenthal MD, Gordon MN, Buescher ES, Slusser JH, Harris LK, Franson RC. Human neutrophils store type II 14-kDa phospholipase A2 in granules and secrete active enzyme in response to soluble stimuli. Biochem Biophys Res Commun. 1995;208(2):650–6. doi: 10.1006/bbrc.1995.1388. [DOI] [PubMed] [Google Scholar]
- 48.Venge P. Eosinophil activity in bronchial asthma. Allergy Proc. 1994;15(3):139–41. doi: 10.2500/108854194778702937. [DOI] [PubMed] [Google Scholar]
- 49.Bowler RP, Reisdorph N, Reisdorph R, Abraham E. Alterations in the human lung proteome with lipopolysaccharide. BMC Pulm Med. 2009;9 doi: 10.1186/1471-2466-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sohma H, Matsushima N, Watanabe T, Hattori A, Kuroki Y, Akino T. Ca(2+)-dependent binding of annexin IV to surfactant protein A and lamellar bodies in alveolar type II cells. Biochem J. 1995;312(Pt 1):175–81. doi: 10.1042/bj3120175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sohma H, Ohkawa H, Akino T, Kuroki Y. Binding of annexins to lung lamellar bodies and the PMA-stimulated secretion of annexin V from alveolar type II cells. J Biochem. 2001;130(3):449–55. doi: 10.1093/oxfordjournals.jbchem.a003005. [DOI] [PubMed] [Google Scholar]
- 52.Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, Hodge CL, Haase J, Janes J, Huss JW, 3rd, Su AI. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009;10(11):R130. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A. 2004;101(16):6062–7. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dezso Z, Nikolsky Y, Sviridov E, Shi W, Serebriyskaya T, Dosymbekov D, Bugrim A, Rakhmatulin E, Brennan RJ, Guryanov A, Li K, Blake J, Samaha RR, Nikolskaya T. A comprehensive functional analysis of tissue specificity of human gene expression. BMC Biol. 2008;6:49. doi: 10.1186/1741-7007-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu C, Gelius E, Liu G, Steiner H, Dziarski R. Mammalian peptidoglycan recognition protein binds peptidoglycan with high affinity, is expressed in neutrophils, and inhibits bacterial growth. J Biol Chem. 2000;275(32):24490–9. doi: 10.1074/jbc.M001239200. [DOI] [PubMed] [Google Scholar]
- 56.Yang D, Chertov O, Oppenheim JJ. Participation of mammalian defensins and cathelicidins in anti-microbial immunity: receptors and activities of human defensins and cathelicidin (LL-37) J Leukoc Biol. 2001;69(5):691–7. [PubMed] [Google Scholar]
- 57.Lehrer RI, Ganz T. Cathelicidins: a family of endogenous antimicrobial peptides. Curr Opin Hematol. 2002;9(1):18–22. doi: 10.1097/00062752-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 58.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5(14):1317–27. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 59.O'Grady NP, Preas HL, Pugin J, Fiuza C, Tropea M, Reda D, Banks SM, Suffredini AF. Local inflammatory responses following bronchial endotoxin instillation in humans. Am J Respir Crit Care Med. 2001;163(7):1591–8. doi: 10.1164/ajrccm.163.7.2009111. [DOI] [PubMed] [Google Scholar]
- 60.de Torre C, Ying SX, Munson PJ, Meduri GU, Suffredini AF. Proteomic analysis of inflammatory biomarkers in bronchoalveolar lavage. Proteomics. 2006;6(13):3949–57. doi: 10.1002/pmic.200500693. [DOI] [PubMed] [Google Scholar]
- 61.Nick JA, Coldren CD, Geraci MW, Poch KR, Fouty BW, O'Brien J, Gruber M, Zarini S, Murphy RC, Kuhn K, Richter D, Kast KR, Abraham E. Recombinant human activated protein C reduces human endotoxin-induced pulmonary inflammation via inhibition of neutrophil chemotaxis. Blood. 2004;104(13):3878–85. doi: 10.1182/blood-2004-06-2140. [DOI] [PubMed] [Google Scholar]
- 62.Theilgaard-Monch K, Jacobsen LC, Nielsen MJ, Rasmussen T, Udby L, Gharib M, Arkwright PD, Gombart AF, Calafat J, Moestrup SK, Porse BT, Borregaard N. Haptoglobin is synthesized during granulocyte differentiation, stored in specific granules, and released by neutrophils in response to activation. Blood. 2006;108(1):353–61. doi: 10.1182/blood-2005-09-3890. [DOI] [PubMed] [Google Scholar]
- 63.Coldren CD, Nick JA, Poch KR, Woolum MD, Fouty BW, O'Brien JM, Gruber MP, Zamora MR, Svetkauskaite D, Richter DA, He Q, Park JS, Overdier KH, Abraham E, Geraci MW. Functional and genomic changes induced by alveolar transmigration in human neutrophils. Am J Physiol Lung Cell Mol Physiol. 2006;291(6):L1267–76. doi: 10.1152/ajplung.00097.2006. [DOI] [PubMed] [Google Scholar]
- 64.Walters EH, Gardiner PV. Bronchoalveolar lavage as a research tool. Thorax. 1991;46(9):613–8. doi: 10.1136/thx.46.9.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haslam PL, Baughman RP. Report of ERS Task Force: guidelines for measurement of acellular components and standardization of BAL. Eur Respir J. 1999;14(2):245–8. doi: 10.1034/j.1399-3003.1999.14b01.x. [DOI] [PubMed] [Google Scholar]
- 66.Baharloo F, Veyckemans F, Francis C, Biettlot MP, Rodenstein DO. Tracheobronchial foreign bodies: presentation and management in children and adults. Chest. 1999;115(5):1357–62. doi: 10.1378/chest.115.5.1357. [DOI] [PubMed] [Google Scholar]
- 67.Qureshi A, Behzadi A. Foreign-body aspiration in an adult. Can J Surg. 2008;51(3):E69–70. [PMC free article] [PubMed] [Google Scholar]
- 68.Bull JL, Tredici S, Komori E, Brant DO, Grotberg JB, Hirschl RB. Distribution dynamics of perfluorocarbon delivery to the lungs: an intact rabbit model. J Appl Physiol. 2004;96(5):1633–42. doi: 10.1152/japplphysiol.01158.2003. [DOI] [PubMed] [Google Scholar]
- 69.Brody E, Gold L, Mehan M, Ostroff R, Rohloff J, Walker J, Zichi D. Life's Simple Measures: Unlocking the Proteome. J Mol Biol. 2012 doi: 10.1016/j.jmb.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 70.Mehan MR, Ayers D, Thirstrup D, Xiong W, Ostroff RM, Brody EN, Walker JJ, Gold L, Jarvis TC, Janjic N, Baird GS, Wilcox SK. Protein signature of lung cancer tissues. PLoS One. 2012;7(4):e35157. doi: 10.1371/journal.pone.0035157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Que LG, Stiles JV, Sundy JS, Foster WM. Pulmonary function, bronchial reactivity, and epithelial permeability are response phenotypes to ozone and develop differentially in healthy humans. J Appl Physiol. 2011;111(3):679–87. doi: 10.1152/japplphysiol.00337.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lucas JE, Thompson JW, Dubois LG, McCarthy J, Tillmann H, Thompson A, Shire N, Hendrickson R, Dieguez F, Goldman P, Schwarz K, Patel K, McHutchison J, Moseley MA. Metaprotein expression modeling for label-free quantitative proteomics. BMC Bioinformatics. 2012;13(1):74. doi: 10.1186/1471-2105-13-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.