Abstract
Exposure of aqueous solutions of DNA to X- or γ-rays, which induces hydroxyl radical as one of the major reactive oxygen species (ROS), can result in the generation of a battery of single-nucleobase and bulky DNA lesions. These include the (5′R) and (5′S) diastereomers of 8,5′-cyclo-2′-deoxyadenosine (cdA) and 8,5′-cyclo-2′-deoxyguanosine (cdG), which were also found to be present at appreciable levels in DNA isolated from mammalian cells and tissues. However, it remains unexplored how efficiently the cdA and cdG can be induced by Fenton-type reagents. By employing HPLC coupled with tandem mass spectrometry (LC-MS/MS/MS) with the use of the isotope-dilution technique, here we demonstrated that treatment of calf thymus DNA with Cu(II) or Fe(II), together with H2O2 and ascorbate, could lead to dose-responsive formation of both the (5′R) and (5′S) diastereomers of cdA and cdG, though the yields of cdG were 2–4 orders of magnitude lower than that of 8-oxo-7,8-dihydro-2′-deoxyguanosine. This result suggests that Fenton reaction may constitute an important endogenous source for the formation of the cdA and cdG. Additionally, the (5′R) diastereomers of cdA and cdG were induced at markedly higher levels than the (5′S) counterparts. This latter finding, in conjunction with the previous observations of similar or greater levels of the (5′S) than (5′R) diastereomers of the two lesions in mammalian tissues, furnishes an additional line of evidence to support the more efficient repair of the (5′R) diastereomers of the purine cyclonucleosides in mammalian cells.
Keywords: 8, 5′-cyclo-2′-deoxyguanosine; 8,5′-cyclo-2′-deoxyadenosine; Fenton reagent; DNA damage; reactive oxygen species; mass spectrometry
Introduction
Reactive oxygen species (ROS) are constantly induced by endogenous and exogenous sources and they can result in damage to DNA.1, 2 For instance, exposure of DNA to γ- or X-rays can lead to the formation of a variety of single-nucleobase and bulky DNA lesions.3–11 During normal aerobic metabolism, electrons leaking from the electron transport chain in mitochondria may couple with molecular O2 to yield superoxide anion radical (O2−•), which can be subsequently converted to H2O2 by superoxide dismutase. Being freely diffusible in the cellular environment, H2O2 may reach the nucleus and react with DNA-bound transition metal ions [e.g. Fe(II) or Cu(II)] to yield the highly reactive hydroxyl radical (•OH) via the Fenton-type reaction.12 Along this line, earlier studies by Linn and coworkers 13–15 demonstrated the capability of Fenton-type reagents in inducing oxidatively generated lesions of DNA.
The importance of Fenton-type reaction in human diseases is manifested by genetic disorders associated with defects in handling transition metal ions, including Wilson’s disease 16 and iron overload disease.17 In this vein, we found previously that deficiency in the ortholog of human Wilson’s disease gene (i.e., Atp7b) and the ensuing aberrant accumulation of copper ions in hepatocytes led to elevated levels of oxidatively induced 8,5′-cyclo-2′-deoxyadenosine (cdA) and 8,5′-cyclo-2′-deoxyguanosine (cdG) lesions in liver tissues of Long-Evans Cinnamon rats.18
The cdA and cdG are unique oxidatively induced DNA lesions owing to the presence of an additional C–C bond between the C8 of the purine base and the C5′ of the 2-deoxyribose in the same nucleoside (Scheme 1). This additional covalent linkage introduces helical distortion to DNA and renders the N-glycosidic bond resistant toward acid-induced hydrolysis.19–22 Thus, cdA and cdG are attractive substrates for nucleotide excision repair (NER), but they are poor substrates for DNA glycosylase-mediated base excision repair (BER).23–25 Both the (5′R) and (5′S) diastereomers of cdA inhibit primer extension by T7 DNA polymerase as well as human DNA polymerase δ.23, 26, 27 Nevertheless, results from steady-state kinetic measurements showed that yeast and human polymerase η-mediated nucleotide incorporation opposite (5′S)-cdA and (5′S)-cdG was largely efficient and accurate.28 In addition, cdA and cdG strongly impede DNA transcription in mammalian cells and induce transcription mutagenesis.23, 24, 26
Scheme 1.
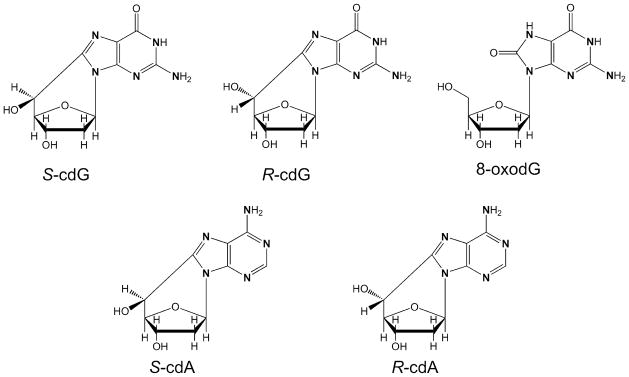
Chemical structures of synthesized 15N-labeled nucleosides. The “N” in bold represents [15N].
Recent studies have shown that both the (5′R) and (5′S) diastereomers of cdA and cdG have been detected in vitro and in vivo,18, 29–37 Nevertheless, it remains to be established how efficiently these lesions can be induced in DNA by Fenton-type reagents. Here we treated calf thymus DNA with Cu(II)/H2O2/ascorbate or Fe(II)/H2O2/ascorbate and analyzed the enzymatic digestion products of DNA by LC-MS/MS or LC-MS/MS/MS. We found that Fenton-type reagents could induce dose-dependent formation of cdA and cdG, with the (5′R) diastereomer being produced much more efficiently than its (5′S) counterpart.
Experimental Procedures
Materials
CuCl2, (NH4)2Fe(SO4)2•6H2O, L-methionine, L-ascorbic acid, calf thymus DNA, nuclease P1, alkaline phosphatase, and phosphodiesterases 1 and 2 were purchased from Sigma-Aldrich (St. Louis, MO). Hydrogen peroxide (30%) and erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA) hydrochloride were obtained from Fisher Scientific (Fair Lawn, NJ) and Tocris Bioscience (Ellisville, MO), respectively. The uniformly [15N]-labeled 8-oxodG, cdA and cdG were synthesized previously (Structures shown in Scheme 1).18, 38
Treatment of Calf Thymus DNA
Commercially available calf thymus DNA was desalted by ethanol precipitation. The DNA pellet was redissolved in a solution containing 25 mM NaCl and 50 mM phosphate (pH 7.0), and the DNA was annealed by heating the solution to 90°C for 5 min followed by cooling slowly to room temperature.
Aliquots of DNA (75 μg) were incubated with CuCl2 or (NH4)2Fe(SO4)2 (12.5–200 μM), H2O2 (0.1–1.6 mM), and ascorbate (1–16 mM) in a 250-μL solution containing 25 mM NaCl and 50 mM phosphate (pH 7.0) at room temperature under aerobic conditions for 60 min. In this vein, ascorbate was added to maintain copper and iron in the reduced state (i.e., Cu+ and Fe2+) so that they could participate in the Fenton reaction. Chemicals used in the Fenton-type reagent treatment of DNA were freshly prepared in doubly distilled water. Detailed concentrations of individual Fenton reagents used for the reactions are shown in Table 1. After 60 min, the reactions were terminated by adding excess L-methionine, and the DNA samples were again desalted by ethanol precipitation.
Table 1.
Concentrations of Fenton Type Reagents Employed for the Treatment of Calf Thymus DNA.a
| Control | A | B | C | D | E | |
|---|---|---|---|---|---|---|
| Cu(II)/Fe(II) (μM) | 200 | 12.5 | 25 | 50 | 100 | 200 |
| H2O2 (μM) | 0 | 100 | 200 | 400 | 800 | 1600 |
| Ascorbate (mM) | 0 | 1.0 | 2.0 | 4.0 | 8.0 | 16.0 |
All reactions were carried out in a 250-μL solution containing 75 μg of calf thymus DNA.
Enzymatic Digestion of DNA
To the above desalted DNA samples were added 8 units of nuclease P1, 0.01 unit of phosphodiesterase 2, 20 nmol of EHNA and a 20-μL solution containing 300 mM sodium acetate (pH 5.6) and 10 mM zinc chloride. EHNA was added to the enzymatic digestion mixture to prevent the possible deamination induced by residual contamination of adenine deaminase present in some commercial preparations of enzymes used in DNA digestion.39 After a 48-hr incubation at 37°C, 8 units of alkaline phosphatase, 0.02 unit of phosphodiesterase 1, and 40 μL of 0.5 M Tris-HCl (pH 8.9) were added to the digestion mixture. The solution was further incubated at 37°C for 2 h, after which the enzymes were removed by chloroform extraction and the solution dried by Speed-vac. DNA samples were then reconstituted in doubly distilled water and their concentration measured using UV-absorption spectrophotometry. To the mixture were then added uniformly [15N]-labeled 8-oxodG (500 fmol), R-cdG (200 fmol), S-cdG (100 fmol), R-cdA (100 fmol), and S-cdA (40 fmol, Scheme 1). The resulting aliquots were subjected directly to LC-MS/MS analysis (for 8-oxodG), or HPLC enrichment prior to LC-MS/MS/MS analysis (for cdA and cdG).
HPLC Enrichment
A 4.6×250 mm Alltima HP C18 column (5 μm in particle size, Grace Davison, Deerfield, IL) was used for the enrichment of the oxidatively induced cdA and cdG lesions from the enzymatic digestion products of DNA. The flow rate was 1 mL/min, and the mobile phases were 10 mM ammonium formate (solution A) and methanol (solution B). A gradient of 25 min 0–2% B, 1 min at 2% B, 14 min 2–5% B, 1 min 5–15% B, and 20 min at 15% B was employed. The HPLC fractions eluting at 12–18, 31.5–41.5, 42.5–50.5, 63–71.5 min were pooled for R-cdG, R-cdA, S-cdG, and S-cdA, respectively (Figure S1). The collected fractions were dried in the Speed-vac, redissolved in H2O, and subjected to LC-MS/MS/MS analysis.
LC-MS/MS Analysis of 8-oxodG
A 3×100 mm Hypersil Gold column (5 μm in particle size, Thermo, San Jose, CA) and an Accela 600 HPLC pump (Thermo) were used, and the flow rate was 50 μL/min. A solution of 0.1% (v/v) formic acid in doubly distilled water (solution A) and a solution of 0.1% (v/v) formic acid in methanol (solution B) were employed as mobile phases. The gradient included 0–90% B in 30 min and 90% B in 5 min. The effluent from the LC column was directed to a TSQ Vantage triple quadrupole mass spectrometer (Thermo). The instrument was operated in multiple-reaction monitoring (MRM) mode, and the MRM transitions for 8-oxodG and its uniformly 15N-labeled standard were m/z 284→168 and m/z 289→173, respectively.
LC-MS/MS/MS Analysis of cdA and cdG
An Agilent 1100 capillary HPLC pump (Agilent Technologies) and a 0.5×250 mm Zorbax SB-C18 column (particle size, 5 μm, Agilent) were used for the separation. A solution of 0.1% (v/v) formic acid in water (solution A) and a solution of 0.1% (v/v) formic acid in methanol (solution B) were used as mobile phases, and the gradient included 20 min 0–15% B, 10 min 15–35% B, and 10 min 35–60% B. The flow rate was 10 μL/min.
The effluent from the LC column was directed to an LTQ linear ion-trap mass spectrometer (Thermo), which monitored the fragmentation of the [M+H]+ ions of labeled and unlabeled R-cdG, S-cdG, R-cdA, S-cdA, and the further fragmentation of the corresponding [M+H–86]+ fragments found in MS/MS.
Results
LC-MS/MS/MS Identification and Quantification of cdA and cdG in Calf Thymus DNA Exposed with Cu(II)/H2O2/Ascorbate
We set out to investigate how efficiently the cdA and cdG lesions could be induced by Fenton-type reagents in isolated DNA. To this end, we treated calf thymus DNA with various concentrations of Cu(II)/H2O2/ascorbate (Table 1), digested the DNA with a cocktail of four enzymes, enriched cdA and cdG from the resulting nucleoside mixture and subjected them to LC-MS/MS or LC-MS3 analysis by using the corresponding uniformly [15N]-labeled nucleosides as internal standards (see Experimental Procedures).
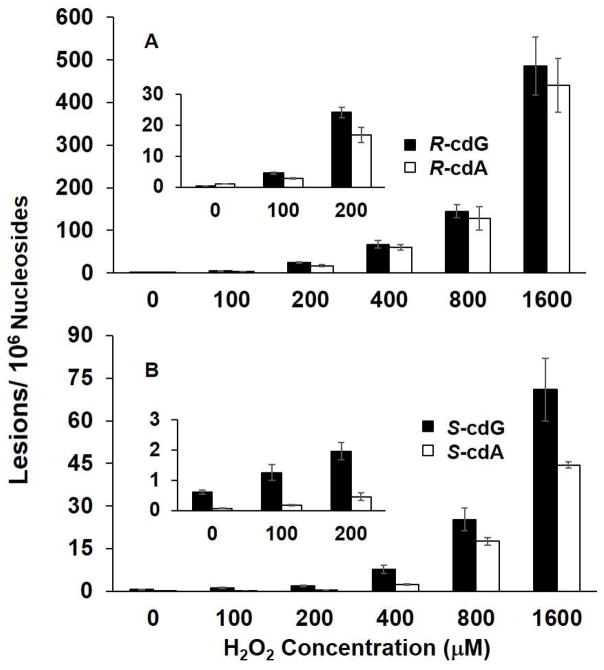
In the LC-MS/MS/MS experiment, we monitored the m/z 250→164→136 and m/z 255→169→141 transitions for (5′S)-cdA and its uniformly [15N]-labeled counterpart, respectively. The selected-ion chromatograms (SICs) and MS3 spectra for the unlabeled and [15N]-labeled (5′S)-cdA are displayed in Figure 1. The identity of the component eluting at 22.6 min in the SIC (Figure 1A&B) was found to be (5′S)-cdA by the co-elution, and similar fragment ions found in MS3, of the analyte and its uniformly [15N]-labeled standard. Ions of m/z 164 and m/z 169 arise from the neutral loss of a 86-Da fragment via cleavages of both the N-glycosidic linkage and the bond between the C5′ and C4′ of the 2-deoxyribose moiety of (5′S)-cdA and uniformly [15N]-labeled (5′S)-cdA, respectively. Further cleavage of the ion of m/z 164 led to the elimination of a CO molecule to give the major fragment ion at m/z 136. Corresponding fragment ions were observed in the MS3 of the uniformly [15N]-labeled (5′S)-cdA. (5′R)-cdA was found to yield the same fragment ions in both MS/MS and MS3 as (5′S)-cdA, though the two diastereomers exhibited different elution time on a reversed phase C18 column (Figure S2). Moreover, the amounts of (5′R)- and (5′S)-cdA are significantly lower in the control samples without hydrogen peroxide treatment (Figure 2 and Table S1), supporting that Cu(II)/H2O2/ascorbate can induce the formation of these two lesions.
Figure 1.
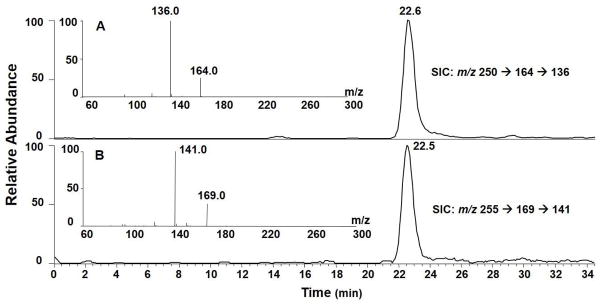
Selected-ion chromatograms (SICs) for monitoring the m/z 250→164→136 [A, for unlabeled (5′S)-cdA] and m/z 255→169→141 [B, for uniformly 15N-labeled (5′S)-cdA] transitions in the Cu(II)/H2O2/ascorbate-treated calf thymus DNA after enzymatic digestion. Shown in the insets are the positive-ion MS3 spectra for the unlabeled and labeled (5′S)-cdA. The sample was treated under Conditions B listed in Table 1.
Figure 2.
Cu(II)/H2O2/ascorbate-induced formation of cPu lesions in calf thymus DNA: A, (5′R)-cdG and (5′R)-cdA; B, (5′S)-cdG and (5′S)-cdA. The values represent the means ± S.D. of results from three independent experiments. The corresponding concentrations of Cu(II) and ascorbate are shown in Table 1.
We also observed the presence of (5′R)- and (5′S)-cdG based on the peaks at 11.6 and 21.4 min found in the SIC for monitoring the m/z 266→180→163 transition (Figures S3&S4). These components exhibit identical retention times as their respective isotope-labeled internal standards. Moreover, the major fragment ion at m/z 180 arises again from the cleavages of the C5′-C4′ bond of 2-deoxyribose and the N-glycosidic bond in cdG.40 Further fragmentation of the ion of m/z 180 yielded three major fragment ions of m/z 163, 152, and 135 in MS/MS/MS, which emanate from the neutral losses of NH3, CO, and [NH3 + CO], respectively (Figures S3&S4). The corresponding fragment ions were found in the MS3 for both diastereomers of the internal standards (Figures S3&S4). LC-MS3 quantification results showed that the treatment of calf-thymus DNA with Cu(II)/H2O2/ascorbate induced the dose-dependent formation of both diastereomers of cdG (Figure 2 and Table S1).
LC-MS/MS/MS Identification and Quantification of cdA and cdG Lesions Formed in Calf Thymus DNA Treated with Fe(II)/H2O2/Ascorbate
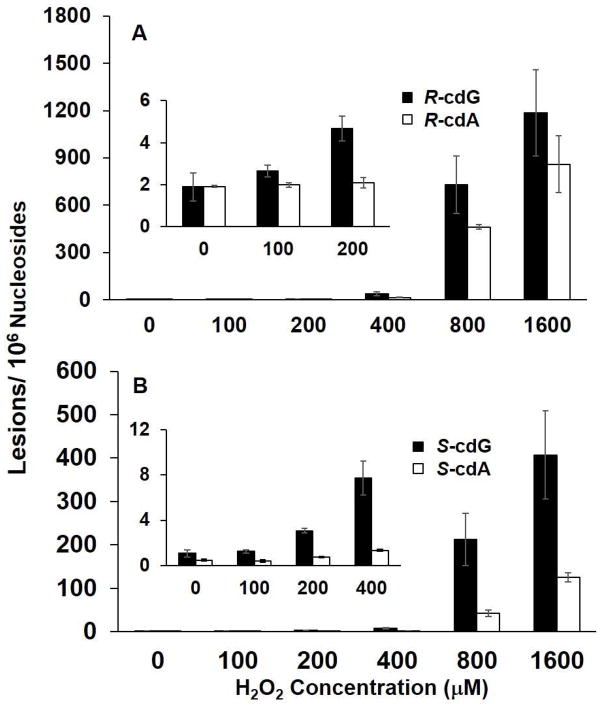
Iron is another biologically important transition metal that can participate in Fenton-type reactions.13, 14, 41 Iron’s ability to generate highly mutagenic lesions in genomic DNA has been linked with iron-induced carcinogenesis in iron-overload diseases.17 Thus, we also assessed the formation of cdA and cdG lesions in calf thymus DNA treated with Fe(II)/H2O2/ascorbate (Table 1). It turned out that there is again a dose-dependent increase in the formation of the (5′R) and (5′S) diastereomers of cdA and cdG (Figure 3 and Table S2).
Figure 3.
Fe(II)/H2O2/ascorbate-induced formation of cPu lesions in calf thymus DNA: A, (5′R)- cdG and (5′R)-cdA; B, (5′S)-cdG and (5′S)-cdA. The values represent the means ± S.D. of results from three independent experiments. The corresponding concentrations of Fe(II) and ascorbate are listed in Table 1.
Comparison of the levels of the cdA and cdG lesions induced by the two Fenton systems revealed that the yields of cdA and cdG were lower when Cu(II) was replaced with Fe(II) under reaction conditions A through C (Figures 2&3 and Tables S1&S2). However, we observed an opposite trend when the transition metal ion concentrations exceeded 100 μM (i.e., conditions D and E, Figures 2&3 and Tables S1&S2).
LC-MS/MS Quantification of 8-oxodG Formed in Calf Thymus DNA Treated with Fe(II)/H2O2/Ascorbate
Ionizing radiation-induced formation of cdA is thought to proceed through an •OH-mediated hydrogen abstraction from the C5′ of the 2-deoxyribose. The resultant C5′ radical then couples with the C8 of the purine base to form the additional covalent bond between the nucleobase and 2-deoxyribose in the same nucleoside. Viewing that •OH can also result in the formation of single-nucleobase lesions, it is important to compare the yields for the formation of cdA and cdG lesions with respect to single-nucleobase lesions in both transition metal systems. Previous studies showed that Fenton reagents, Cu(II)/H2O2/ascorbate, can induce single-nucleobase lesion, 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) in isolated DNA.38, 42 A comparison of the quantification results for Cu(II)/H2O2/ascorbate-induced cdG with these previously published quantification data of 8-oxodG under the same experimental conditions38 showed that cdG was induced at a level that is 2–4 orders of magnitude lower than 8-oxodG. Here we further assessed the generation of 8-oxodG via the Fe(II)/H2O2/ascorbate system, and our results revealed that the yield of 8-oxodG was ~50–2500 fold greater than the combined yield of the two diastereomers of cdG (Figures 3&4 and Tables S2&S3).
Figure 4.
Fe(II)/H2O2/ascorbate-induced formation of 8-oxodG in calf thymus DNA. The values represent the means ± S.D. of results from three independent oxidation and quantification experiments.
It is worth noting that Cu2+/H2O2 was found to induce singlet oxygen (1O2) generation43, 44 and the incubation of Cu+ or Cu2+ complex with H2O2 was observed to induce one-electron oxidation of DNA 45. Both singlet oxygen and one-electron oxidation could result in the formation of 8-oxodG, but not cdA or cdG. Ascorbate, however, is a known scavenger for singlet oxygen 46 and the inclusion of excess amount of ascorbate in the reaction (Table 1) should also minimize one-electron oxidation of DNA by maintaining copper ion in the +1 oxidation state. Thus, we reason that the markedly lower yield observed for cdA and cdG than 8-oxodG is unlikely due to the involvement of singlet oxygen or one-electron oxidation.
Discussion
Here we demonstrated, by using LC-MS/MS/MS with the standard isotope dilution technique, that the treatment of isolated DNA with Cu(II)/H2O2/ascorbate or Fe(II)/H2O2/ascorbate can lead to the formation of both the (5′R) and (5′S) diastereomers of cdA and cdG. Additionally, the yields of the cdA and cdG lesions exhibit a dose-dependent increase at low concentration ranges followed by a marked increase at 800 μM Cu(II) or Fe(II) (Figures 2&3 and Table S1&S2). These results are reminiscent of previous findings about the formation of intrastrand cross-link lesions in calf thymus DNA exposed with Fenton reagents under similar conditions,38, 42 suggesting that binding to Cu(II) and Fe(II) may result in an alteration in DNA conformation.41 Although cdA and cdG can be detected in calf thymus DNA without treatment with Fenton reagents, which is consistent with the previous finding,34 it is apparent that Fe(II)/H2O2/ascorbate and Cu(II)/H2O2/ascorbate could induce the dose-responsive formation of these lesions. Our results also revealed that Fe(II)/H2O2/ascorbate at the two highest concentrations (conditions D and E) were at least ~ 2–8 fold as effective as Cu(II)/H2O2/ascorbate system in inducing the cdA and cdG lesions, though the latter is more efficient in inducing these lesions at lower concentrations. Our observations with calf thymus DNA suggested that Fenton reaction may constitute an important endogenous source for the formation of cdA and cdG in mammalian tissues.
In keeping with previous results from treatment with γ rays,29 our data demonstrated that treatment with Fenton reagents also led to the preferential formation of (5′R)- over (5′S)-diastereomers of both cdA and cdG. However, these results were inconsistent with another study showing the preferential formation of (5′S)- over (5′R)-cdG in calf thymus DNA upon exposure to γ rays.40 The exact reason for this discrepancy is unclear, though it is possible that less specific LC-MS or GC-MS technique used in the previous study40 may lead to inaccurate measurements of the cdA and cdG lesions. Recent LC-MS/MS/MS quantification studies revealed that the (5′S) diastereomers of cdA and cdG are present at similar or higher levels than the corresponding (5′R) diastereomers in DNA isolated from mammalian tissues.18, 36, 37 This result is in line with the more efficient repair of the (5′R) diastereomer than its (5′S) counterpart; indeed it was observed that a substrate housing a (5′R)-cdA was cleaved more efficiently than the corresponding (5′S)-cdA substrate by NER activities in mammalian nuclear extract.23
cdA and cdG are known substrates for NER, but not BER.23, 24, 47 Our results showed that these lesions could be induced by Fenton-type reagent as efficiencies that are 2–4 orders of magnitude lower than that of 8-oxodG under the same experimental conditions (Figures 2–4). The detection of appreciable levels of the cdA and cdG lesions in DNA isolated from mammalian tissues18, 36, 37, 48 suggests that these lesions might be more resistant to repair than the oxidatively generated single-nucleobase lesions (e.g., 8-oxodG). In addition, cdA was a strong block to DNA polymerase δ,23 and both cdA and cdG strongly inhibit transcription by RNA polymerase II in vitro and in mammalian cells.23, 24, 26 Thus, these lesions could accumulate in patients with deficiency in NER and become cytotoxic. Neurons consume a vast amount of oxygen, rendering the central nervous system susceptible to ROS-induced DNA damage. In this vein, xeroderma pigmentosum (XP) patients suffer from a progressive, yet massive, neuron loss, which is accompanied with mental deterioration over several decades, and these patients also manifest an elevated frequency of internal cancers.49 The induction of cdA and cdG lesions by Fenton-type reagents reported here provide important new knowledge toward understanding the role of these lesions in the pathological symptoms of XP patients or with deficiencies in handling transition metal ions, such as Cu(II) or Fe(II).
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by the National Institutes of Health (R01 CA101864).
Abbreviations
- ROS
reactive oxygen species
- cdA
8,5′-cyclo-2′-deoxyadenosine
- cdG
8,5′-cyclo-2′-deoxyguanosine
- 8-oxodG
8-oxo-7,8-dihydro-2′-deoxyguanosine
- EHNA
erythro-9-(2-hydroxy-3-nonyl)adenine
- SIC
selected-ion chromatogram
- NER
nucleotide excision repair
- BER
base excision repair
- XP
xeroderma pigmentosum
Footnotes
Supporting Information Available: HPLC enrichment trace, LC-MS/MS/MS data, calibration curves, and the numerical values about the levels of DNA lesions. This material is available free of charge via Internet at http://pubs.acs.org.
References
- 1.Lindahl T. DNA lesions generated in vivo by reactive oxygen species, their accumulation and repair. NATO ASI Ser Ser A. 1999;302:251–257. [Google Scholar]
- 2.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 3.Box HC, Budzinski EE, Dawidzik JD, Wallace JC, Evans MS, Gobey JS. Radiation-induced formation of a crosslink between base moieties of deoxyguanosine and thymidine in deoxygenated solutions of d(CpGpTpA) Radiat Res. 1996;145:641–643. [PubMed] [Google Scholar]
- 4.Box HC, Budzinski EE, Dawidzik JB, Gobey JS, Freund HG. Free radical-induced tandem base damage in DNA oligomers. Free Radic Biol Med. 1997;23:1021–1030. doi: 10.1016/s0891-5849(97)00166-4. [DOI] [PubMed] [Google Scholar]
- 5.Budzinski EE, Dawidzik JB, Rajecki MJ, Wallace JC, Schroder EA, Box HC. Isolation and characterization of the products of anoxic irradiation of d(CpGpTpA) Int J Radiat Biol. 1997;71:327–336. doi: 10.1080/095530097144210. [DOI] [PubMed] [Google Scholar]
- 6.Box HC, Budzinski EE, Dawidzik JB, Wallace JC, Iijima H. Tandem lesions and other products in X-irradiated DNA oligomers. Radiat Res. 1998;149:433–439. [PubMed] [Google Scholar]
- 7.Romieu A, Bellon S, Gasparutto D, Cadet J. Synthesis and UV photolysis of oligodeoxynucleotides that contain 5-(phenylthiomethyl)-2′-deoxyuridine: A specific photolabile precursor of 5-(2′-deoxyuridilyl)methyl radical. Org Lett. 2000;2:1085–1088. doi: 10.1021/ol005643y. [DOI] [PubMed] [Google Scholar]
- 8.Bellon S, Ravanat JL, Gasparutto D, Cadet J. Cross-linked thymine-purine base tandem lesions: synthesis, characterization, and measurement in gamma-irradiated isolated DNA. Chem Res Toxicol. 2002;15:598–606. doi: 10.1021/tx015594d. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Q, Wang Y. Independent generation of 5-(2′-deoxycytidinyl)methyl radical and the formation of a novel cross-link lesion between 5-methylcytosine and guanine. J Am Chem Soc. 2003;125:12795–12802. doi: 10.1021/ja034866r. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, Wang Y. Independent generation of the 5-hydroxy-5,6-dihydrothymidin-6-yl radical and its reactivity in dinucleoside monophosphates. J Am Chem Soc. 2004;126:13287–13297. doi: 10.1021/ja048492t. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y. Bulky DNA lesions induced by reactive oxygen species. Chem Res Toxicol. 2008;21:276–281. doi: 10.1021/tx700411g. [DOI] [PubMed] [Google Scholar]
- 12.Cadet J, Delatour T, Douki T, Gasparutto D, Pouget JP, Ravanat JL, Sauvaigo S. Hydroxyl radicals and DNA base damage. Mutat Res. 1999;424:9–21. doi: 10.1016/s0027-5107(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 13.Henle ES, Luo Y, Gassmann W, Linn S. Oxidative damage to DNA constituents by iron-mediated fenton reactions. The deoxyguanosine family. J Biol Chem. 1996;271:21177–21186. [PubMed] [Google Scholar]
- 14.Imlay JA, Chin SM, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240:640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 15.Imlay JA, Linn S. DNA damage and oxygen radical toxicity. Science. 1988;240:1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- 16.Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. Wilson’s disease. Lancet. 2007;369:397–408. doi: 10.1016/S0140-6736(07)60196-2. [DOI] [PubMed] [Google Scholar]
- 17.Toyokuni S. Iron-induced carcinogenesis: the role of redox regulation. Free Radic Biol Med. 1996;20:553–566. doi: 10.1016/0891-5849(95)02111-6. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Yuan B, Guerrero C, Bahde R, Gupta S, Wang Y. Quantification of oxidative DNA lesions in tissues of Long-Evans Cinnamon rats by capillary high-performance liquid chromatography-tandem mass spectrometry coupled with stable isotope-dilution method. Anal Chem. 2011;83:2201–2209. doi: 10.1021/ac103099s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das RS, Samaraweera M, Morton M, Gascon JA, Basu AK. Stability of N-glycosidic bond of (5′S)-8,5′-cyclo-2′-deoxyguanosine. Chem Res Toxicol. 2012;25:2451–2461. doi: 10.1021/tx300302a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang H, Das RS, Basu AK, Stone MP. Structure of (5′S)-8,5′-cyclo-2′-deoxyguanosine in DNA. J Am Chem Soc. 2011;133:20357–20368. doi: 10.1021/ja207407n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theruvathu JA, Jaruga P, Dizdaroglu M, Brooks PJ. The oxidatively induced DNA lesions 8,5′-cyclo-2′-deoxyadenosine and 8-hydroxy-2′-deoxyadenosine are strongly resistant to acid-induced hydrolysis of the glycosidic bond. Mech Ageing Dev. 2007;128:494–502. doi: 10.1016/j.mad.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaliznyak T, Lukin M, de los Santos C. Structure and stability of duplex DNA containing (5′S)-5′,8-cyclo-2′-deoxyadenosine: an oxidatively generated lesion repaired by NER. Chem Res Toxicol. 2012;25:2103–2111. doi: 10.1021/tx300193k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuraoka I, Bender C, Romieu A, Cadet J, Wood RD, Lindahl T. Removal of oxygen free-radical-induced 5′,8-purine cyclodeoxynucleosides from DNA by the nucleotide excision-repair pathway in human cells. Proc Natl Acad Sci USA. 2000;97:3832–3837. doi: 10.1073/pnas.070471597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooks PJ, Wise DS, Berry DA, Kosmoski JV, Smerdon MJ, Somers RL, Mackie H, Spoonde AY, Ackerman EJ, Coleman K, Tarone RE, Robbins JH. The oxidative DNA lesion 8,5′-(S)-cyclo-2′-deoxyadenosine is repaired by the nucleotide excision repair pathway and blocks gene expression in mammalian cells. J Biol Chem. 2000;275:22355–22362. doi: 10.1074/jbc.M002259200. [DOI] [PubMed] [Google Scholar]
- 25.Pande P, Das RS, Sheppard C, Kow YW, Basu AK. Repair efficiency of (5′S)-8,5′-cyclo-2′-deoxyguanosine and (5′S)-8,5′-cyclo-2′-deoxyadenosine depends on the complementary base. DNA Repair (Amst) 2012;11:926–931. doi: 10.1016/j.dnarep.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marietta C, Brooks PJ. Transcriptional bypass of bulky DNA lesions causes new mutant RNA transcripts in human cells. EMBO Rep. 2007;8:388–393. doi: 10.1038/sj.embor.7400932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuraoka I, Robins P, Masutani C, Hanaoka F, Gasparutto D, Cadet J, Wood RD, Lindahl T. Oxygen free radical damage to DNA. Translesion synthesis by human DNA polymerase eta and resistance to exonuclease action at cyclopurine deoxynucleoside residues. J Biol Chem. 2001;276:49283–49288. doi: 10.1074/jbc.M107779200. [DOI] [PubMed] [Google Scholar]
- 28.Swanson AL, Wang J, Wang Y. Accurate and efficient bypass of 8,5′-cyclopurine-2′-deoxynucleosides by human and yeast DNA polymerase η. Chem Res Toxicol. 2012;25:1682–1691. doi: 10.1021/tx3001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belmadoui N, Boussicault F, Guerra M, Ravanat JL, Chatgilialoglu C, Cadet J. Radiation-induced formation of purine 5′,8-cyclonucleosides in isolated and cellular DNA: high stereo specificity and modulating effect of oxygen. Org Biomol Chem. 2010;8:3211–3219. doi: 10.1039/c004531d. [DOI] [PubMed] [Google Scholar]
- 30.Dizdaroglu M. Free-radical-induced formation of an 8,5′-cyclo-2′-deoxyguanosine moiety in deoxyribonucleic acid. Biochem J. 1986;238:247–254. doi: 10.1042/bj2380247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dizdaroglu M, Dirksen ML, Jiang HX, Robbins JH. Ionizing-radiation-induced damage in the DNA of cultured human cells. Identification of 8,5-cyclo-2-deoxyguanosine. Biochem J. 1987;241:929–932. doi: 10.1042/bj2410929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Errico M, Parlanti E, Teson M, de Jesus BM, Degan P, Calcagnile A, Jaruga P, Bjoras M, Crescenzi M, Pedrini AM, Egly JM, Zambruno G, Stefanini M, Dizdaroglu M, Dogliotti E. New functions of XPC in the protection of human skin cells from oxidative damage. EMBO J. 2006;25:4305–4315. doi: 10.1038/sj.emboj.7601277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez H, Jaruga P, Leber D, Nyaga SG, Evans MK, Dizdaroglu M. Lymphoblasts of women with BRCA1 mutations are deficient in cellular repair of 8,5′-cyclopurine-2′-deoxynucleosides and 8-hydroxy-2′-deoxyguanosine. Biochem J. 2007;46:2488–2496. doi: 10.1021/bi062022p. [DOI] [PubMed] [Google Scholar]
- 34.Jaruga P, Dizdaroglu M. 8,5′-Cyclopurine-2′-deoxynucleosides in DNA: mechanisms of formation, measurement, repair and biological effects. DNA Repair (Amst) 2008;7:1413–1425. doi: 10.1016/j.dnarep.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Kirkali G, de Souza-Pinto NC, Jaruga P, Bohr VA, Dizdaroglu M. Accumulation of (5′S)-8,5′-cyclo-2′-deoxyadenosine in organs of Cockayne syndrome complementation group B gene knockout mice. DNA Repair (Amst) 2009;8:274–278. doi: 10.1016/j.dnarep.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Clauson CL, Robbins PD, Niedernhofer LJ, Wang Y. The oxidative DNA lesions 8,5′-cyclopurines accumulate with aging in a tissue-specific manner. Aging Cell. 2012;11:714–716. doi: 10.1111/j.1474-9726.2012.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tilstra JS, Robinson AR, Wang J, Gregg SQ, Clauson CL, Reay DP, Nasto LA, St Croix CM, Usas A, Vo N, Huard J, Clemens PR, Stolz DB, Guttridge DC, Watkins SC, Garinis GA, Wang Y, Niedernhofer LJ, Robbins PD. NF-κB inhibition delays DNA damage–induced senescence and aging in mice. J Clin Invest. 2012;122:2601–2612. doi: 10.1172/JCI45785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong H, Cao H, Wang Y, Wang Y. Identification and quantification of a guanine-thymine intrastrand cross-link lesion induced by Cu(II)/H2O2/ascorbate. Chem Res Toxicol. 2006;19:614–621. doi: 10.1021/tx060025x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim KS, Jenner A, Halliwell B. Quantitative gas chromatography mass spectrometric analysis of 2′-deoxyinosine in tissue DNA. Nat Protoc. 2006;1:1995–2002. doi: 10.1038/nprot.2006.301. [DOI] [PubMed] [Google Scholar]
- 40.Jaruga P, Birincioglu M, Rodriguez H, Dizdaroglu M. Mass spectrometric assays for the tandem lesion 8,5′-cyclo-2′-deoxyguanosine in mammalian DNA. Biochemistry. 2002;41:3703–3711. doi: 10.1021/bi016004d. [DOI] [PubMed] [Google Scholar]
- 41.Rai P, Wemmer DE, Linn S. Preferential binding and structural distortion by Fe2+ at RGGG-containing DNA sequences correlates with enhanced oxidative cleavage at such sequences. Nucleic Acids Res. 2005;33:497–510. doi: 10.1093/nar/gki192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao H, Wang Y. Quantification of oxidative single-base and intrastrand cross-link lesions in unmethylated and CpG-methylated DNA induced by Fenton-type reagents. Nucleic Acids Res. 2007;35:4833–4844. doi: 10.1093/nar/gkm497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto K, Kawanishi S. Hydroxyl free radical is not the main active species in site-specific DNA damage induced by copper (II) ion and hydrogen peroxide. J Biol Chem. 1989;264:15435–15440. [PubMed] [Google Scholar]
- 44.Ma WJ, Cao EH, Qin JF. The involvement of singlet oxygen in copper-phenanthroline/H2O2-induced DNA base damage: a chemiluminescent study. Redox Rep. 1999;4:271–276. doi: 10.1179/135100099101535115. [DOI] [PubMed] [Google Scholar]
- 45.Frelon S, Douki T, Favier A, Cadet J. Hydroxyl radical is not the main reactive species involved in the degradation of DNA bases by copper in the presence of hydrogen peroxide. Chem Res Toxicol. 2003;16:191–197. doi: 10.1021/tx025650q. [DOI] [PubMed] [Google Scholar]
- 46.Shi M, Xu B, Azakami K, Morikawa T, Watanabe K, Morimoto K, Komatsu M, Aoyama K, Takeuchi T. Dual role of vitamin C in an oxygen-sensitive system: discrepancy between DNA damage and cell death. Free Radic Res. 2005;39:213–220. doi: 10.1080/10715760400022129. [DOI] [PubMed] [Google Scholar]
- 47.You C, Dai X, Yuan B, Wang J, Wang J, Brooks PJ, Niedernhofer LJ, Wang Y. A quantitative assay for assessing the effects of DNA lesions on transcription. Nat Chem Biol. 2012;8:817–822. doi: 10.1038/nchembio.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitra D, Luo X, Morgan A, Wang J, Hoang MP, Lo J, Guerrero CR, Lennerz JK, Mihm MC, Wargo JA, Robinson KC, Devi SP, Vanover JC, D’Orazio JA, McMahon M, Bosenberg MW, Haigis KM, Haber DA, Wang Y, Fisher DE. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature. 2012;491:449–453. doi: 10.1038/nature11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cleaver JE, Kraemer KH. The Metabolic Basis of Inherited Disease. McGraw-Hill; New York: 1989. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.