Abstract
A 75-year-old man presented to the emergency department (ED) for syncope and was found to have intermittent runs of torsades de pointes (TdP). The patient had a medical history significant for disseminated coccidiomycosis and was on prophylactic fluconazole. Forty-eight hours prior to presentation, the patient had intractable nausea and vomiting and was unable to take anything orally. He eventually presented to the ED with severe hypokalaemia and hypomagnesaemia with repeat symptomatic runs of TdP, which required overdrive transvenous pacing. During the patient’'s admission, his electrolytes were aggressively replete. Fluconazole was discontinued, and prior to discharge, the patient recovered fully with ECGs showing a normalisation of the QT interval.
Background
Torsades de pointes (TdP) is a life-threatening form of polymorphic ventricular tachycardia that requires prompt interventions. TdP can be caused by an acquired or congenitally prolonged QT interval. Acquired cases are often caused by drugs and exacerbated by electrolyte abnormalities, most frequently hypokalaemia or hypomagnesaemia. This case illustrates the risk factors for acquired TdP and the critical life-saving maneuveres in TdP. In addition, this case illustrates the importance of considering the cause of ventricular tachycardia. While monomorphic tachycardias are often related to ischaemia, in TdP, the aetiology is often related to a prolonged QT or electrolyte abnormality and requires different management as a result.1
Case presentation
A 75-year-old African-American man with a medical history of hypertension, hyperlipidemia and disseminated coccidioidomycosis presented to the emergency department (ED) with syncope. Forty-eight hours prior to presentation, the patient had intractable nausea and vomiting resulting in him being unable to take anything orally. On presentation to the ED he was bradycardic and found to have incessant episodes of TdP occurring every couple of minutes. The shorter episodes were a few seconds and the longer episodes persisted up to 20s. Each of these longer episodes would result in loss of consciousness. He denied chest pain or pressure, diaphoresis, palpitations, a history of arrhythmias or previous similar episodes.
He had developed hypokalaemia in the past and had been treated with supplemental potassium and diet modifications to include high potassium foods such as bananas and orange juice. Several weeks earlier, his potassium was noted to be 3.5 mmol/L.
His ED course was notable for a blood pressure of 180/64 mm Hg, heart rate of 35 bpm, respiratory rate of 20 breaths/min, oxygen saturation of 99% on room air and a temperature of 36.5°C. He had recurrent episodes of polymorphic ventricular tachycardia, consistent with TdP, occurring every couple of minutes each lasting approximately 10 s (figure 1). During each of these episodes, he would lose and regain consciousness. His potassium was noted to be low at 2.1 mmol/L (normal 3.5–5.1 mmol/L) and the magnesium was also low at 1.7 mg/dL (normal 1.7–2.6 mg/dL). He was given 80 mEq of potassium and 2 gm of magnesium intravenously and admitted to the intensive care unit.
Figure 1.
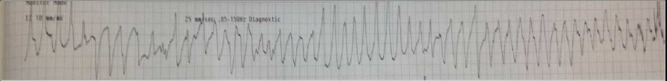
Patient's telemetry strip demonstrating polymorphic ventricular tachycardia consistent with torsades de pointes.
Investigations
His ECG was significant for sinus bradycardia, a QT interval of 600 ms and no ST elevations or depressions. A chest X-ray was unremarkable. Laboratories were significant for potassium of 2.1 mmol/L and magnesium of 1.7 mg/dL. An indeterminate level troponin-T of 0.012 ng/mL remained stable on repeat labs. Acute coronary syndrome as a potential aetiology was ruled out based on history, physical examination and a lack of dynamic ECG changes or significant cardiac markers.
After the patient was stabilised, an echocardiogram revealed a decreased ejection fraction and global hypokinesis. A cardiac nuclear perfusion stress test was performed with Regadenoson, which confirmed global hypokinesis with ejection fraction of 34% without reversible ischaemic aetiology. His hypokinesis and cardiomyopathy was presumed to be secondary to his tachycardia.
Treatment
In a patient with incessant TdP, it is important to treat the underlying cause, while at the same time, preventing any further episodes of TdP. In our patient, the fluconazole was discontinued and his potassium and magnesium were aggressively replaced. The patient was unable to tolerate oral potassium replacement secondary to his persistent nausea and vomiting, so he was initially replete with 20 mEq/h of potassium chloride administered through a central line and 10 mEq/h running in two peripheral lines for a total of 40 mEq/h. Within the first 12h of arrival to the hospital, a total of 200 mEq of intravenous potassium chloride was administered increasing the potassium from 2.1 to 2.4 mmol/L. He also received a total of 6 g of magnesium sulfate concurrently.
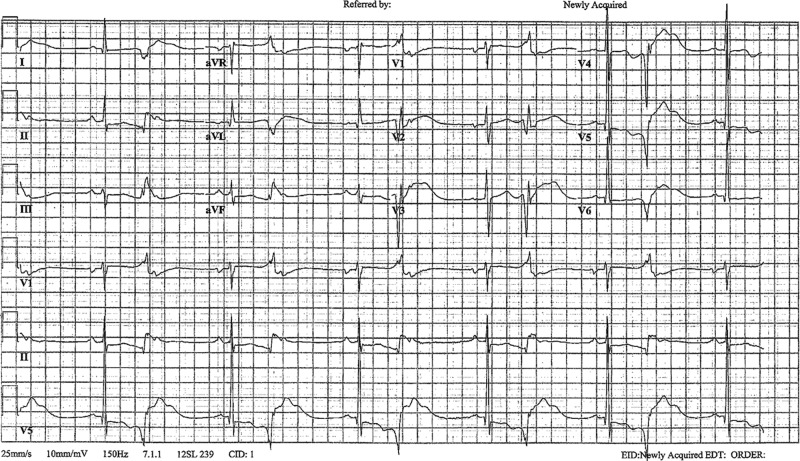
ECGs between episodes showed sinus bradycardia and a prolonged QT with ventricular premature contractions in a pattern of bigeminy (figure 2). This ectopy is superimposed on the vulnerable T wave repolarisation of the preceding beat causing a phenomenon known as R-on-T.2 He continued to have multiple intermittent runs of prolonged TdP up to 15s. On every occasion the arrhythmia spontaneously resolved before any attempt at defibrillation. Given the severity of the TdP and the slow correction of electrolytes, it was determined that temporary overdrive pacing would be necessary. A transjugular temporary pacer was placed and the patient was overdrive paced at a rate of 110 bpm with successful resolution of ectopy and TdP. The rate of 110 bpm was the slowest rate to eliminate allectopy.
Figure 2.
Presenting ECG: sinus bradycardia showing prolonged QT with bigeminy with K 2.1 mmol/L and Mg 1.7 mg/dL.
Outcome and follow-up
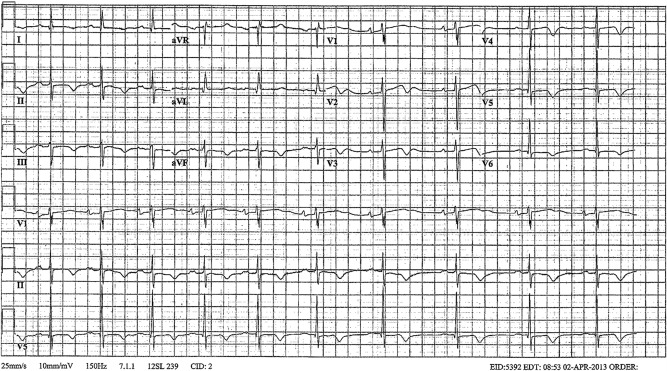
With the overdrive pacing, there was no further episodes of TdP. On hospital day 1, the patient continued to have ectopy with the pacemaker turned off. Therefore, we decided to continue the overdrive pacing to avoid any further R-on-T phenomena. The electrolytes on this day also continued to improve with the potassium at 3.7 mmol/L and the magnesium at 2.3 mg/dL. By hospital day 2, the potassium and magnesium normalised to 4.4 and 2 mg/dL, respectively. Now there was no further ectopy and the temporary pacemaker was turned off. A repeat ECG showed sinus bradycardia with P mitrale, and a prolonged QTc of 516 ms (figure 3).
Figure 3.
Repeat ECG: sinus bradycardia with prolonged QTc (516 ms) with K 3.7 mmol/L and Mg 2.3 mg/dL.
The patient had no further episodes of TdP during the remainder of his hospital admission. A transthoracic echo showed moderate left ventricular systolic dysfunction with an ejection fraction of 35% and global hypokinesis. Nuclear stress imaging showed fixed defects with global hypokinesis and no areas of reversible ischaemia. These findings were not felt to be directly related to the patient's episodes of TdP, and were likely related to prolonged hypertension. The patient was started on metoprolol for persistent hypertension. Losartan and spironolactone were added to help maintain blood control with the added effect of increasing the patient’'s serum potassium.
Primary mineralocorticoid excess and renovascular disease were considered in the setting of metabolic alkalosis with hypertension and hypokalaemia; however, renin and aldosterone levels were normal. Infectious disease was consulted for his disseminated cocciodomycosis with recommendations to discontinue fluconazole, with plans to try another antifungal for prophylaxis as an outpatient.
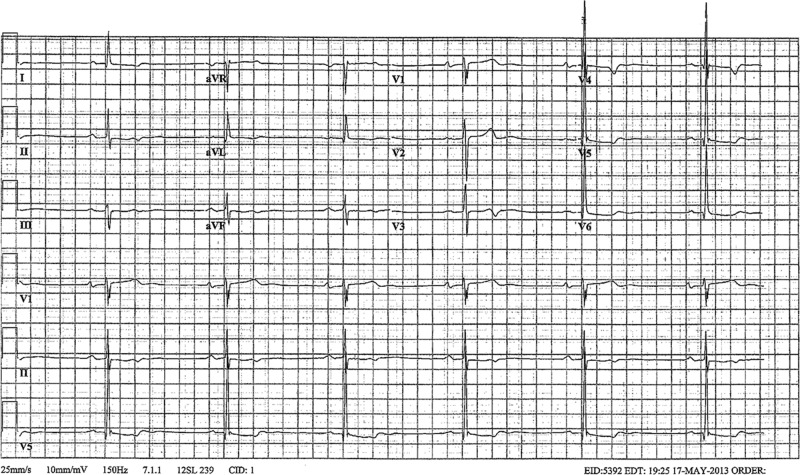
The patient's prolonged QT interval was a combination of the patient's chronic fluconazole therapy exacerbated by the hypokalaemia and hypomagnesia caused by the patient's vomiting. At the time of discharge, the patient was haemodynamically stable, tolerating oral medications, had normal electrolytes and a normal QT interval on ECG (figure 4). He was counselled to avoid QT prolonging medications in the future.
Figure 4.
ECG on discharge: normal QTc interval (401 ms) with normal K and Mg.
Discussion
TdP is a relatively rare life-threatening arrhythmia often associated with the use of QT prolonging drugs. Between 1983 and 1999, only 761 cases of drug-induced TdP were reported to the WHO Drug Monitoring Centre.3 While the number of documented cases likely underestimates the true incidence of drug-induced TdP, the actual number of events remains low. An observational study performed in the Netherlands found that only 3.1% of patients with sudden cardiac death were using QT prolonging drugs.4
Many drugs are associated with a prolonged QT interval with the major classes including antiarrhythmic drugs, macrolide antibiotics and certain psychotropic medications, gastric motility agents and non-sedating antihistamines.3 The azole class of antifungals has been implicated in QT prolongation with fluconazole accounting for the most frequently reported of this class.5 In almost all of these cases, concurrent use of additional QT prolonging agents and/or other risk factors for the development of a prolonged QT and TdP were present.6
The initial assessment in evaluating the risk for developing TdP begins with calculating the heart rate-corrected QT interval (QTc). A prolonged QTc is generally accepted as >470 ms for men and >480 ms for women. Studies have shown a gradual increase in risk for TdP as the QTc increases. A QTc >500 ms is considered highly abnormal for men as well as women and is associated with a twofold to threefold increased risk for developing TdP.7
The risk of TdP appears to increase in patients with multiple risk factors. A literature review showed that 97% of patients with TdP associated with non-cardiac drugs had at least one other risk factor and 71% had had at least two.8 In addition to the use of fluconazole, our patient had several risk factors that may have contributed to the development of TdP. These include advanced age, underlying heart disease, a baseline prolonged QT interval, sinus bradycardia and premature ventricular contractions.9 In addition, he was found to be significantly hypokalaemic at presentation which was likely a combination of recent vomiting and diuretic use. It has also been suggested that diuretic use may be considered a risk factor as well.9
While TdP is generally short lived with spontaneous termination, most patients experience multiple episodes in rapid succession, as illustrated by our case.10 The potential degeneration to ventricular fibrillation and sudden cardiac death makes the prompt delivery of therapy imperative. Immediate defibrillation is warranted in unstable patients. Administration of intravenous magnesium sulfate is considered as first-line therapy.10 11 The 2006 ACC/AHA/ESC guidelines for the acute management of TdP recommends withdrawal of any offending drugs and correction of electrolyte abnormalities as well as intravenous magnesium sulfate and the use of pacing for patients with heart block or symptomatic bradycardia.11 Isoproterenol can be used for patients with recurrent pause-dependent TdP without congenital long QT syndrome to increase the heart rate, thus reducing the QT interval.11 In patients with long QT syndrome, recurrent presyncope or syncope refractory to optimal medical management may require pacemaker and implantable cardioverter–defibrillator.10 The benefit of intravenous magnesium sulfate was even seen in patients with normal serum magnesium concentrations.10 12 Temporary transvenous overdrive pacing at rates of approximately 100 bpm has been demonstrated to be highly effective at terminating TdP and reducing recurrence of ectopic ventricular beats associated with drug induced long QT syndrome.10 11 Similar results have also been seen with the administration of isoproterenol to achieve a heart rate of 100 bpm. Amiodarone is a common treatment for monomorphic ventricular tachycardia, but may be contraindicated in cases of polymorphic ventricular tachycardia. Because amiodarone markedly prolongs the QT interval, it should be used cautiously, especially in the setting of a prolonged QT.1
Learning points.
Emergent maneuveres for refractory TdP involve cardioversion and magnesium sulfate intravenously.
In cases of recurrent and persistent TdP, evaluation of the underlying aetiology is important for management.
In cases of acquired long QT from a drug and/or severe electrolyte disturbances, overdrive pacing can provide a temporary solution until the drug metabolises and electrolytes normalise.
In the age of polypharmacy, the risks/benefits of QT prolonging agents must be carefully considered, especially in patients with congenitally prolonged QT syndrome or risk factors for acquired prolonged QT.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Neumar RW, Otto CW, Link MS, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010;2013(Suppl 3):S729–67 [DOI] [PubMed] [Google Scholar]
- 2.Engel TR, Meister SG, Frankl WS. The ‘R-on-T’ phenomenon: an update and critical review. Ann Intern Med 1978;2013:221–5 [DOI] [PubMed] [Google Scholar]
- 3.Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart 2003;2013:1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Straus SM, Sturkenboom MC, Bleumink GS, et al. Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur Heart J 2005;2013:2007. [DOI] [PubMed] [Google Scholar]
- 5.Poluzzi E, Raschi E, Motola D, et al. Antimicrobials and the risk of torsades de pointes: the contribution from data mining of the US FDA adverse event reporting system. Drug Saf 2010;2013:303–14 [DOI] [PubMed] [Google Scholar]
- 6.Pham CP, de Feiter PW, van der Kuy PH. Long QTc interval and torsade de pointes caused by fluconazole. Ann Pharmacother 2006;2013:1456–61 [DOI] [PubMed] [Google Scholar]
- 7.Drew BJ, Ackerman MJ, Funk M, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation 2010;2013:1047–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeltser D, Justo D, Halkin A, et al. Torsade de pointes due to non-cardiac drugs: most patients have easily identifiable risk factors. Medicine (Baltimore) 2003;2013:282. [DOI] [PubMed] [Google Scholar]
- 9.Drew BJ, Ackerman MJ, Funk M, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation 2010;2013:1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan IA. Long QT syndrome: diagnosis and management. Am Heart J 2002;2013:7. [DOI] [PubMed] [Google Scholar]
- 11.European Heart Rhythm Association, Heart Rhythm Society, Zipes DP, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for practice guidelines (Writing Committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death). J Am Coll Cardiol 2006;2013:e247. [DOI] [PubMed] [Google Scholar]
- 12.Tzivoni D, Banai S, Schuger C, et al. Treatment of torsade de pointes with magnesium sulfate. Circulation 1988;2013:392. [DOI] [PubMed] [Google Scholar]