Abstract
Proteins that switch between distinct conformational states are ideal to monitor and control molecular processes within the complexity of biological systems. Inspired by the modular architecture of natural signaling proteins, our group explores generic design strategies for the construction of FRET-based sensor proteins and other protein switches. Here I show that designing FRET sensors based on mutually exclusive domain interactions provides a robust method to engineer sensors with predictable properties and an inherently large change in emission ratio. The modularity of this approach should make it easily transferable to other applications of protein switches in fields ranging from synthetic biology, optogenetics and molecular diagnostics.
Keywords: fluorescent sensors, FRET, protein engineering, synthetic biology, intracellular imaging
Introduction
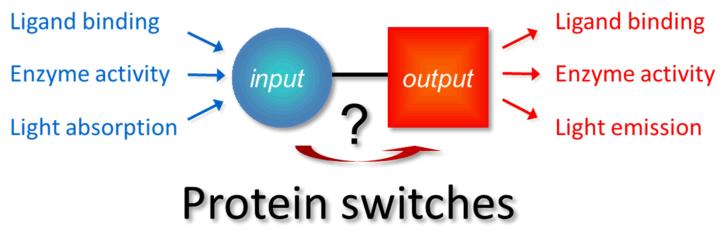
The availability of robust and generally applicable strategies to construct protein switches is vital for many areas of life sciences, providing essential tools for molecular imaging, synthetic biology and molecular diagnostics [1, 2]. The design of protein switches can be discussed in terms of an input module where molecular recognition takes place, an output module to generate a signal, and a mechanism through which the changes in the input module are translated to changes in the output function (Fig.1). Modular approaches in which the input and output functions are part of separate domains are attractive, because they enable a synthetic biology type of protein engineering in which proteins switches are constructed from a toolbox of structurally and functionally well-defined building blocks. Genetically-encoded fluorescent sensor proteins that use Förster Resonance Energy Transfer (FRET) between donor and acceptor fluorescent domains provide an excellent and very successful example of this modular design approach [3]. The distance and orientation dependence of FRET make it an attractive mechanism to translate conformational changes at an input domain into a change in fluorescence output, irrespective of the receptor domain and the nature of the protein-ligand interaction. An additional advantage of FRET-based fluorescent sensors is that their response is ratiometric, i.e. the ratio of donor and acceptor emission provides a measure of the ligand concentration that is independent of the absolute sensor concentration. This is an important practical benefit when measuring in complex biological samples where the sensor concentration cannot be easily controlled and background fluorescence is always present.
Figure 1.
Protein switches can be described as consisting of input and output modules. The protein engineering challenge is to ensure that a conformational change in the input module is efficiently translated into a robust change in the output function.
Despite the strong dependence of FRET on the distance and orientation between donor and acceptor domain and the modular architecture of FRET-based sensor proteins, it has proven difficult to translate conformational changes at a receptor domain into changes in emission ratio that are larger than 50% [3]. One of the reasons is that changes in distance are detected with the greatest sensitivity around the Förster distance, which is ~50 Å for the most common FRET pairs. Because the fluorophores are buried inside the fluorescent domains and the domains are separated by receptor domain(s), energy transfer is relatively inefficient in most sensors, resulting in small changes in FRET. Moreover, flexible linkers of at least a few amino acids are typically required between two domains to allow proper folding of each domain. The conformational flexibility of these linkers strongly attenuates effective translation of a conformational changes between domains, however. As a result the performance of FRET sensors typically depends on subtle conformational effects that are not well-understood. The most common approach in the development of FRET sensors has therefore been one of trial and error in which many constructs varying in linker lengths, receptor domains and fluorescent domains need to be tested in order to obtain a FRET sensor with a sufficiently large dynamic response [4, 5]. One of the ‘tricks’ that has been found to affect the performance of FRET sensor proteins is the use of circularly permuted fluorescent protein domains, but no general correlation seems to exist between the type of circularly permuted protein and its effect on the sensors dynamic range.
Using ‘sticky’ fluorescent domains in FRET sensor design
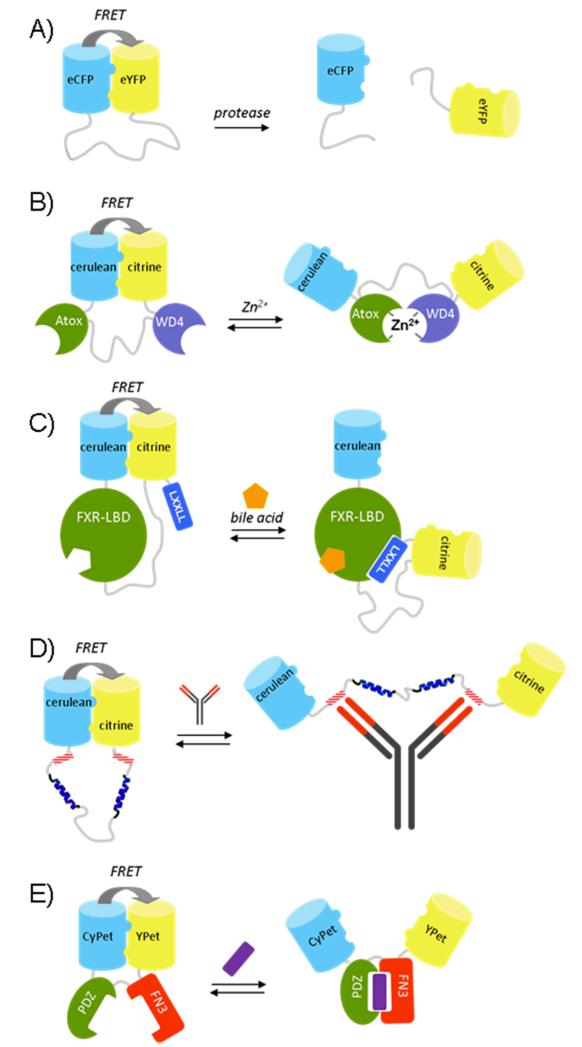
An important challenge for the rational design of FRET sensors is the difficulty to control the distribution of conformations in both the ‘on’ and the ‘off’ state. In recent years our group therefore pursued a different approach in which the in- and output domains are arranged in such way that the donor and acceptor fluorescent domains can interact in one state of the sensor, but not in the other state. This approach was inspired by a study of Daugherty and coworkers who used Fluorescence Assisted Cell Sorting (FACS) to evolve variants of CFP and YFP (CyPet and YPet) that, when linked via a flexible caspase recognition sequence, yielded a FRET sensor with a 20-fold increase in ratiometric change upon cleavage by caspase-3 [6]. The molecular origin for this spectacular increase in sensor performance initially remained unclear [7], but we and others subsequently showed that these improved sensor properties were caused by mutations at the protein exterior that promoted the formation of an intramolecular complex between the fluorescent domains [8, 9]. Whereas wild-type ECFP and EYFP do not form an intramolecular complex when connected by a long flexible linker, substitution of a single hydrophilic residue at the GFP dimerization interface (Ser208Phe) was sufficient to promote intramolecular association of the two domains, resulting in a large increase in energy transfer (Fig. 2A) [8, 10]. The introduction of a second mutation at the dimerization interface, V224L, did not by itself enhance intramolecular interactions between ECFP and EYFP, but it did induce a further increase in energy transfer efficiency in the presence of the S208F mutation. Cleavage of the linker by a protease resulted in a 16-fold decrease in emission ratio. The absence of any FRET after cleavage of the linker between the FP domains shows that interaction between these self-associating variants of ECFP and EYFP was readily disrupted, and no intermolecular complex was formed at the low micromolar concentrations used in these experiments.
Figure 2.
FRET sensor proteins based on self-associating donor and acceptor fluorescent domains: a protease sensor (A), the eCALWY Zn2+ sensor (B), a bile acid sensor based on the ligand binding domain of the nuclear receptor FXR (C) a FRET sensor that allows specific antibody detection in solution (D) and a FRET sensor based on an affinity clamp protein (E).
Can self-associating FPs also be used to increase the response of more typical FRET sensors, e.g. those that dependent on a ligand-induced interaction between two input domains? The first system in which we explored this concept was a genetically encoded FRET sensor for Zn2+ called CALWY. The initial version of this sensor consisted of two small metal binding domains (Atox1 and WD4) linked via a long flexible linker, with each domain providing two 2 cysteines to form a tetrahedral zinc binding pocket [11]. Remarkably, the Zn2+-induced intramolecular complex formation between Atox1 and WD4 resulted in a decrease in energy transfer. The emission ratio in the unbound state was strongly dependent on the linker length, whereas similar emission ratios were observed for the Zn2+-bound state. This behavior can be readily understood by considering the conformational behavior of the linker. The distance between the C-terminus of Atox1 and the N-terminus of WD4 in the Zn2+-complex is 50 Å. Even for the longest linker, the average end-to-end distance is smaller than 50 Å, resulting in a predicted net decrease in energy transfer upon formation of the Zn2+-bound complex. Since the low amount of FRET in the Zn2+-bound state is dictated by the structure of the metal-bound Atox-WD4 complex, we reasoned that the best way to improve the dynamic range of the CALWY sensors would be to promote the interaction of the fluorescent domains in the absence of Zn2+. Indeed, introduction of the S208F/V224L mutations on cerulean and citrine (improved variants of ECFP and EYFP) dramatically increased the emission ratio in the absence of Zn2+, while still allowing a switch to the low FRET state upon addition of Zn2+, corresponding to a 6-fold improvement in dynamic range to 240% (Fig. 2B) [12]. Interestingly, introduction of self-associating mutations in the FPs resulted in a 10-fold decrease in Zn2+-affinity, which is consistent with a mechanism that requires that the intramolecular interaction (ΔG0 of 1.7 kcal/mole, unpublished results) between the FPs needs to be broken before ligand binding can occur. Mutation of one of the cysteines in the WD4 domain, yielded a variant (eCALWY-4) with a 300-fold lower Zn2+ affinity, but with a similar change in emission ratio. Subsequent shortening of the linker length between the metal binding domains yielded a series of sensor variants (eCALWY1-6) with Zn2+ affinities ranging between 2 pM and 5 nM, while all displaying at least a two-fold change in emission ratio.
More recently, a similar design strategy was used to develop a FRET-sensor for bile acids that can be used to monitor bile acid transport in single living cells (Fig. 2C) [13]. This Bile Acid Sensor (BAS) contains the ligand binding domain (LBD) of FXR, a nuclear receptor that binds bile acids and regulates transcriptional control of bile acids synthesis, import and export. Upon binding bile acid, the FXR-LBD undergoes a conformational change that creates a binding pocket for the characteristic LXXLL motif present in co-activator proteins. In the absence of ligand, self-associating variants of cerulean and citrine form an intramolecular complex, ensuring a high amount of FRET. Bile-acid binding induces the formation of a co-activator binding site at opposite site of the FXR-LBD, resulting in a conformational switch in which the interaction between the FPs is disrupted to allow the LXXLL peptide motif at the C-terminus of citrine to interact with the LBD. To ensure proper separation between the FPs in the ligand bound state, the last 9 amino acids of the flexible C-terminal tails of cerulean and citrine were deleted. In vitro titration experiments showed a 2-fold decrease in emission ratio upon titration of a number of different bile acid species and a synthetic agonist. Importantly, the same sensor construct lacking the mutations that promote FP association (Q204F in this case), showed a low level of FRET in the ligand-free state and no significant FRET response upon addition of bile acids. Robust changes in emission ratio were also observed for a range of cell lines and subcellular locations, although in situ the sensor response was reversed and an increase in FRET is observed upon ligand binding, which may result from interactions with endogenous co-activator proteins in a ligand-dependent manner.
The concept of designing FRET sensors based on mutually exclusive domain interactions should be a generic strategy to rationally design FRET sensors, provided that the N- and C-termini of the ligand-binding domain(s) are sufficiently spatially separated to prevent the fluorescent domains from forming a complex in both the on- and the off-state. The characteristic Y-shape architecture of antibodies with two identical antigen binding sites separated by a distance of ~ 100 Å, is ideal to be used in FRET sensors based on this principle [14]. Antibody-specific FRET sensors have been constructed by fusing self-associating donor and acceptor fluorescent domains together via a long flexible linker that includes two antibody-binding epitopes adjacent to the fluorescent domains (Fig. 2D). Binding of a single antibody to the two peptide epitopes disrupts the interaction between the two fluorescent domains, resulting in a substantial decrease in energy transfer. In this case effective bivalent binding was only observed after introduction of two 45 Å α-helical blocks in an otherwise flexible linker, illustrating the importance of proper linker design. Another interesting class of input domains is provided by the so-called ‘affinity clamp’ proteins developed by Koide and coworkers [15]. Affinity clamps provide an alternative scaffold to antibody (fragments) for the evolution of novel ligand binders. Unlike antibody domains and other ligand binding proteins which consists of a single rigid scaffold domain, affinity clamps consists of two domains, a PDZ domain and a fibronection type III domain, linked by a flexible hinge region. Affinity clamps derive their high affinity from their ability to bind a peptide target by forming a complex in which the peptide is sandwiched in between the two domains. The intrinsic conformational rearrangement upon ligand binding in these ligand binding proteins makes them attractive input domains for switches based on mutually exclusive domain interactions. Functionalization of an affinity clamp for a C-terminal peptide motif with CyPet and Ypet yielded a sensor with a 200% decrease in emission ratio, consistent with a model in which an interaction between the two fluorescent domain is sterically incompatible with formation of the peptide-complex (Fig 2E) [16].
The importance of fluorescent domain interactions in other FRET sensors
Whereas the deliberate use of intramolecular domain interactions in FRET sensor design is relatively new, interactions between fluorescent domains may play an important role in the performance of many previously developed FRET sensors. Kotera and coworkers showed that introduction of the A206K mutation, which suppresses the weak dimerization properties of GFP-derived fluorescent domains [17], severely attenuated the dynamic range of three highly optimized FRET sensors, suggesting that reversible intramolecular interactions are important to create FRET sensors with a large dynamic range [18] [19]. This phenomenon could also provide a rationale for the sometimes dramatic improvement in dynamic range when using circular permuted variants of one of the fluorescent domains. Such effects can be explained when the fluorescent domains are constrained in at least one of the two senor states, e.g. because circular permutation allows an interaction between donor and acceptor fluorescent domains that is not possible when using normal fluorescent domains. Kotera and coworkers also reported that introduction of the S208F/V224L mutations in the same FRET sensors typically also resulted in a decrease in ratiometric change. The latter observation illustrates that successful implementation of self-associating fluorescent domains requires careful consideration of the sensor’s architecture.
Future perspectives
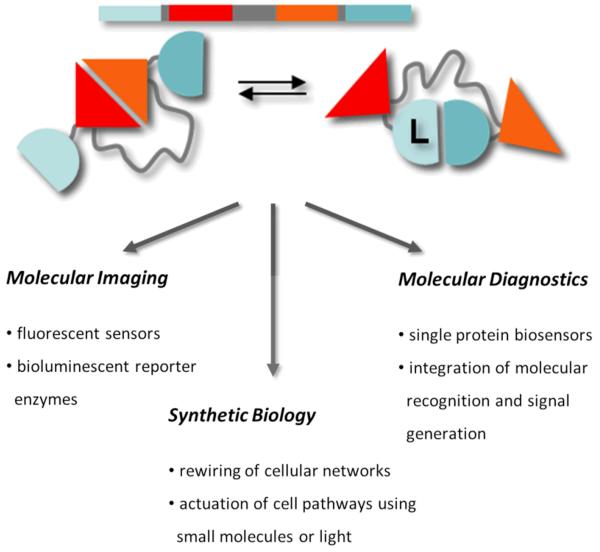
The examples discussed above show that designs based on mutually exclusive domain interactions allow the construction of FRET sensors with predictable and robust changes in emission ratio. The modular nature of these switches should in principle also allow easy exchange of the fluorescent output domains. One obvious future direction is to expand the palette of self-associating fluorescent domains beyond the classical FRET pair of CFP and YFP derivatives. We recently developed self-associating variants of mOrange and mCherry, an attractive FRET pair to use in combination with existing CFP-YFP based FRET sensors for multiparameter imaging (unpublished work). Another attractive class of fluorescent output domains are the dimerization dependent green, yellow and red fluorescent proteins that were recently introduced by Campbell and coworkers. These new fluorescent proteins are non/weakly fluorescent as monomers, but show a strong increase in fluorescence intensity upon dimerization. Interestingly, the Kd values for these protein pairs vary between 10 and 30 μM [20, 21], which is similar to the interaction strength between self-associating variants of CFP and YFP. Although they have thus far only be used to detect intermolecular protein-protein interactions, their moderate affinities should render them also suitable for use in single-chain sensors based on mutually exclusive interactions. The modular design of these FRET sensors not only allows easy exchange for new fluorescent domains, but in principle should also allow one to control other output function such as enzyme activity or ligand binding (Fig. 3). In general, using mutually exclusive domain interactions represents an attractive strategy to efficiently translate a conformational change in an input domain to different types of output functions, providing a true synthetic biology-type of approach to engineer protein switches for a broad range of applications ranging from intracellular imaging, synthetic biology, optogenetics and molecular diagnostics.
Figure 3.
Using mutually exclusive interactions between input and output domains provides a generic, modular design approach for the construction of sensors and actuators for use intracellular imaging, synthetic biology and molecular diagnostics.
Acknowledgements
I thank all the coworkers who have contributed to the work discussed in this review. Our current work in this area is supported by grants from the Netherlands Organization of Scientific Research (VIDI grant 700.56.428 and ECHO grant 700.59.013) and an ERC starting grant (ERC-2011-StG 280255).
Abbreviations
- CFP
Cyan Fluorescent Proteins
- FP
Fluorescent Protein
- FRET
Forster Resonance Energy Transfer
- YFP
Yellow Fluorescent Protein
REFERENCES
- 1.Ha JH, Loh SN. Protein conformational cwitches: from nature to design. Chem-Eur J. 2012;18:7984–7999. doi: 10.1002/chem.201200348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golynskiy MV, Koay MS, Vinkenborg JL, Merkx M. Engineering protein switches: sensors, regulators, and spare parts for biology and biotechnology. ChemBioChem. 2011;12:353–361. doi: 10.1002/cbic.201000642. [DOI] [PubMed] [Google Scholar]
- 3.Campbell RE. Fluorescent-protein-based biosensors: modulation of energy transfer as a design principle. Anal. Chem. 2009;81:5972–5979. doi: 10.1021/ac802613w. [DOI] [PubMed] [Google Scholar]
- 4.Piljic A, de Diego I, Wilmanns M, Schultz C. Rapid development of genetically encoded FRET Reporters. ACS Chem. Biol. 2011;6:685–691. doi: 10.1021/cb100402n. [DOI] [PubMed] [Google Scholar]
- 5.Ibraheem A, Yap H, Ding YD, Campbell RE. A bacteria colony-based screen for optimal linker combinations in genetically encoded biosensors. BMC Biotechnol. 2011;11 doi: 10.1186/1472-6750-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen AW, Daugherty PS. Evolutionary optimization of fluorescent proteins for intracellular FRET. Nat. Biotechnol. 2005;23:355–360. doi: 10.1038/nbt1066. [DOI] [PubMed] [Google Scholar]
- 7.Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nat Methods. 2005;2:905–909. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- 8.Vinkenborg JL, Evers TH, Reulen SWA, Meijer EW, Merkx M. Enhanced sensitivity of FRET-based protease sensors by redesign of the GFP dimerization interface. ChemBioChem. 2007;8:1119–1121. doi: 10.1002/cbic.200700109. [DOI] [PubMed] [Google Scholar]
- 9.Ohashi T, Galiacy SD, Briscoe G, Erickson HP. An experimental study of GFP-based FRET, with application to intrinsically unstructured proteins. Protein Sci. 2007;16:1429–1438. doi: 10.1110/ps.072845607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evers TH, van Dongen E, Faesen AC, Meijer EW, Merkx M. Quantitative understanding of the energy transfer between fluorescent proteins connected via flexible peptide linkers. Biochemistry. 2006;45:13183–13192. doi: 10.1021/bi061288t. [DOI] [PubMed] [Google Scholar]
- 11.van Dongen E, Evers TH, Dekkers LM, Meijer EW, Klomp LWJ, Merkx M. Variation of linker length in ratiometric fluorescent sensor proteins allows rational tuning of Zn(II) affinity in the picomolar to femtomolar range. J Am Chem Soc. 2007;129:3494–3495. doi: 10.1021/ja069105d. [DOI] [PubMed] [Google Scholar]
- 12.Vinkenborg JL, Nicolson TJ, Bellomo EA, Koay MS, Rutter GA, Merkx M. Genetically encoded FRET sensors to monitor intracellular Zn2+ homeostasis. Nat Methods. 2009;6:737–740. doi: 10.1038/nmeth.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Velden LM, Golynskiy MV, Bijsmans ITGW, van Mil SWC, Klomp LWJ, Merkx M, van de Graaf SFJ. Monitoring bile acid transport in single living cells using a genetically encoded Forster Resonance Energy Transfer sensor. Hepatology. 2013;57:740–752. doi: 10.1002/hep.26012. [DOI] [PubMed] [Google Scholar]
- 14.Golynskiy MV, Rurup WF, Merkx M. Antibody detection by using a FRET-based protein conformational switch. ChemBioChem. 2010;11:2264–2267. doi: 10.1002/cbic.201000143. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Koide A, Makabe K, Koide S. Design of protein function leaps by directed domain interface evolution. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6578–6583. doi: 10.1073/pnas.0801097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J, Koide S. Rational conversion of affinity reagents into label-free sensors for peptide motifs by designed allostery. ACS Chem. Biol. 2010;5:273–277. doi: 10.1021/cb900284c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 18.Kotera I, Iwasaki T, Imamura H, Noji H, Nagai T. Reversible dimerization of Aequorea victoria fluorescent proteins increases the dynamic range of FRET-based indicators. ACS Chem. Biol. 2010;5:215–222. doi: 10.1021/cb900263z. [DOI] [PubMed] [Google Scholar]
- 19.Jost CA, Reither G, Hoffmann C, Schultz C. Contribution of fluorophores to protein kinase CFRET probe performance. ChemBioChem. 2008;9:1379–1384. doi: 10.1002/cbic.200700728. [DOI] [PubMed] [Google Scholar]
- 20.Alford SC, Ding YD, Simmen T, Campbell RE. Dimerization-dependent green and yellow fluorescent proteins. ACS Synth. Biol. 2012;1:569–575. doi: 10.1021/sb300050j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alford SC, Abdelfattah AS, Ding YD, Campbell RE. A fluorogenic red fluorescent protein heterodimer. Chem. Biol. 2012;19:353–360. doi: 10.1016/j.chembiol.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]