Abstract
A 53-year-old man presented with sudden painless diminution of vision in his right eye for 3 days. His fundus examination showed diffuse whitening of the retina with a cherry red spot at the fovea with cilioretinal artery sparing. On fluorescein angiography delayed arteriovenous transit was observed. Three-dimensional spectral domain optical coherence tomography was used to assess retinal nerve fibre layer thickness and average macular central subfield thickness on days 3, 7, 30 and 90. Marked retinal oedema due to ischaemia was observed on day 3 of occurrence of central retinal artery occlusion. On day 7, significant decrease in retinal nerve fibre thickness and macular thickness was noted suggestive of acute reperfusion injury. Retinal nerve fibre layer thickness and macular thickness returned to near normal on day 30 due to restoration of blood supply with wash out of stress mediators. Retinal atrophy was observed on day 90.
Background
Central retinal artery occlusion (CRAO) is a grave ocular emergency resulting in poor visual outcome. Prolonged retinal ischaemia leads to irreversible injury to the retina. Experimental CRAO in rhesus monkeys by temporary clamping of the central retinal artery, was performed. Irreversible damage to the ganglion cell layer and the inner nuclear layer was observed.1 Total or almost total optic nerve atrophy and nerve fibre damage has also been observed.2 In the acute stage intracellular oedema is observed as a thickening of the inner retinal layers on spectral domain optical coherence tomography (SD-OCT). Subsequently, atrophic areas appear at 1 month (‘pseudonormalisation’) and the retina becomes atrophic at 3 months.3
We report retinal ischaemia-reperfusion injury in a case of CRAO for the first time. This report highlights the importance of reperfusion injury in CRAO. Appropriate intervention within a critical time period may help salvage vision.
Case presentation
A 53-year-old man presented with a sudden painless diminution of vision in his right eye for 3 days. His right eye visual acuity was hand movement with defective projection of rays. His left eye visual acuity was 20/40. No abnormality was detected on systemic examination. An anterior segment slit lamp biomicroscopic examination was unremarkable. On fundus examination, there was diffuse whitening of the retina with a cherry red spot at the fovea with cilioretinal artery sparing (figure 1). On the fluorescein angiography (delayed arm-to-retina time—18 s) delayed arteriovenous transit was observed. Late staining of the disc was also observed. Sparing of papillomacular fibres was demonstrated by the early perfusion of the cilioretinal artery (figure 2A,B).
Figure 1.
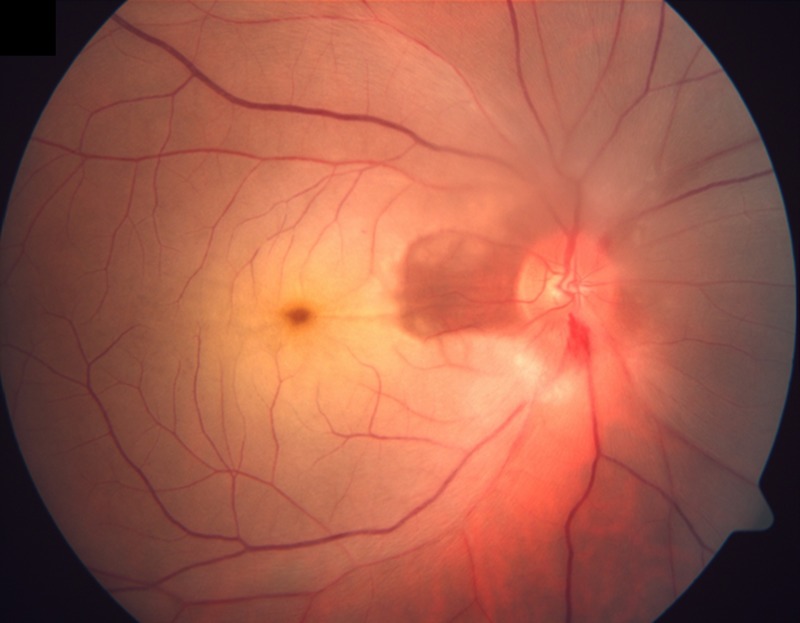
Coloured fundus photograph of the right eye showing diffuse whitening of the retina with a cherry red spot at the fovea with cilioretinal artery sparing.
Figure 2.
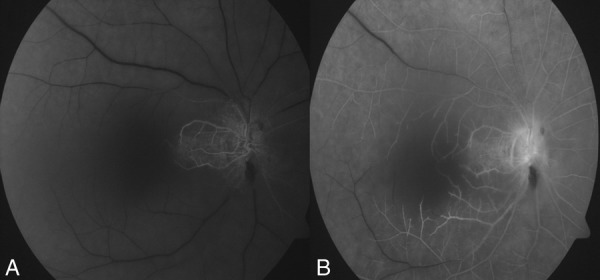
(A) Fundus fluorescein angiography showing early perfusion of the cilioretinal artery. (B) Late phase showing delayed arteriovenous transit and late staining of the disc.
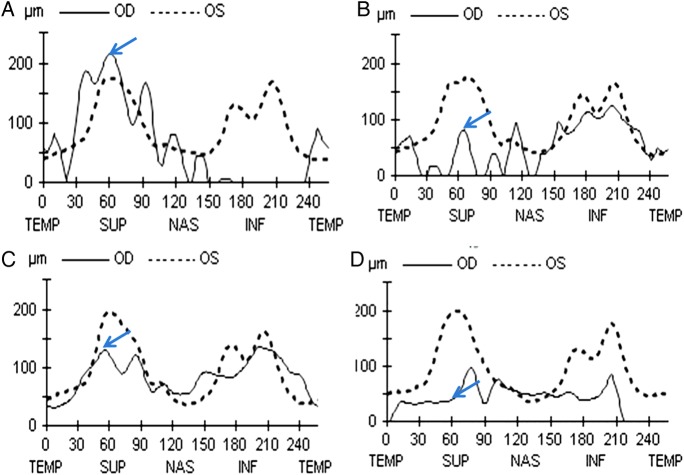
SD-OCT was used to assess the average peripapillary retinal nerve fibre thickness, nerve fibre thickness at sample 60 (nerve fibre layer thickness at sample 60 denotes thickness of nerve fibre layer at the 60th A scan among the 256 A scans taken along the peripapillary circle of reference and corresponds to the superior half of peripapillary area of retinal nerve fibre layer (RNFL)) and average macular central sub field thickness on days 3, 7, 30 and 90. Retinal nerve fibre thickness at sample 60 and average sub field thickness showed a common pattern of initial rise due to a retinal oedema on day 3. A sudden decrease in all the three values was noted on day 7 suggestive of an acute injury to inner retinal layers. This was followed by return of near normal values of all the three parameters by day 30. Marked decrease in the value of average retinal nerve fibre thickness occurred by day 90 (table 1 and figure 3). Inner retinal layers were found to have increased hyper-reflectivity and thickness. Outer retinal layers were intact. Hyper-reflectivity of inner retinal layers decreased on the third visit on day 30 with near normal appearance of retinal layers at day 90 (figure 4). No intervention was performed and no improvement of vision was observed in subsequent follow-up visits.
Table 1.
Changes in retinal nerve fibre layer thickness and central sub field macular thickness.
| Serial number | Time period from the day of occurrence of CRAO | Average retinal nerve fibre layer thickness (µm) OD | Average retinal nerve fibre layer thickness (µm) OS | Retinal nerve fibre thickness at sample 60 (µm) OD | Central subfield macular thickness (µm) OD |
|---|---|---|---|---|---|
| 1 | Day 3 | 64 | 91 | 217 | 664 |
| 2 | Day 7 | 55 | 92 | 64 | 91 |
| 3 | Day 30 | 86 | 91 | 123 | 134 |
| 4 | Day 90 | 41 | 92 | 42 | 184 |
CRAO, Central retinal artery occlusion. Table shows average retinal nerve fibre layer thickness in the affected right eye and normal left eye. Retinal nerve fibre thickness at sample 60 and central subfield macular thickness of the affected eye are also shown. Occurrence of ischaemia-reperfusion retinal injury after day 3 is observed as decrease in retinal nerve fibre thickness at sample 60 leading to final atrophy at day 90.
Figure 3.
Retinal nerve fibre layer (RNFL) thickness profiles of both eyes noted on days 3 (A), 7 (B), 30 (C) and 90 (D) showing TSNIT values. Blue arrows show sample 60 values that is the RNFL thickness in the superior quadrant. RNFL profile precisely highlights initial rise due to retinal oedema on day 3 followed by marked decrease on day 7 suggestive of acute reperfusion injury. (C) Return to nearly normal values on day 30 signifying return of circulation and wash out of reperfusion stress mediators. Marked decrease in the value of average retinal nerve fibre thickness can be noted by day 90 due to setting in of atrophy.
Figure 4.
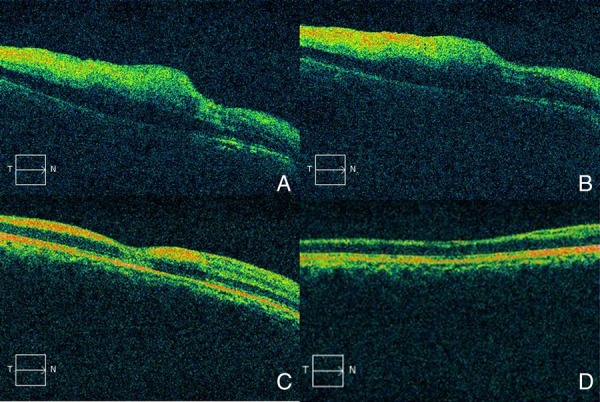
Advanced macular visualisation shows increased hyper-reflectivity and thickness of inner retinal layer with low reflectivity of outer retinal layer. Hyper-reflectivity of inner layers decreased by day 30 with near normal appearance of retinal layers on day 90 (A—day 3, B—day 7, C—day 30 and D—day 90).
Table 1 shows average RNFL thickness in the affected right eye and normal left eye. Retinal nerve fibre thickness at sample 60 and central subfield macular thickness of the affected eye are also shown. Occurrence of ischaemia-reperfusion retinal injury after day 3 is observed as decrease in retinal nerve fibre thickness at sample 60 leading to final atrophy at day 90.
Investigations
Fundus photography
Fluorescein angiography
Three dimensional SD-OCT (Cirrus high definition OCT (Carl Zeiss Meditec Inc, California, USA)
Discussion
The central retinal artery is an end artery providing blood supply to the inner layers of the retina. Central retinal artery occlusion thus may lead to damage to the inner retinal layers. Retinal nerve fibre layer thinning was noted in the macular and peripapillary area in CRAO by Leung et al.4 They further found that on OCT, thinning was limited to inner retinal layers while the outer retinal layers were spared.
A study on rhesus monkey investigated the retinal survival time following CRAO. Massive irreversible retinal damage was noted in the arterial occlusion lasting for about 240 min.1 The ganglion cell layer and inner nuclear layer were found to be damaged proportionally to the duration of CRAO. Outer nuclear and plexiform layers and photoreceptors showed no damage at all with CRAO. In another OCT-based study based on quantitative evaluation of ischaemia-reperfusion injury performed in black Norway rat retina model, mean retinal thickness after different duration of occurrence of CRAO was evaluated. The OCT-determined mean retinal thickness was found to be 177±2.16, 170±7.55, 159±5.34 and 140±5.56 µm on days 1, 2, 4 and 7, respectively, in the ischaemia-reperfusion group. The OCT determined retinal thickness correlated well with the histologically determined thickness.5
In our case we observed marked retinal oedema due to ischaemia on day 3 of occurrence of CRAO. There was significant increase in RNFL thickness at sample 60 (217 µm) and central subfield macular thickness (664 µm). On the other hand on day 7, a decrease was observed in RNFL thickness at sample 60 (68 µm) and central subfield macular thickness (134 µm) which is suggestive of reperfusion injury resulting in acute damage and cell death. Similar reperfusion injury has been noted in brain following ischaemic stroke and heart following myocardial infarction.6 7 By day 30, values of average RNFL thickness (86 µm) and RNFL thickness at sample 60 (123 µm) improved indicating ‘pseudo normalisation’.2 Marked atrophic changes were observed on the fourth visit on day 90 with noticeable diminution of average RNFL thickness (41 µm) and nerve fibre layer thickness at sample 60 (42 µm). Meticulous observation at regular period of time helped us to conclude that after initial period of ischaemia, which results in retinal oedema, there occurs acute thinning of inner retinal layers due to reperfusion acute oxidative injury. But by 4 weeks, there occurs recovery of the thickness of inner retinal layers with the restoration of blood supply and wash out of free radicals and other oxidative stress mediators.8 Final retinal atrophy sets in by 3 months. In terms of structural and functional relationship, the association of degree of macular thickness and peripapillary RNFL thickness and visual outcome is not linear.4 Although by 1 month, ‘pseudo normalisation’ of thickness of nerve fibre layer and macular thickness occurred there was no gain in visual acuity further supporting the damage by reperfusion injury. Inspite of reoccurrence of perfusion, retinal functions remained compromised and eventually led to global atrophy of RNFL.
The reperfusion injury in a case of CRAO is being reported for the first time in human and corroborates well with the study on a rat model where similar findings were observed on day 4 and 7, respectively.5 Timeline of reperfusion injury in CRAO gives us a novel insight into the critical period for treatment. The first 72 h following CRAO may be considered to be most crucial following which reperfusion damage occurs resulting in irreversible changes in retinal layers. Corrective measures9 10 for salvaging vision in CRAO, should be started within first 72 h of occurrence.
SD-OCT proves to be an invaluable tool for not only monitoring retinal changes and documentation of nerve fibre layer thickness on successive visits but also in highlighting the role of reperfusion injury in CRAO.
Learning points.
Central retinal artery occlusion (CRAO) affects mainly the inner retinal layers and the outer retinal layers remain intact.
CRAO, if followed in its natural course without any intervention, shows initial oedema of inner retinal layers followed by marked decrease in thickness of inner retinal layers due to reperfusion injury. In due course there is return to near normal values followed by eventual atrophy of inner retinal nerve fibres.
Initial 72 h from occurrence of CRAO are critical for initiation of therapy after which reperfusion injury occurs.
Spectral domain optical coherence tomography serves as invaluable tool for monitoring changes in inner retinal layers and retinal nerve fibre layer thickness assessment.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hayreh SS, Zimmerman MB, Kimura A, et al. Central retinal artery occlusion. Retinal survival time. Exp Eye Res 2004;2013:723–36 [DOI] [PubMed] [Google Scholar]
- 2.Hayreh SS, Jonas JB. Optic disk and retinal nerve fiber layer damage after transient central retinal artery occlusion: an experimental study in rhesus monkeys. Am J Ophthalmol. 2000;2013:786–95 [DOI] [PubMed] [Google Scholar]
- 3.Cornut PL, Bieber J, Beccat S, et al. Spectral domain OCT in eyes with retinal artery occlusion. J Fr Ophtalmol 2012;2013:606–13 [DOI] [PubMed] [Google Scholar]
- 4.Leung CKS, Tham CCY, Mohammed S, et al. In vivo measurements of macular and nerve fibre layer thickness in retinal arterial occlusion. Eye 2007;2013:1464–8 [DOI] [PubMed] [Google Scholar]
- 5.Sho K, Takahashi K, Fukuchi T, et al. Quantitative evaluation of ischemia–reperfusion injury by optical coherence tomography in the rat retina. Jpn J Ophthalmol. 2005;2013:109–13 [DOI] [PubMed] [Google Scholar]
- 6.White BC, Sullivan JM, DeGracia DJ, et al. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci 2000;2013:1–33 [DOI] [PubMed] [Google Scholar]
- 7.Eltzschig K, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull 2004;2013:71–86 [DOI] [PubMed] [Google Scholar]
- 8.Cervia D, Casini G. Recent advances in cellular and molecular aspects of mammalian retinal ischemia. World J Pharmacol 2012;2013:30–43 [Google Scholar]
- 9.Junk AK, Mammis A, Savitz SI, et al. Rosenbaum: erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc Natl Acad Sci USA 2002;2013:10659–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werner D, Michalk F, Harazny J, et al. Accelerated reperfusion of poorly perfused retinal areas in central retinal artery occlusion and branch retinal artery occlusion after a short treatment with enhanced external counterpulsation. Retina 2004;2013:541–7 [DOI] [PubMed] [Google Scholar]