Abstract
Background
The role of weight training in the primary prevention of type 2 diabetes is largely unknown. We examined the association of weight training with risk of type 2 diabetes among U.S. men and assessed the influence of combining weight training and aerobic exercise.
Methods
We performed a prospective cohort study among 32,002 men from the Health Professionals Follow-up Study that were followed from 1990 until 2008. Weekly time spent on weight training and aerobic exercise (including brisk walking, jogging, running, bicycling, swimming, tennis, squash, calisthenics/rowing) was obtained from questionnaires at baseline and biennially during follow-up.
Results
During 508,332 person years of follow-up (18 years), we documented 2,278 new cases of type 2 diabetes. In multivariable adjusted models, we observed a dose-response relationship between an increasing amount of time spent on weight training or aerobic exercise and lower risk of diabetes (p for trend<0.001). Engaging in weight training or aerobic exercise of at least 150 min/week was independently associated with a lower risk of diabetes of 34% (95% CI 7 – 54%) and 52% (95% CI 45 – 58%), respectively. Men who engaged in both aerobic exercise and weight training of at least 150 min/week had the greatest reduction in type 2 diabetes risk (59%, 95% CI 39 – 73%).
Conclusions
Weight training was associated with a significantly lower risk of type 2 diabetes, independent of aerobic exercise. The combination of weight training and aerobic exercise conferred a greater benefit.
Introduction
Regular physical activity (PA) is a cornerstone in the prevention and management of type 2 diabetes (T2DM). Achieving a daily amount of moderate or vigorous PA of at least 30 min/day is associated with a substantial reduction in the risk of T2DM.1–4 This is broadly consistent with the current recommendations on PA among adults.5 More recently, evidence from randomized controlled trials have shown that resistance training can improve glycemic control in patients with T2DM, even in the absence of aerobic training.6 This has led to the recommendation for resistance training 3 times per week among individuals with T2DM.7;8 However, whereas the evidence that regular aerobic exercise can prevent T2DM is compelling, to our knowledge, no previous studies have examined the role of weight training in the primary prevention of T2DM.
In this study we examined the association of weight training with the risk of T2DM among men followed biennially for 18 years in the Health Professionals Follow-up Study (HPFS). In particular, we examined whether the influence of weight training is independent of aerobic exercise and assessed the combined influence of weight training and aerobic exercise on T2DM risk.
Methods
Study population
The HPFS is an ongoing prospective cohort study of 51,529 male health professionals aged 40 to 75 years at baseline in 1986. Every two years the cohort participants are sent a questionnaire on diseases and personal- and lifestyle characteristics such as height, weight, smoking status, dietary intake (food frequency questionnaire (FFQ)), and PA. Ninety-four percent of the cohort has completed at least one follow-up questionnaire. For this analysis we excluded those men who reported a history of diabetes, cancer, myocardial infarction, angina, coronary artery bypass graft, other heart conditions, stroke, or pulmonary embolism on the baseline questionnaire (1986) and in 1988, and 1990, leaving a study population of 32,002 participants with information on exposures and covariates. This study was approved by the Harvard School of Public Health Institutional Review Board.
Assessment of weight training, other physical activity and TV viewing
From 1990 and onwards, the participants reported their average weekly amount of weight training, other physical activities, and TV viewing biennially. Other physical activities included walking, jogging, running, bicycling, swimming, tennis, squash, calisthenics/rowing, and heavy outdoor work. There were 13 response categories ranging from none to >40 hours/week for weight training and other PA’s. Participants were also asked about the daily number of flights of stairs climbed, and usual walking pace. Of these other physical activities brisk walking, jogging, running, bicycling, swimming, tennis, squash, calisthenics/rowing was considered aerobic exercise of at least moderate intensity (≥3 METs). We used these activities because they are often performed repetitively and produce dynamic contractions of large muscle groups for an extended period of time.5 We calculated the total time spent on aerobic exercise of at least moderate intensity (≥3 METs) and grouped participants into four categories: none, 1–59, 60–149, and ≥150 min/week. We grouped participants in the same categories for weight training. We also constructed a variable representing unstructured PA of at least moderate intensity consisting of MET-hours per week of activity heavy outdoor work and stair climbing as previously described.9;10 The reproducibility and validity of the PA questionnaire have been assessed in a sub-sample of the HPFS participants. The Pearson correlation between PA of vigorous intensity from diaries for 4 weeks across different seasons and that from the questionnaire was 0.58.11 For weight training, the correlation was 0.79.11 Reproducibility of vigorous physical activities and weight training from two questionnaires were 0.52 and 0.50 respectively. Another study has reported a correlation of 0.54 between PA score obtained from a similar questionnaire and maximum oxygen uptake.12
Assessment of type 2 diabetes and death
We ascertained T2DM that occurred between return of the questionnaire in 1990 and January 31 in 2008. Men who reported a diagnosis of diabetes in the biennual follow-up questionnaires were sent a supplementary questionnaire to confirm the diagnosis obtaining information on symptoms, treatment, and diagnostic tests. From 1990 to 1996 the criteria from the National Diabetes Data Group was used to confirm self-reported diagnosis of T2DM and from 1998 we used the American Diabetes Association criteria. Ninety seven percent (57 of 59) of self-reported T2DM cases were confirmed by means of medical record review in a validation study in a sub-group of HPFS participants.10 We identified deaths by reports by searching the National Death Index, next of kin or from postal authorities. Death due to cardiovascular disease (CVD) was classified using International Classification of Diseases (eighth revision). The National Death Index has an estimated sensitivity of at least 98%.13
Assessment of covariates
Family history of T2DM, hypertension, or coronary heart disease was assessed at baseline by self-report. Smoking status and BMI were assessed at baseline and biannually thereafter. Dietary factors were assessed in 1990, 1994, 1998, 2002, and 2006 using a 131-item validated FFQ.14 Daily intake of total energy (cal/d), saturated fat to polyunsaturated fat ratio, trans fat (% of total energy), alcohol intake, coffee intake, cereal fiber (g/d), whole grains (g/d), and glycemic load were considered as covariates in the analyses. We also calculated a dietary index composed of polyunsaturated fat to saturated fat ratio, trans fat (inverted), cereal fiber, whole grains, and glycemic load (inverted) by standardizing and summarizing the respective continuously scaled dietary variables.15
Statistical analysis
Person-time at risk was calculated from the return of the 1990 questionnaire until January 31 2008, death, loss to follow-up, or whichever occurred first. Relative risks (RRs) of T2DM by categories of weight training and aerobic exercise were estimated using time dependent cox proportional-hazard regression. To control for calendar time and age the analyses were stratified jointly by age (in months) at start of follow-up and the year of questionnaire return. We calculated cumulative averages of weight training and aerobic PA from baseline (1990) to censoring time to minimize measurement error and to characterize long term exposure status. In multivariable analysis we additionally adjusted for aerobic exercise, other PA, TV viewing, alcohol intake, coffee intake, smoking, ethnicity, family history of diabetes, and the dietary variables total calorie intake, saturated fat to polyunsaturated fat ratio, trans fat, cereal fiber, whole grains, and glycemic load. Tests for trend were performed by assigning the median value of each category of the exposure and treating this variable as continuous. To examine the combined association of weight training and aerobic exercise, we constructed a joint variable of weight training (4 categories) and aerobic exercise (2 categories representing adherence to current recommendations) and associated that with T2DM risk. Test for multiplicative interaction was done with the likelihood ratio test by comparing models with main effects and interaction terms and models only containing the main effects. We did not see indications that the proportional hazard assumption was violated based on interaction test between follow-up time and weight training.
We also examined the nature of the possible dose-response relationship between weight training and T2DM by using restricted cubic spline regression with 4 knots.16 Deviation from linearity was tested using the likelihood ratio test by comparing models with cubic spline terms and models only containing the linear term.
We performed several sensitivity analyses to assess the robustness of the results. Firstly, we used the simple update- and the baseline information respectively on weight training as an alternative for the cumulative average. Secondly, we performed an analysis using a 4-year lag in exposure classification to assess the possibility of reverse causality. Thirdly, we included confounding variables assessed on the continuous scale in this form in the models to address the possibility of residual confounding. Finally, we repeated the analysis with death from all causes treated as a competing risk according to the method of Fine and Gray.17
All analyses were conducted in the Statistical Analysis Systems 8 software package, version 9.2 (SAS Institute, Inc., Cary, North Carolina).
Results
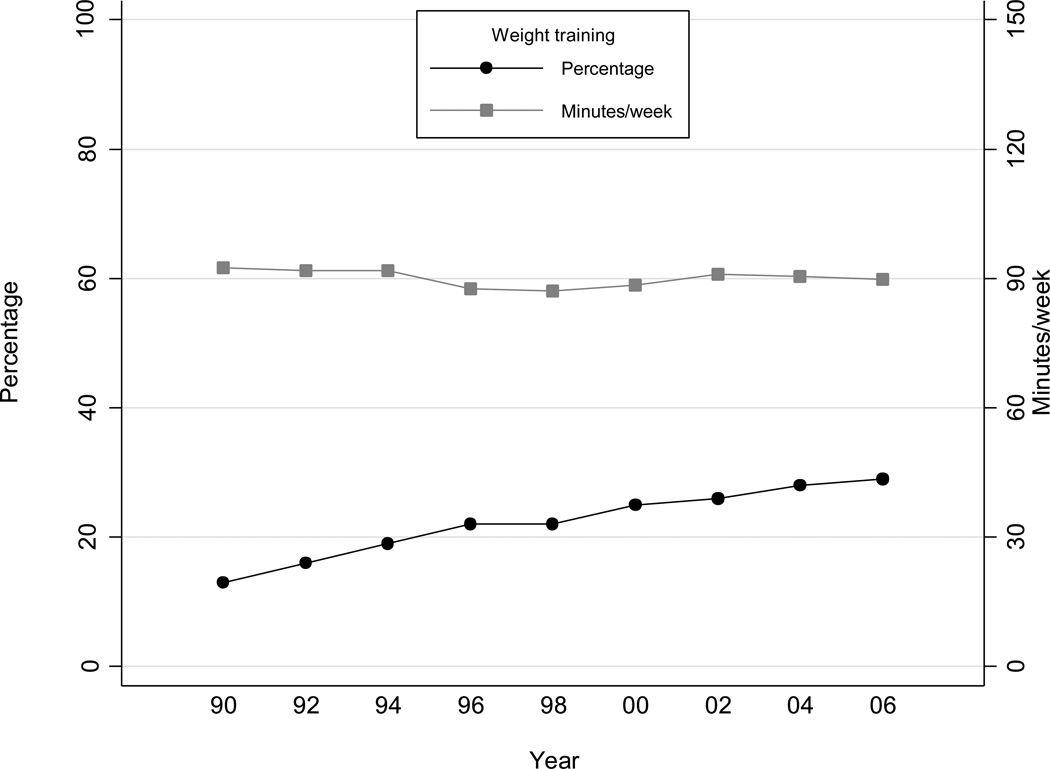
During 508,332 person years of follow-up (18 years), we documented 2,278 new cases of T2DM. Table 1 shows the baseline characteristics of the study population by levels of weight training per week. Fourteen percent of men reported weight training at baseline. Whereas the age adjusted percentage that engaged in weight training increased with time to 29% in 2006, the average time spent weight training among these individuals appeared stable over time (Figure 1). Men who reported weight training ≥150 min/week at baseline performed more aerobic exercise, viewed less TV, drank less alcohol, were less likely to smoke, and had a healthier dietary intake profile (except for glycemic load) compared to men reporting no weight training.
Table 1.
Age-adjusted baseline (1990) characteristics of the study population by levels of weight training per week.
| Minutes per week of weight training | ||||
|---|---|---|---|---|
| None | 1 – 59 | 60 – 149 | ≥150 | |
| No | 26,439 | 2,068 | 2,078 | 1,417 |
| BMI (kg/m2) | 25.6(3.3) | 25.1(2.7) | 24.9(2.6) | 24.9(2.7) |
| Aerobic exercise* (h/w) | 3.2(5.1) | 4.4(5.1) | 5.5(5.4) | 6.9(14.2) |
| Other physical activity (MET-h/w)** | 9.1(22.3) | 5.5(13.5) | 6.1(14.6) | 8.4(16.8) |
| TV viewing (h/w) | 10.3(8.4) | 9.4(8.0) | 9.5(7.7) | 9.6(7.7) |
| Alcohol intake (g/d) | 10.2(14.5) | 10.6(14.1) | 10.8(13.1) | 9.8(12.5) |
| Coffee intake (cups/d) | 1.3(1.6) | 1.1(1.5) | 1.2(1.6) | 1.1(1.5) |
| P:S ratio | 0.6(0.2) | 0.6(0.2) | 0.6(0.2) | 0.7(0.2) |
| Transfat (% of total energy) | 1.5(0.6) | 1.4(0.6) | 1.4(0.6) | 1.3(0.6) |
| Cereal fiber (g/d) | 6.3(4.3) | 7.1(4.6) | 7.1(4.7) | 7.2(5.0) |
| Whole grains (g/d) | 24.6(20.3) | 28.9(22.7) | 29.2(21.6) | 31.8(29.2) |
| Glycemic load | 125(47) | 130(48) | 129(47) | 132(49) |
| Total energy intake (kcal/d) | 1928(600) | 1943(602) | 1937(596) | 1942(598) |
| Dietary index z-score (SD)*** | −0.1(2.5) | 0.5(2.6) | 0.7(2.6) | 0.9(3.0) |
| Current smoking, % | 8 | 4 | 4 | 5 |
| Race, % white | 96 | 94 | 97 | 96 |
| Family history of diabetes, % | 15 | 15 | 15 | 14 |
Values are means(SD) or percentages and are standardized to the age distribution of the study population. P:S ratio, polyunsaturated fat to saturated fat ratio; MET, metabolic equivalent task.
Aerobic exercise consists of walking with brisk pace, jogging, running, bicycling, swimming, tennis, squash, and calisthenics/rowing.
Other physical activity consists of heavy outdoor work and stair climbing.
Dietary index is the sum of standardized saturated fat to polyunsaturated fat ratio, trans fat (inverted), cereal fiber, whole grains, and glycemic load (inverted).
Figure 1. Participation in weight training over time (1990 – 2006).
Data are age adjusted percentage of men engaged in weight training and mean minutes/week of weight training among men engaged in weight training across study year.
Table 2 shows the association of weight training and aerobic exercise with the risk of T2DM. In multivariable adjusted analysis including aerobic exercise men performing weight training 1–59, 60–149, and ≥150 min/week had RRs of 0.88, 0,75, and 0.66 lower risk of T2DM (p<0.001 for trend), respectively, compared to men reporting no weight training. The RR for T2DM for men performing 1–59, 60–149, and ≥150 min/week of aerobic exercise respectively compared to men reporting no aerobic exercise was 0.93, 0.69, and 0.48 (p<0.001 for trend) in multivariable adjusted analysis. When using the baseline information only or the simple updated information on weight training (instead of the cumulatively updated) results modestly attenuated (baseline: multivariable adjusted RR=0.67 (95%CI 0.51–0.88), simple updated: multivariable adjusted RR=0.75 (95%CI 0.60–0.94) for the highest categories of weight training). Using a 4 year lag in exposure classification strengthened the association (multivariable adjusted RR=0.50 (95%CI 0.33–0.76) for the highest category of weight training). To assess the possibility of residual confounding, we included covariates as continuous variables where possible, but this did not materially change the results. To further address the possibility that the association of weight training with risk of T2DM was due to confounding by aerobic exercise, we restricted the analysis to men who reported no aerobic exercise. This analysis showed that any weight training was associated with 48% (95% CI 1–72) lower risk compared to no weight training in multivariable adjusted analysis. In a secondary analysis, we also analyzed if weight training was associated with mortality from CVD (n=1,901 deaths) and all-causes (n=6,251 deaths). The age-adjusted RRs across categories of weight training were 0.76, 0.79, and 0.78 (p=0.009 for trend) for CVD mortality and 0.75, 0.82, and 0.89 (p=0.002 for trend) for all-cause mortality. After multivariable adjustment including aerobic exercise, the corresponding RRs were 0.90, 1.00, and 0.98 (p=0.82 for trend) for CVD mortality and 0.88, 1.04, and 1.11 (p=0.38 for trend) for all-cause mortality. Treating death from all causes as a competing risk gave similar results to the standard cox model in the analysis with T2DM as outcome.
Table 2.
Weight training, aerobic exercise and risk of type 2 diabetes in men from Health Professional Follow-up Study (1990–2008).
| Minutes per week of activity | |||||
|---|---|---|---|---|---|
| None | 1 – 59 | 60 – 149 | ≥150 | p trend | |
| Weight training | |||||
| Median time (minutes/week) | 0 | 17 | 85 | 193 | |
| Number of cases | 1,630 | 507 | 109 | 32 | |
| Person years | 322,984 | 130,190 | 39,936 | 15,221 | |
| Age adjusted | 1 | 0.72 (0.65–0.80) | 0.53 (0.44–0.65) | 0.46 (0.32–0.65) | <.001 |
| Multivariable adjusted model 1* | 1 | 0.78 (0.71–0.87) | 0.61 (0.50–0.75) | 0.53 (0.37–0.76) | <.001 |
| Multivariable adjusted model 2** | 1 | 0.88 (0.79–0.98) | 0.75 (0.61–0.92) | 0.66 (0.46–0.93) | <.001 |
| Multivariable adjusted model 3*** | 1 | 0.92 (0.82–1.02) | 0.82 (0.67–1.00) | 0.71 (0.49–1.00) | 0.009 |
| Aerobic exercise† | |||||
| Median time (minutes/week) | 0 | 27 | 97 | 360 | |
| Number of cases | 395 | 589 | 445 | 849 | |
| Person years | 56,897 | 85,616 | 94,942 | 270,877 | |
| Age adjusted | 1 | 0.93 (0.79–1.03) | 0.63 (0.54–0.72) | 0.39 (0.35–0.45) | <.001 |
| Multivariable adjusted model 1* | 1 | 0.92 (0.81–1.05) | 0.67 (0.58–0.78) | 0.46 (0.40–0.52) | <.001 |
| Multivariable adjusted model 2** | 1 | 0.93 (0.81–1.06) | 0.69 (0.60–0.80) | 0.48 (0.42–0.55) | <.001 |
| Multivariable adjusted model 3*** | 1 | 1.00 (0.88–1.15) | 0.80 (0.69–0.92) | 0.61 (0.53–0.70) | <.001 |
Data are relative risks (95% CI).
adjusted for age (months), smoking (never, past, or current with cigarette use of 1–14, 15–24, ≥25 per day), alcohol consumption (0, 1–5, 6–10, 11–15, >15 g/d), coffee intake (0, <1, 1–3, 3–5, >5 cups/day), race (white, non-white), family history of diabetes, intake of total energy, trans fat, polyunsaturated fat to saturated fat ratio, cereal fiber, wholegrain, and glycemic load (all dietary factors in quintiles).
additionally adjusted for aerobic exercise (or weight training if aerobic exercise was exposure), other physical activity of at least moderate intensity (quintiles), and TV viewing (quintiles).
additionally adjusted for body mass index.
Aerobic exercise consists of walking with brisk pace, jogging, running, bicycling, swimming, tennis, squash, and calisthenics/rowing.
Adjusting for BMI moderately attenuated the associations for both weight training (multivariable adjusted RR=0.71 (95%CI 0.49–1.00) for the highest category) and aerobic exercise (multivariable adjusted RR=0.61 (95%CI 0.53–0.70) for the highest category) with T2DM risk. A sub-sample of the participants also had information on waist circumference in 1987 and 1996 (total of 413,890 person-years and 1,850 cases). Using this to assess mediation by adiposity attenuated the association of weight training and aerobic exercise to a larger extent (weight training RR=0.76 (95%CI 0.51–1.14) and aerobic exercise RR=0.62 (95%CI 0.53–0.73) for the highest categories, although the trend across categories were still present for both exercise types (p<0.05 for trend).
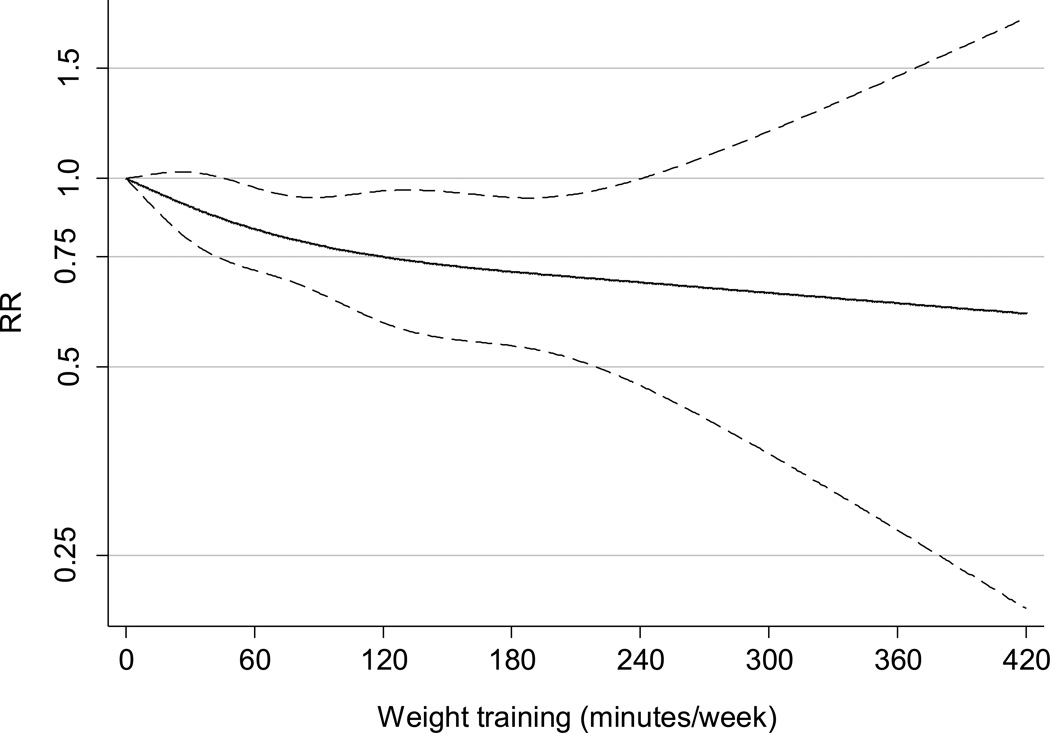
Results from the multivariable adjusted restricted cubic spline regression showed that the risk of T2DM decreased linearly with increasing time spent weight training (p=0.59 for non-linear response) (Figure 2). For each 60 min of weight training per week the risk of T2DM decreased by 13 percent (95%CI 6 – 19, p<0.001). For aerobic exercise the relationship clearly appeared non-linear with the strongest association at the lower level of aerobic exercise (p<0.001 for non-linear response) (eFigure).
Figure 2. Dose response relationship between weight training (min/week) and risk of type 2 diabetes.
Dotted lines are 95% CI for the trend obtained from restricted cubic spline regression (4 knots). The model included the following covariates: age (months), aerobic exercise (0, 1–59, 60–149, ≥150 min/week), other physical activity of at least moderate intensity (quintiles), TV viewing (quintiles), smoking (never, past, or current with cigarette use of 1–14, 15–24, ≥25 per day), alcohol consumption (0, 1–5, 6–10, 11–15, >15 g/d), coffee intake (0, <1, 1–3, 3–5, >5 cups/day), race (white, non-white), family history of diabetes, intake of total energy, trans fat, polyunsaturated fat to saturated fat ratio, cereal fiber, wholegrain, and glycemic load (all dietary factors in quintiles). The analysis was truncated to men reporting ≤420 min/week. P=0.59 for non-linear relationship.
We then examined the association of weight training and aerobic exercise stratified by age (<65, ≥65 years), BMI (<30, ≥30 kg/m2), family history of T2DM (yes, no), and dietary index score (below and above the median) (Table 3 and eTable). The association of weight training with T2DM were stronger among men below 65 years of age (p<0.001 for multiplicative interaction). There was also evidence that the association was stronger among men with no family history of T2DM (p=0.04 for multiplicative interaction). This was less apparent for aerobic exercise where associations were fairly similar across these strata (eTable).
Table 3.
Weight training and risk of type 2 diabetes in men from Health Professional Follow-up Study (1990–2008) stratified by age, body mass index, family history of type 2 diabetes, and dietary index score.
| Weight training (minutes/week) | |||||||
|---|---|---|---|---|---|---|---|
| None | 1 – 59 | 60 – 149 | ≥150 | p trend | RR per 60 min/week | p interaction | |
| Age (years) | |||||||
| <65 (1,125 cases, 289,111 person years) | 1 | 0.90 (0.77–1.04) | 0.64 (0.48–0.85) | 0.54 (0.33–0.86) | 0.002 | 0.79 (0.69–0.89) | <0.001 |
| ≥65 (1,153 cases, 219,221 person years) | 1 | 0.87 (0.75–1.01) | 0.92 (0.69–1.22) | 0.95 (0.56–1.62) | 0.56 | 0.96 (0.84–1.10) | |
| BMI (kg/m2) | |||||||
| <30 (1,499 cases, 455,664 person years) | 1 | 0.87 (0.76–0.99) | 0.75 (0.59–0.95) | 0.79 (0.53–1.18) | 0.023 | 0.90 (0.82–0.98) | 0.50 |
| ≥30 (779 cases, 52,668 person years) | 1 | 1.00 (0.83–1.21) | 0.99 (0.68–1.42) | 0.40 (0.18–0.90) | 0.055 | 0.87 (0.76–1.00) | |
| Family history of type 2 diabetes | |||||||
| Negative (1,687 cases, 436,300 person years) | 1 | 0.88 (0.78–1.00) | 0.69 (0.54–0.88) | 0.59 (0.38–0.90) | <0.001 | 0.85 (0.78–0.94) | 0.043 |
| Positive (591 cases, 72,032 person years) | 1 | 0.86 (0.70–1.07) | 0.88 (0.62–1.26) | 0.93 (0.49–1.75) | 0.55 | 0.92 (0.80–1.06) | |
| Dietary index score | |||||||
| < Median (1,376 cases, 253,486 person years) | 1 | 0.91 (0.79–1.04) | 0.72 (0.53–0.96) | 0.71 (0.43–1.16) | 0.012 | 0.89 (0.79–0.99) | 0.52 |
| > Median (902 cases, 254,847 person years) | 1 | 0.86 (0.73–1.01) | 0.77 (0.58–1.01) | 0.62 (0.37–1.19) | 0.012 | 0.86 (0.77–0.96) | |
Data are relative risks (95% CI). All models included age (months), smoking (never, past, or current with cigarette use of 1–14, 15–24, ≥25 per day), alcohol consumption (0, 1–5, 6–10, 11–15, >15 g/d), coffee intake (0, <1, 1–3, 3–5, >5 cups/day), race (white, non-white), family history of diabetes, intake of total energy, trans fat, polyunsaturated fat to saturated fat ratio, cereal fiber, wholegrain, glycemic load (all dietary factors in quintiles), aerobic exercise, other physical activity of at least moderate intensity (quintiles), and TV viewing (quintiles)
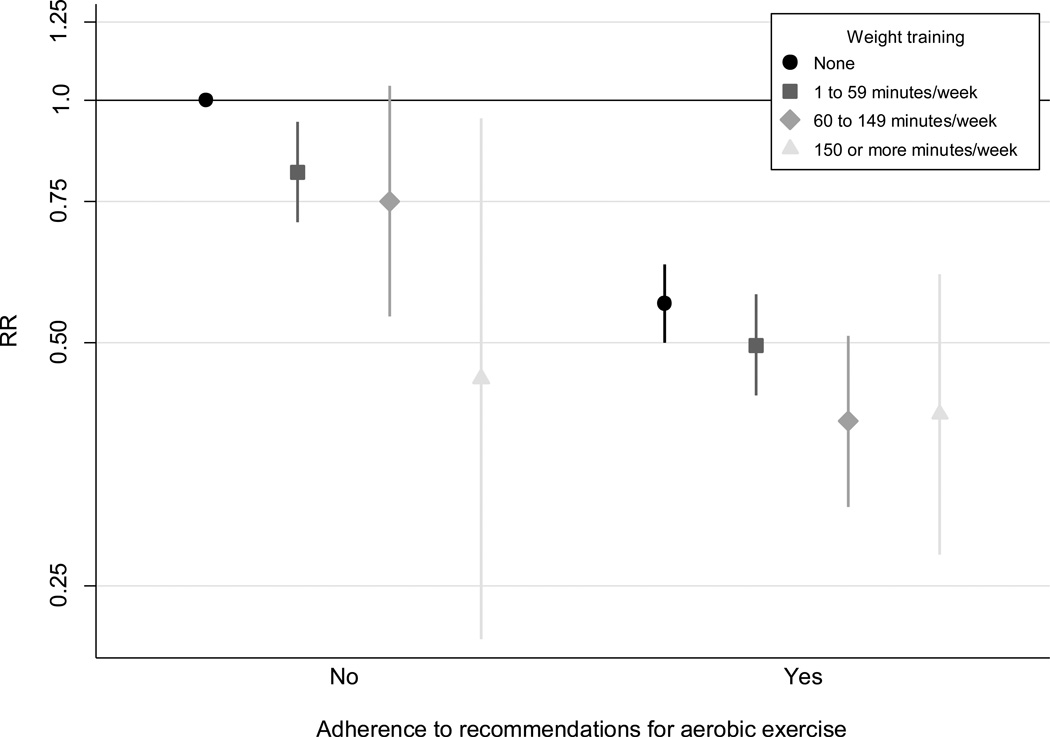
Finally we examined the joint association of weight training and aerobic exercise with the risk of T2DM (Figure 3). Men who adhered to the current recommendations on aerobic exercise (at least 150 min/week) and engaged in weight training of at least 150 min/week had the greatest reduction in T2DM risk (RR=0.41, 95%CI 0.27–0.61), p=0.26 for multiplicative interaction.
Figure 3. Joint association of weight training and aerobic exercise with the risk of type 2 diabetes.
Data are estimates of RR with 95% CI from multivariate cox regression models adjusted for age (months), other physical activity of at least moderate intensity (quintiles), TV viewing (quintiles), smoking (never, past, or current with cigarette use of 1–14, 15–24, ≥25 per day), alcohol consumption (0, 1–5, 6–10, 11–15, >15 g/d), coffee intake (0, <1, 1–3, 3–5, >5 cups/day), race (white, non-white), family history of diabetes, intake of total energy, trans fat, polyunsaturated fat to saturated fat ratio, cereal fiber, wholegrain, and glycemic load (all dietary factors in quintiles). Adherence to the recommendations on aerobic exercise is at least 150 min/week.
Comment
In this large prospective cohort study with biannual follow-up over 18 years, men who engaged in weight training had a reduced risk of T2DM. The association was independent of aerobic exercise, and even a modest amount of time engaged in weight training appeared to be beneficial. The risk reduction associated with weight training was comparable in magnitude to that of aerobic exercise, with risk reductions of about 35% and 50% among men performing at least 150 min/week of either weight training or aerobic exercise respectively. Our results support that weight training serves as an important alternative for individuals having difficulty in adhering to aerobic exercise, but the combination of weight training with aerobic exercise conferred even a greater benefit.
Our findings are in agreement with those obtained from a recent meta-analysis of randomized controlled trials showing that resistance training can improve glycemic control among individuals with T2DM.6 However, no previous studies have examined the association of weight training with the risk of T2DM. A number of cross sectional studies have shown that weight training, muscle strength, or muscle mass is associated with greater insulin sensitivity or pre-diabetes.18–21 In addition, two prospective cohort studies reported that greater muscle strength was associated with a lower risk of incident metabolic syndrome, although association was attenuated with adjustment for aerobic fitness in both reports.22;23 Finally, in a previous study from HPFS we have reported an inverse association of weight training with the risk of coronary heart disease independent of other physical activities.24 Further studies are needed to examine the associations between weight training and other outcomes, including total and cause-specific mortality.
The two largest trials performed on resistance training among individuals with T2DM showed that the combination of aerobic exercise and resistance training conferred further benefit for glycemic control among individuals with T2DM than either type of exercise alone.25;26 We observed that combining aerobic exercise and weight training was associated with the largest reduction in the risk of T2DM. While we observed that the time spent engaged in weight training provided a fairly comparable reduction in risk compared to the time spent in aerobic exercise, it is unclear if the total energy expenditure plays the same role for the two types of exercise. Because the anaerobic energy expenditure contribution during weight training can be substantial, the energy requirements for weight training may be grossly underestimated when comparing it to aerobic exercise using MET values. Furthermore, we did not obtain specific information on the type and intensity of weight training. Thus, it is uncertain if the altered daily total energy expenditure from engaging in aerobic exercise is comparable to those from weight training in our study.
Although many of the acute and chronic physiological responses induced by resistance training and aerobic exercise are similar, there are also clear distinct effects of each exercise type.27 On the cellular level engagement in aerobic exercise increases mitochondrial density and oxidative enzyme activity thereby facilitating improved ability of fatty acid oxidation, whereas resistance training increases the glycolytic capacity and promotes type-II muscle fiber abundance and growth which enhances the capacity of glucose utilization.28 In turn, aerobic exercise leads to greater improvements in aerobic fitness while resistance training favors increases lean body mass and muscle strength.29;30 Beyond improving glycemic control, both exercise types have been shown to reduce adiposity and improve blood pressure- and lipid levels.31–34
We did not observe a strong attenuation of the association with weight training after additional adjustment for BMI. This may be attributable to weight training being able to increase lean mass and reduce fat mass without a major change in body weight, as previously indicated in trials among individuals with T2DM.25;26 However, using waist circumference indicated that part of the beneficial effect of weight training was mediated by abdominal adiposity. In our previous analysis, weight training was associated with a smaller increase in waist circumference over time in men.35
We found that the association of weight training with T2DM risk was attenuated among men 65 years of age and above and among men with a family history of T2DM. The attenuation of association in these subgroups may be attributed to power. An alternative explanation could be that the intensity of weight training is decreased at old age. However, we do not have data to test this hypothesis. The possible weakened relationship between weight training and T2DM risk among men with a positive family history deserves more attention in future studies.
The strengths of this study include the large sample size, long follow-up time, and the biannual assessment of exposures and most confounders including important dietary factors. We were also able to show that associations were robust to a number of sensitivity analyses including an analysis using a 4-year lag in exposure classification. Limitations include that the study only comprised men who were working health professionals and mostly white. The findings may therefore not be generalizable to women and other ethnic or racial groups of men. Furthermore, we were not able to explore the importance of type and intensity of weight training as we only obtained information on weekly non-specific weight training. Finally, there is a possibility of residual and unknown confounding. Because we observed risk reduction with any weight training among individuals reporting no aerobic exercise it is unlikely that the association of weight training can be explained by residual confounding by aerobic exercise.
In conclusion, this prospective cohort study showed that weight training was associated with a reduced risk of T2DM in a dose response manner independent of aerobic exercise level. The magnitude of risk reduction associated with weight training was close to that of aerobic exercise. Our results support that weight training is a valuable alternative for individuals having difficulty adhering to aerobic exercise and adding weight training to aerobic exercise appears to give further protection from T2DM. Further research should examine the influence of duration, type and intensity of weight training on T2DM risk in greater detail.
Supplementary Material
Acknowledgement
Funding/Support
The study is supported by NIH grants DK58845 and CA55075.
Role of sponsors
There was no sponsor involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Author contributions
Mr. Grøntved and Dr. Hu had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Mr. Grøntved, Dr. Rimm, Dr. Willett, Dr. Andersen, and Dr. Hu. Acquisition of data: Dr. Rimm, Dr. Willett, and Dr. Hu. Analysis and interpretation of data: Mr. Grøntved, Dr. Rimm, Dr. Willett, Dr. Andersen, and Dr. Hu. Drafting of manuscript: Mr. Grøntved and Dr. Hu. Critical revision of manuscript for important intellectual content: Mr. Grøntved, Dr. Rimm, Dr. Willett, Dr. Andersen, and Dr. Hu. Statistical analysis: Mr. Grøntved and Dr. Hu. Obtained funding: Dr. Hu. Administrative, technical, or material support: Dr. Rimm, Dr. Willett, and Dr. Hu. Study supervision: Dr. Andersen and Dr. Hu.
Conflict of Interest
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. None of the authors have any conflict of interest to declare.
References
- 1.Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical Activity of Moderate Intensity and Risk of Type 2 Diabetes. Diabetes Care. 2007;30:744–752. doi: 10.2337/dc06-1842. [DOI] [PubMed] [Google Scholar]
- 2.Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, Hennekens CH. A Prospective Study of Exercise and Incidence of Diabetes Among US Male Physicians. JAMA. 1992;268:63–67. [PubMed] [Google Scholar]
- 3.Waller K, Kaprio J, Lehtovirta M, Silventoinen K, Koskenvuo M, Kujala U. Leisure-time physical activity and type 2 diabetes during a 28 year follow-up in twins. Diabetologia. 2010;53:2531–2537. doi: 10.1007/s00125-010-1875-9. [DOI] [PubMed] [Google Scholar]
- 4.Meisinger C, Löwel H, Thorand B, Döring A. Leisure time physical activity and the risk of type 2 diabetes in men and women from the general population. Diabetologia. 2005;48:27–34. doi: 10.1007/s00125-004-1604-3. [DOI] [PubMed] [Google Scholar]
- 5.Washington, DC: U S Department of Health and Human Services; 2008. Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. [Google Scholar]
- 6.Umpierre D, Ribeiro PAB, Kramer CK, et al. Physical Activity Advice Only or Structured Exercise Training and Association With HbA1c Levels in Type 2 Diabetes. JAMA. 2011;305:1790–1799. doi: 10.1001/jama.2011.576. [DOI] [PubMed] [Google Scholar]
- 7.Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and Type 2 Diabetes. Diabetes Care. 2010;33:e147–e167. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Standards of Medical Care in Diabetes – 2011. Diabetes Care. 2011;34:S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: A Second Update of Codes and MET Values. Med Sci Sports Exerc. 2011 doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 10.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical Activity and Television Watching in Relation to Risk for Type 2 Diabetes Mellitus in Men. Arch Intern Med. 2001;161:1542–1548. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 11.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and Validity of a Self-Administered Physical Activity Questionnaire for Male Health Professionals. Epidemiology. 1995;7:81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs DR, Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140:1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 14.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 15.Hu FB, Manson JE, Stampfer MJ, et al. Diet, Lifestyle, and the Risk of Type 2 Diabetes Mellitus in Women. New England Journal of Medicine. 2001;345:790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 16.Greenland S. Dose-Response and Trend Analysis in Epidemiology: Alternatives to Categorical Analysis. Epidemiology. 1995;6:356–365. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999 Jun;94(446):496–509. [Google Scholar]
- 18.Cheng YJ, Gregg EW, De Rekeneire N, et al. Muscle-Strengthening Activity and Its Association With Insulin Sensitivity. Diabetes Care. 2007;30:2264–2270. doi: 10.2337/dc07-0372. [DOI] [PubMed] [Google Scholar]
- 19.Srikanthan P, Karlamangla AS. Relative Muscle Mass Is Inversely Associated with Insulin Resistance and Prediabetes. Findings from The Third National Health and Nutrition Examination Survey. Journal of Clinical Endocrinology & Metabolism. 2011 doi: 10.1210/jc.2011-0435. [DOI] [PubMed] [Google Scholar]
- 20.Barzilay JI, Cotsonis GA, Walston J, et al. Insulin Resistance Is Associated With Decreased Quadriceps Muscle Strength in Nondiabetic Adults Aged ≥70 Years. Diabetes Care. 2009;32:736–738. doi: 10.2337/dc08-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steene-Johannessen J, Anderssen SA, Kolle E, Andersen LB. Low muscle fitness is associated with metabolic risk in youth. Med Sci Sports Exerc. 2009;41:1361–1367. doi: 10.1249/MSS.0b013e31819aaae5. [DOI] [PubMed] [Google Scholar]
- 22.Wijndaele K, Duvigneaud N, Matton L, et al. Muscular strength, aerobic fitness, and metabolic syndrome risk in Flemish adults. Med Sci Sports Exerc. 2007;39:233–240. doi: 10.1249/01.mss.0000247003.32589.a6. [DOI] [PubMed] [Google Scholar]
- 23.Jurca R, Lamonte MJ, Barlow CE, Kampert JB, Church TS, Blair SN. Association of muscular strength with incidence of metabolic syndrome in men. Med Sci Sports Exerc. 2005;37:1849–1855. doi: 10.1249/01.mss.0000175865.17614.74. [DOI] [PubMed] [Google Scholar]
- 24.Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA: The Journal of the American Medical Association. 2002;288:1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- 25.Church TS, Blair SN, Cocreham S, et al. Effects of Aerobic and Resistance Training on Hemoglobin A1c Levels in Patients With Type 2 Diabetes. JAMA. 2010;304:2253–2262. doi: 10.1001/jama.2010.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sigal RJ, Kenny GP, Boulé NG, et al. Effects of Aerobic Training, Resistance Training, or Both on Glycemic Control in Type 2 Diabetes. Ann Intern Med. 2007;147:357–369. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 27.Hawley JA. Molecular responses to strength and endurance training: are they incompatible? Appl Physiol Nutr Metab. 2009;34:355–361. doi: 10.1139/H09-023. [DOI] [PubMed] [Google Scholar]
- 28.LeBrasseur NK, Walsh K, Arany Z. Metabolic benefits of resistance training and fast glycolytic skeletal muscle. American Journal of Physiology - Endocrinology And Metabolism. 2011;300:E3–E10. doi: 10.1152/ajpendo.00512.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc. 2011;43:249–258. doi: 10.1249/MSS.0b013e3181eb6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson MD, Rhea MR, Sen A, Gordon PM. Resistance exercise for muscular strength in older adults: A meta-analysis. Ageing Research Reviews. 2010;9:226–237. doi: 10.1016/j.arr.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelley GA, Kelley KS. Impact of progressive resistance training on lipids and lipoproteins in adults: A meta-analysis of randomized controlled trials. Preventive Medicine. 2009;48:9–19. doi: 10.1016/j.ypmed.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Kraus WE, Houmard JA, Duscha BD, et al. Effects of the Amount and Intensity of Exercise on Plasma Lipoproteins. New England Journal of Medicine. 2002;347:1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 33.Cornelissen VA, Fagard RH. Effect of resistance training on resting blood pressure: a meta-analysis of randomized controlled trials. Journal of Hypertension. 2005;23 doi: 10.1097/00004872-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Whelton SP, Chin A, Xin X, He J. Effect of Aerobic Exercise on Blood Pressure: A Meta-Analysis of Randomized, Controlled Trials. Ann Intern Med. 2002;136:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 35.Koh-Banerjee P, Chu NF, Spiegelman D, et al. Prospective study of the association of changes in dietary intake, physical activity, alcohol consumption, and smoking with 9-y gain in waist circumference among 16 587 US men. The American Journal of Clinical Nutrition. 2003;78:719–727. doi: 10.1093/ajcn/78.4.719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.