Abstract
Heterotopic ossification is an observable phenomenon in the setting of abdominal wounds, estimated to effect 25% of all patients after midline abdominal surgery. The development of acellular dermal matrices has revolutionised the approach in repairing abdominal hernias, especially for potentially contaminated wounds. We describe a case of heterotopic bone formation incorporating the whole of an acellular dermal matrix in a patient on chronic steroid therapy.
Background
Reconstruction of complex abdominal hernias can be challenging in patients with diseases such as diabetes, systemic lupus erythematosus (SLE) and malnutrition.1 Difficult cases involving radiated or contaminated wounds are facilitated by acellular dermal matrices (ADMs), boasting overall success rates greater than 90%.2 In the setting of infected surgical fields, these biological materials aid the healing process by promoting host collagen deposition and neovascularisation.3 While heterotopic ossification (HO) in abdominal incisions has been well documented, we report an interesting case of a hernia repair using fetal bovine ADM, which to our knowledge is the first case of heterotopic bone formation involving the whole of a biological mesh.
Case presentation
A 70-year-old woman with SLE on daily prednisone therapy presented for reversal of a diverting ostomy and complex abdominal wall reconstruction. Her extensive surgical history began on 30 June 2011 when a bilateral salpingo-oophorectomy, partial sigmoidectomy, sigmosigmoidostomy and diverting ileostomy were complicated by evisceration and enterotomy. In multiple stages, the ileostomy was reversed, cutaneous flaps were advanced, and negative pressure therapy was initiated over the open abdomen. Eight days after the initial injury, a 16×20 cm piece of fetal bovine ADM was placed as an interposition underlay between rectus muscles. Shortly thereafter, an anastomotic leak warranted resection of 15 cm of small bowel, placement of an end ileostomy in the left upper quadrant, resection of eroded dermal matrix and reinforcement with a polyglactin mesh underlay. The patient continued negative pressure therapy and a split-thickness skin graft was subsequently placed on the abdominal wound.
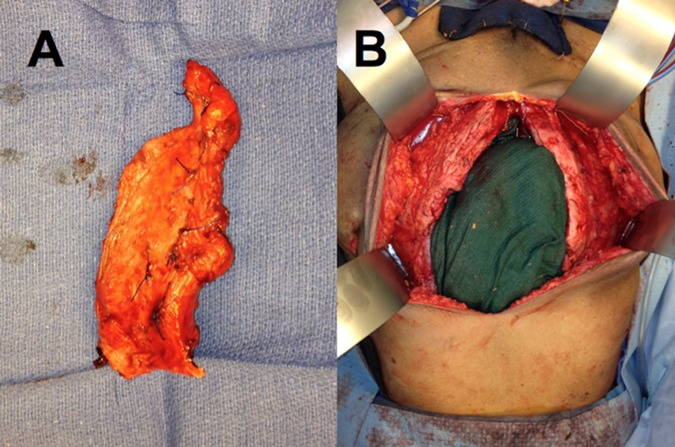
Nine months later an exploratory laparotomy with extensive lysis of adhesions, ileocecostomy, appendectomy, drainage of internal abscess and side-to-side jejunocolic anastomosis was performed. Exploration of the ventral hernia defect revealed portions of bone that were fractured and enucleated from a periosteum-like sheath. The distribution of bony material approximated the margin where the remnant ADM was previously spared, forming an immobile ring (figure 1). The remaining rectus muscle did not appear to be calcified. The extent of heterotopic bone formation in our patient measured approximately 16.4×4.1 cm in length (figure 2A) for the right segment and 6.8×4.7 cm for the left. Our findings represent the largest reported ossified mass observed in an abdominal wound, which historically has not exceeded 15.5×4 cm.4
Figure 1.
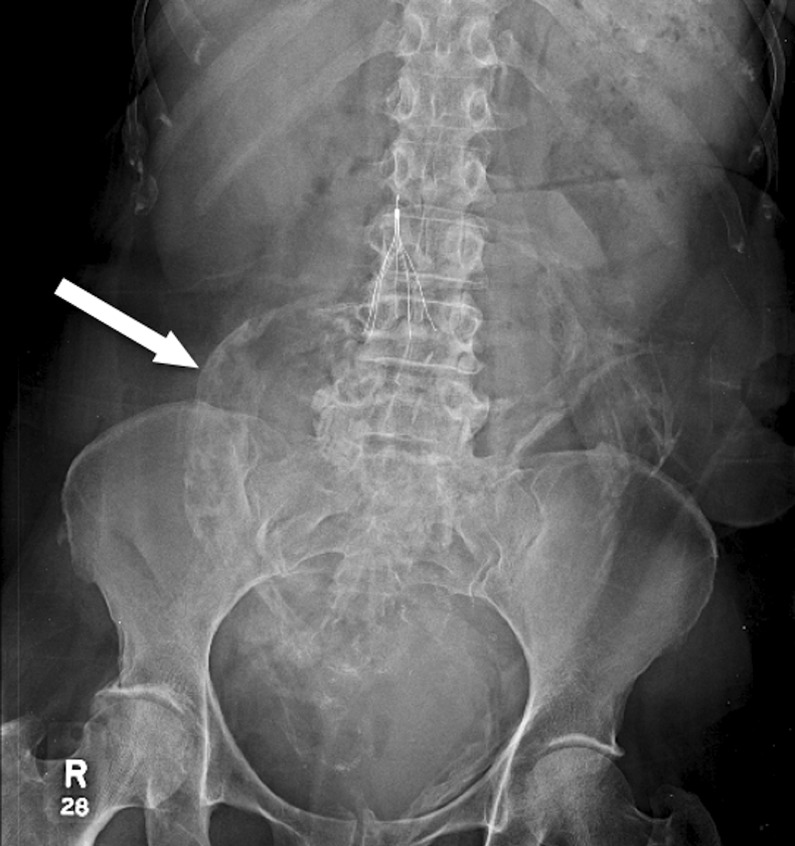
Anteroposterior radiograph of the abdomen. Note radio-opaque shadow in midabdomen (white arrow) that was initially read by the radiologist as contrast extravasation.
Figure 2.
(A) Close-up view of partially resected ossified material. (B) Intraoperative view of abdomen following lysis of adhesions, bowel resection and partial resection of ossified acellular dermal matrix.
Treatment
The ossified mesh was resected to healthy margins (figure 2B). After bilateral separation of components, the defect was addressed with a 13×25 cm×3 mm ADM inlay and a 13×25 cm×1.1 mm onlay between external obliques. There were no procedural variations between the original and the most recent reconstruction. The ADM product used was also the same as in the original operation. Pathological analysis of the calcified abdominal material was consistent with HO.
Outcome and follow-up
An abdominal CT scan 17 days after surgery demonstrated an intact reconstruction without evidence of remnant or new HO. At 6 months, there was no clinical evidence of HO and there were no ventral hernias or bulges. On follow-up with the patient's family approximately 8 months following her surgery, we discovered that the patient passed away at an outside hospital due to septic shock from bacteraemia, likely unrelated to her abdominal wall reconstruction.
Discussion
Bone formation outside the skeletal system is extensively described in the orthopaedic literature. In the abdomen, HO is classified as a subset of myositis ossificans traumatica (MOT) first described by Askanazy in 1901. It is estimated that 25% of all patients develop MOT after midline abdominal surgery.1 The majority of cases occur in males (89%) between the ages of 18 and 81 years, within the first year after surgery, and exclusively in vertical midline scars.5 6 We describe a patient on chronic steroids who developed ossification of an ADM-based hernia repair. To our knowledge, there has been only one similar case report. Nagesawaran7 reported a patient with acute abdominal pain secondary to fracture of heterotopic bone enveloping 17-year-old prolene mesh. The bone had a periosteal layer, nutrient artery and histologically confirmed lamellar bone and bone marrow.
Although the mechanism of abdominal wound calcification is poorly understood, there are two existing theories. The first describes inoculation of periosteal or perichondrial particles during surgery into the surgical wound that subsequently develop into bone. The second theory proposes that pluripotent mesenchymal cells differentiate into osteoblasts or condroblasts as an inflammatory response, a process known as osteogenic induction.6 Our patient was on chronic steroids, making this case fascinating because inflammation triggers local bone remodelling following trauma; daily steroid therapy would be expected to curb HO.
Many features of ADMs make them ideal for contaminated wounds with low short-term recurrence rates.3 The ADM used contains type III collagen which may promote wound healing. Biological meshes have been shown to promote neovascularisation and migration of host fibroblasts and macrophages. The role of the biological mesh in ossification, and its potential in the differentiation of pluripotent cells, remains to be elucidated.
Although HO is infrequently clinically significant, it should be considered in all patients after abdominal wound surgery. It is important to recognise on imaging as it can be mistaken for a retained foreign body, contrast extravasation or neoplasm. There is insufficient evidence to support the prevention or early treatment of HO with non-steroidal anti-inflammatory drugs such as indomethacin and bisphosphonates.5 In the patient, 5 mg of prednisone daily did not curb disease progression.
Our preferred approach to HO is surgical excision in patients with symptomatic disease. However, the surgical resection needs to take into consideration what is being removed, as abdominal wall competence is compromised with excessive tissue resection. In this case, the abdominal wall was riddled with heterotopic ossification. Careful resection to healthy margins—and nothing more—led to an excellent functional outcome at 2 months. Overly liberal resection may have led to irreparable domain loss.
ADMs are infection resistant but not immune to HO. We feel replacement of ossified biological material with like material will not lead to disease recurrence in this patient. Our rationale is the surgical environment of an abdominal wall reconstruction, and not the material used, predisposes to HO. Future studies may elucidate why the HO was contained within the ADM and did not involve nearby structures like the rectus and bowel. In other areas of the body this is not the case.
Learning points.
Heterotopic ossification is common after midline abdominal surgery, affecting up to 25% of patients.
Most patients are asymptomatic but may present with abdominal pain secondary to fracture of heterotopic bone.
Radiographically, heterotopic ossification can mimic cancer recurrence, contrast extravasation or retained foreign bodies.
There is insufficient evidence to support heterotopic ossification (HO) prevention with non-steroidal anti-inflammatory drugs and bisphosphonates.
HO occurrence in biological materials may result from hostile or less-than-ideal healing conditions.
Acknowledgments
The authors would like to thank the family of the patient for their cooperation and willingness to share the story of their beloved family member.
Footnotes
Contributors: VT drafted the original manuscript. JZ and JMS edited the manuscript. Photos were taken by JMS.
Competing interests: JMS is a consultant, speaker and is receiving an ongoing grant from LifeCell Corps.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kim J, Kim Y, Jeong WK, et al. Heterotopic ossification developing in surgical incisions of the abdomen: analysis of its incidence and possible factors associated with its development. J Comput Assist Tomogr 2008;2013:872–6 [DOI] [PubMed] [Google Scholar]
- 2.Hiles M, Record Ritchie RD, Altizer AM. Are biologic grafts effective for hernia repair? A systematic review of the liteature. Surg Innov 2009;2013:26–37 [DOI] [PubMed] [Google Scholar]
- 3.Janfaza M, Martin M, Skinner R. A preliminary comparison study of two noncrosslinked biologic meshes used in complex ventral hernia repairs. World J Surg 2012;2013:1760–4 [DOI] [PubMed] [Google Scholar]
- 4.Pearson J, Clark OH. Heterotopic calcification in abdominal wounds. Surg Gynecol Obstet 1978;2013:371–4 [PubMed] [Google Scholar]
- 5.Koolen PG, Schreinemacher MH, Peppelenbosch AG. Heterotopic ossifications in midline abdominal scars: a critical review of the literature. Eur J Vasc Endovasc Surg 2010;2013:155–9 [DOI] [PubMed] [Google Scholar]
- 6.Reardon MJ, Tillou A, Mody DR, et al. Heterotopic calcification in abdominal wounds. Am J Surg 1997;2013:145–7 [DOI] [PubMed] [Google Scholar]
- 7.Nageswaran H, Dunkley A. Acute abdominal pain following fracture of a heterotopically formed bone incorporating a prolene mesh. Ann R Coll Surg Engl 2010;2013:W1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]