Abstract
Facial cutaneous metastasis from carcinoma is a rare and late clinical finding that is associated with disseminated disease and a poor prognosis. Skin metastases predominantly originate from primary tumours of the lung and melanoma. Skin metastases from colon tumours occur in only 4–6.5% of cases of metastatic colorectal cancer. They are most often located on the abdominal skin. We present an unusual case in which a primary colorectal adenocarcinoma metastasised to the face. This cutaneous lesion occurred 4 years after diagnosis of the primary tumour. This case highlights the importance of prompt investigation of new or evolving skin lesions in patients with a history of malignancy. Early detection and initiation of treatment may prevent development of widespread skin metastases and extend life expectancy.
Background
Facial cutaneous metastasis is a rare but important clinical finding, occurring in less than 0.5% of patients with metastatic cancer.1 Cutaneous metastases are discovered in only 1.3% of cases at the time of presentation of the primary tumour, but may be the first sign of recurrence.1
Cancers with a high propensity to metastasise to the skin include melanoma and cancers of the lung and breast.2 3 Cutaneous metastases from colorectal adenocarcinoma also occur in 4–6.5% of cases, with abdominal skin being the most common site. These lesions present on average 33 months after diagnosis of the internal primary tumour.3
We present an unusual case of facial cutaneous metastasis of colorectal adenocarcinoma occurring 4 years after diagnosis of the primary tumour.
Case presentation
A previously fit and healthy 70-year-old Caucasian man was seen in February 2008 by his general practitioner (GP), presenting with a 3 week history of change in bowel habit. His GP subsequently referred him for urgent colonoscopy under the 2 week wait rule in order to rule out a malignant cause. His medical history included type II diabetes mellitus, ischaemic heart disease and hypertension, all of which were well controlled pharmacologically.
On colonoscopy, a suspicious lesion was discovered, which warranted further investigation by the use of CT and MRI scans. Imaging showed a moderately differentiated Dukes B adenocarcinoma of the lower third of the rectum (T2 N0 M0), as well as an incidental abdominal aortic aneurysm measuring 38 mm.
The patient underwent downstaging neo-adjuvant chemotherapy prior to having an abdomino-perineal resection with colostomy formation in May 2008 with the aim of curing the underlying malignant disease.
However, 2 years post surgery, a surveillance CT of the chest/abdomen/pelvis revealed a solitary cavitating metastatic lung nodule in the right lower lobe. This was managed with a wedge resection and further chemotherapy (oxaliplatin and modified de Gramont regimen).
The patient remained well for the next 2 years, with no evidence of disease progression. In June 2012, he was referred to a dermatology clinic by his GP after nicking an area of skin over his left cheek above his upper lip while shaving. The patient was noted to have a type II skin with moderate sun exposure over the years.
The area of injured skin became raw and inflamed, with reoccurring breaks in the skin and intermittent bleeding. Swabs had been taken by the GP to exclude infective pathologies.
The patient described the lesion as non-healing. For 2 months since the initial injury, the area had grown into an ulcerated nodule which was tender on gentle palpation.
A 10×8 mm white ulcerated nodule of the cheek was surgically excised with clear margins. Histological sections and immunohistochemistry of this facial skin lesion revealed moderately differentiated adenocarcinoma, likely a metastatic lesion from the previous tumour of the colon.
Investigations
Skin biopsy for histology and immunohistochemistry.
Differential diagnosis
Metachronous primary skin cancer is the major differential diagnosis, with basal cell carcinoma most commonly occurring in individuals with similar demographics to our patient. Squamous cell carcinoma and malignant melanoma were also considered due to the rapid progression of the lesion, with the latter having a potential amelanotic presentation.
The second differential was infective skin pathology such as impetigo or reactivation of herpes zoster. Such infective causes may occur secondary to immunocompromise caused by the underlying malignant disease.4
Treatment
Surgical excision of the cutaneous lesion.
Outcome and follow-up
The patient recovered fully from total excision of the skin lesion. No further distant metastases have been observed since. However, the underlying malignant disease has continued to progress and is currently being managed by palliative care teams.
Discussion
Cutaneous metastases predominantly originate from primary tumours of the lung, breast and malignant melanoma, although they can also occur from colonic tumours.2 3 Skin metastases from primary colonic tumours occur in only 4–6.5% of cases of metastatic colorectal cancer.3 5 The most frequent site is the abdominal skin, in particular that of postoperative incisional scars, due to direct spread from the neoplasm or lymphatic dissemination. Invasion of veins may lead to distant cutaneous metastases.6 Cutaneous metastasis of the face occurs in less than 0.5% of patients with metastatic cancer.1
Ulceration, nodules, bullae or fibrotic processes are the most common presentations of cutaneous metastases. The median time interval between the diagnosis of internal malignancy and the presentation of metastasis to the skin is 33 months.3
Histology and immunohistochemistry of the skin lesion aids diagnosis of the primary site of malignancy. In our case, histological sections with H&E staining detected tubular differentiation of the infiltrative tissue, suggesting that the skin lesion was of colorectal adenocarcinoma origin (figures 1 and 2). Immunohistochemical staining was used to detect markers for colorectal adenocarcinoma, including cytokeratin 20 (CK20), a major cellular protein of mature enterocytes and goblet cells, specifically found in the gastric and intestinal mucosa, and the CDX-2 homeobox protein biomarker for gastrointestinal differentiation, in particular colorectal adenocarcinoma (figures 3 and 4).
Figure 1.
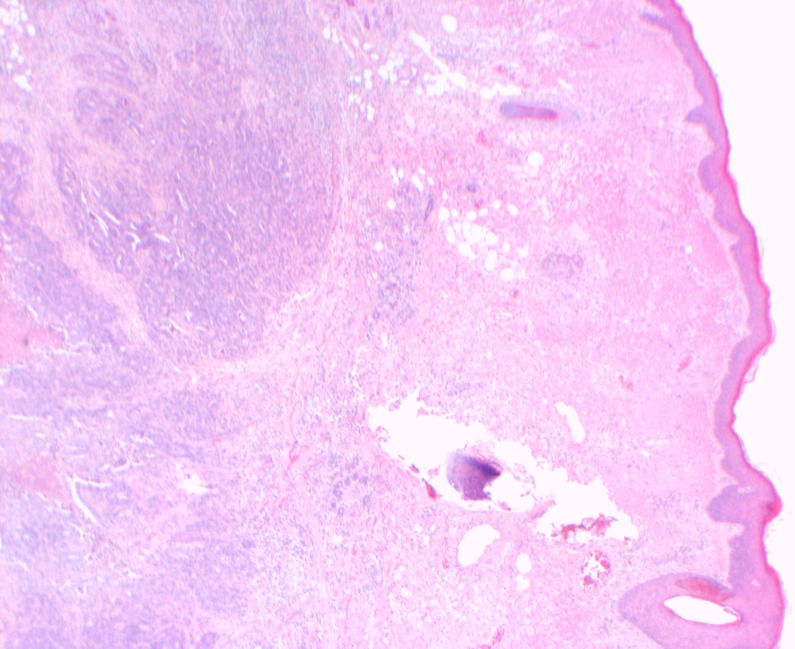
Low-powered H&E stain showing a cross-section of the excision biopsy. The epidermis is seen at the top with normal dermal tissue, hair follicle and sebaceous gland. Infiltration of the abnormal deep dermal tissue is stained blue.
Figure 2.
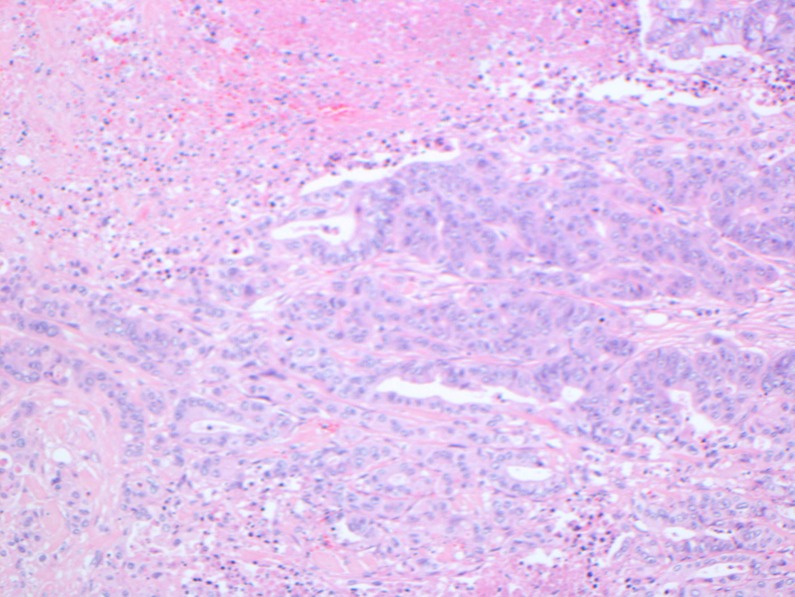
High-powered H&E staining of the same slide showing tubular differentiation of the infiltrative tissue (stained blue) consistent with adenocarcinoma.
Figure 3.
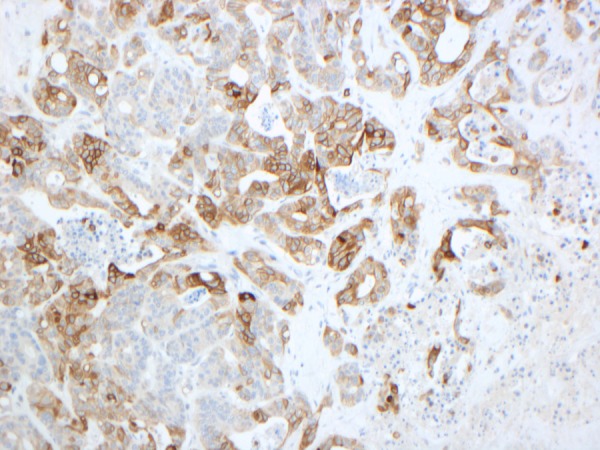
Cytokeratin 20 (CK20) is a protein that in humans is encoded by the KRT20 gene. CK20 is a type I cytokeratin. It is a major cellular protein of mature enterocytes and goblet cells and is specifically found in the gastric and intestinal mucosa. It is used as a marker for colorectal adenocarcinoma and can be seen here with the positive brown staining of the cytoplasm.
Figure 4.
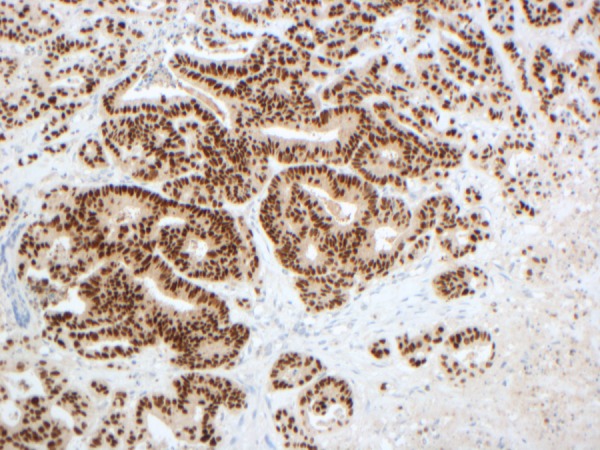
Homeobox protein CDX-2 is a protein that in humans is encoded by the CDX2 gene. CDX2 is used in diagnostic surgical pathology as a marker for gastrointestinal differentiation, especially colorectal adenocarcinoma and the positive result can be seen here with the nuclei of cells stained brown. The cytoplasm remains unchanged stained blue.
Subcutaneous metastases are rarely identified as a first sign of internal malignancy, and may signify disseminated disease. More frequently, skin metastases are identified as a late-developing sign of disease recurrence, identified approximately 4.9 years after excision of a primary visceral tumour.3
A high index of clinical suspicion is required for prompt investigation and diagnosis of new or evolving skin lesions in patients with a history of cancer. Cutaneous metastasis from primary cancers including the lung, cervix and oesophagus is generally preterminal, with a life expectancy of 3 months from diagnosis.7 Schoenlaub et al8 studied 200 cases of cancers with cutaneous or subcutaneous metastasis. Of the patients with colorectal primary tumours, median survival was 4.4 months. On the other hand, a retrospective study by Lookingbill et al showed a median survival of 18 months in patients with similar characteristics.1
Following the diagnosis of cutaneous metastasis, the patient should undergo surgical excision of the skin lesion. In addition, local radiotherapy and systemic chemotherapy may be used. Prompt detection and initiation of treatment may prevent the development of widespread cutaneous metastases and extend life expectancy.
Learning points.
Cutaneous metastasis is a rare clinical finding, occurring in 4.5–6% of cases of colorectal adenocarcinoma. It is usually discovered several years after resection of the primary tumour.
Skin metastasis may be the first sign of recurrence of a previously treated cancer. This clinical finding is late to develop, and is generally associated with disseminated disease and a poor prognosis.
This case highlights the importance of intensive regular follow-up for patients with a history of cancer. A high index of clinical suspicion is required to investigate and diagnose cutaneous metastases when patients with a history of cancer present with new or evolving skin lesions.
Early detection and aggressive treatment may prevent the development of widespread cutaneous metastases and extend life expectancy.
Acknowledgments
With thanks to Dr Abraham, Dr Molyneux and Peter Mooney for immunohistological micrographs and interpretations.
Footnotes
Contributors: YH and SD looked after the patient. YH wrote the first draft of the manuscript and SD contributed to the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol 1993;2013:228–36 [DOI] [PubMed] [Google Scholar]
- 2.Hu SC, Chen GS, Wu CS, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol 2009;2013:379–87 [DOI] [PubMed] [Google Scholar]
- 3.Saeed S, Keehn CA, Morgan MB. Cutaneous metastasis: a clinical, pathological and immunohistochemical appraisal. J Cutan Pathol 2004;2013:419–30 [DOI] [PubMed] [Google Scholar]
- 4.Eichinger J, George B, Myhand R. Cutaneous metastatic rectal carcinoma masquerading as herpes zoster. South Med J 2011;2013:233–5 [DOI] [PubMed] [Google Scholar]
- 5.Fyrmpas G, Barbetakis N, Efstathiou A, et al. Cutaneous metastasis to the face from colon adenocarcinoma. Case report. Int Semin Surg Oncol 2006;2013:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browstein MH, Helwig EG. Patterns of cutaneous metastasis. Arch Dermatol 1972;2013:862–8 [PubMed] [Google Scholar]
- 7.Brady LW, O'Neill EA, Farber SH. Unusual sites of metastases. Semin Oncol 1977;2013:59–64 [PubMed] [Google Scholar]
- 8.Schoenlaub P, Sarraux A, Grosshans E, et al. Survival after cutaneous metastasis: a study of 200 cases (in French). Ann Dermatol Venereol 2001;2013:1310–15 [PubMed] [Google Scholar]


