Summary
Anemia is common in intensive care unit (ICU) patients. Red blood cell (RBC) transfusions are mainstays of their treatment and can be life-saving. Allogeneic blood components inherently bear risks of infection and immune reactions. Although these risks are rare in developed countries, recombinant human erythropoietin (rhEpo) and other erythropoiesis-stimulating agents (ESAs) have been considered alternative anti-anemia treatment options. As summarized herein, however, most of the clinical studies suggest that ESAs are not usually advisable in ICU patients unless approved indications exist (e.g., renal disease). First, ESAs act in a delayed way, inducing an increase in reticulocytes only after a lag of 3–4 days. Second, many critically ill patients present with ESA resistance as inflammatory mediators impair erythropoietic cell proliferation and iron availability. Third, the ESA doses used for treatment of ICU patients are very high. Fourth, ESAs are not legally approved for general use in ICU patients. Solely in distinct cases, such as Jehovah's Witnesses who refuse allogeneic blood transfusions due to religious beliefs, ESAs may be considered an exceptional therapy.
KeyWords: Anemia, Blood transfusion, Hypoxia, Recombinant human erythropoietin, Red blood cells, Critical care unit, Intensive care unit
Introduction
Intensive care units (ICUs) provide full-scale monitoring and treatment of patients in a critically ill or unstable condition, as it may result from a severe accident (e.g., cranial trauma), cardiovascular disease (e.g., myocardial infarction (MI) or stroke), major surgery (e.g., Whipple operation), multiple organ failure (e.g., following major surgery) or sepsis, which is often combined. Anemia is a major issue in ICU patients. In a multicenter study of 145 European ICUs, 63% of the total of >1,100 patients had blood hemoglobin (Hb) concentrations ≤ 120 g/l on admission, including 29% of patients presenting with Hb concentrations ≤ 100 g/l [1]. The CRIT Study (‘Anemia and Blood Transfusion in the Critically Ill –Current Clinical Practice in the United States’), published by Corwin et al. in 2004 [2], has shown that almost all patients are anemic by day 3 of their ICU stay. Both an increased loss and a decreased production of red blood cells (RBCs) contribute to ICU-associated anemia. Almost 50% of ICU patients receive allogeneic RBCs, the number of transfusions correlates with longer hospitalization and increased mortality [2].
Because allogeneic RBC transfusions inherently bear risks of transmission of infectious diseases, acute and chronic hemolytic transfusion reactions and transfusion-related lung injury [3], transfusion practices have become more restrictive over time [4, 5, 6]. Though these adverse events are rare in developed countries (cf. German Haemovigilance Report 2010 of the Paul-Ehrlich-Institut; www.pei.de), recombinant human erythropoietin (rhEpo) and other erythropoiesis-stimulating agents (ESAs) have been considered alternative treatment options in euvolemic ICU patients [7, 8]. At present, the use of ESAs in ICU is off-label, unless the patients present with an approved clinical indication [9]. Depending on individual country regulations, ESAs are approved for anemic patients with chronic kidney disease (CKD), cancer patients on myelosuppressive chemotherapy, HIV-infected patients on zidovudine treatment, patients undergoing autologous blood collection or elective surgery, and anemic preterm infants [10].
Evidence pro and against ESA therapy in ICU patients is the focus of the present article. In addition, some background information on the anemia in ICU patients is provided. Owing to the limited space, in many cases review articles have been cited. Although rhEpo has been approved for treatment of the anemia of prematurity in certain countries, its use in neonatal ICUs is not described here but the information is found elsewhere [11].
Pathophysiology of Anemia in ICU Patients
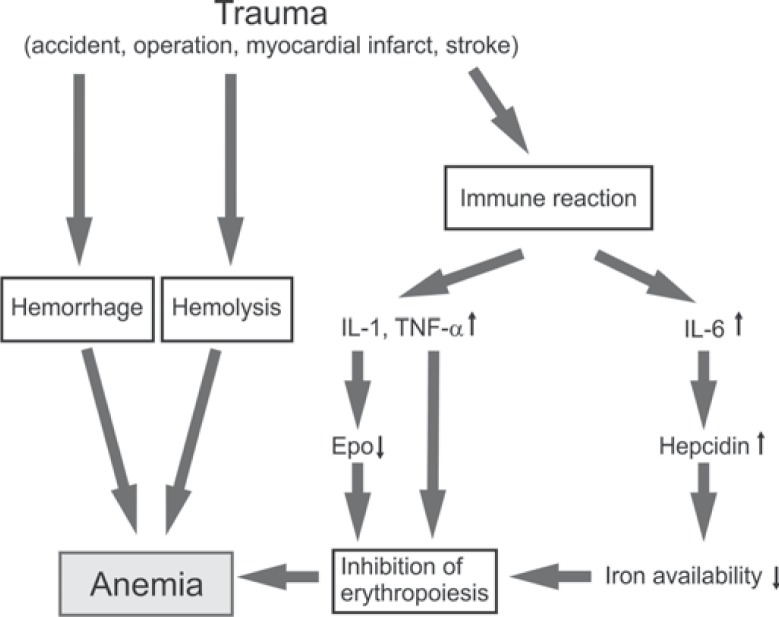
The World Health Organization (WHO) has earlier defined anemia as Hb concentration ≤ 120 g/l in women and ≤ 130 g/l in men [12], and these limits are still accepted [13]. Accordingly, almost all patients (∼97%) are anemic after their first week in the ICU [2]. The anemia in critical illness involves several pathogenetic factors (fig. 1). RBC survival is shortened due to pathogen- and immune reaction-associated hemolysis [14]. The anemic state is aggravated by disorders of hemostasis such as the trauma-induced coagulopathy, gastrointestinal or other occult bleedings, and sequential diagnostic blood sampling. These reactions do not only occur in traumatic or surgical patients but also in medical ICU patients [15]. The daily replacement rate for RBCs under physiological conditions is about 20 ml in healthy persons, but daily phlebotomy may amount to 40–70 ml in ICU patients [16].
Fig. 1.
Causes of anemia in critical illness. Hemorrhage is often the primary factor. Hemolysis is increased by pathogens and immune mediators. Proinflammatory cytokines such as interleukin 1 (IL-1) and tumor necrosis factor α (TNF-α) suppress EPO expression and inhibit the proliferation of erythrocytic progenitors. The iron availability is greatly reduced by the acute-phase protein hepcidin.
The erythropoietic response to anemia is blunted in critical illness [17], sharing pathogenetic mechanisms with the anemia of chronic disease. The anemia is usually normochromic and normocytic. Inflammatory cytokines such as interleukin 1 (IL-1) and tumor necrosis factor α (TNF-α) suppress Epo gene (EPO) expression [18]. Relatively low levels of circulating Epo were measured in studies of mixed populations of critically ill patients [19, 20] and in multiply traumatized patients [21]. The term ‘relatively low’ indicates that the Epo levels were low when related to the patients’ Hb concentrations. In absolute terms Epo levels were approximately 10-fold higher than those in non-anemic healthy subjects. In addition, IL-1 and TNF-α, in concert with interferon γ (IFN-γ), lower the sensitivity of the erythrocytic progenitors towards Epo [22]. The role of interleukin-6 (IL-6) is more complex. IL-6 is thought to stimulate erythropoiesis, directly in the bone marrow, and indirectly through enhancing hepatic Epo synthesis. For example, extremely high plasma Epo levels were measured in patients with lethal sepsis [23]. Most importantly, IL-6 stimulates the production of the iron regulatory hepatic hormone hepcidin [24]. This acute-phase protein mediates the degradation and internalization of ferroportin-1 in enterocytes, hepatocytes, and macrophages. Normally ferroportin-1 transports iron from inside to the outside of the cells. In case of ferroportin-1 degradation, iron cannot be absorbed in the gut, and cannot be released from iron stores. As a result, heme synthesis is greatly impaired [25].
RBC Transfusion
Almost 50% of critically ill patients receive RBC transfusions [2], and >70% of the patients who are resident in the ICU for a week or longer are transfused. Whether benefit is bound to occur from RBC transfusions is a controversial issue, but apparently tradition and the theoretical appeal of abundant oxygen supply have made it standard practice [16]. In viewing the different types of diseases responsible for the admission to an ICU, it seems clear that patient-specific anti-anemia treatment options are to be considered.
Diagnosis of anemia is based on Hb concentration and hematocrit (Hct), i.e., it reflects the relationship between RBC mass and blood plasma volume [26]. On acute hemorrhage, it will take several hours for Hb concentration and Hct to decline. The ‘critical Hb concentration’ is commonly defined as the value below which O2 consumption is limited by O2 supply, this is about <50 g/l in healthy persons [26]. Survival is possible at lower Hb concentrations, as demonstrated for anemic Jehovah's Witnesses [for example see 27]. Based on thoughtful review of the transfusion literature, Walsh and Saleh [26] have stated: ‘…although clinicians frequently transfuse because they are concerned about inadequate oxygen delivery to tissues, this does not usually result in measurable improvements …’.
The Transfusion Requirements in Critical Care (TRICC) trial, published by Hebert et al. in 1999 [28], first provided evidence that Hb concentrations in the 70–90 g/l range are relatively well tolerated by most ICU patients. The restrictive RBC transfusion strategy (i.e., threshold Hb concentration ≥70 g/l for the restrictive group vs. ≥90 g/l for the liberal group) was basically safe, with the possible exception of critically ill patients with acute MI and unstable angina [29]. In 2011, Carson et al. [30] published the results of another trial investigating whether a higher threshold for blood transfusion would improve recovery in patients who had undergone surgery for hip fracture. The study compiled 2,016 patients who were 50 years of age or older, who had either a history of or risk factors for cardiovascular disease, and whose postoperative Hb level was <100 g/l [30]. The patients were assigned to a liberal transfusion strategy (Hb concentration threshold of 100 g/l) or a restrictive transfusion strategy (symptoms of anemia or at physician discretion for an Hb level of <80 g/l). A median of 2 units of RBCs were transfused in the liberal-strategy group and none in the restrictive-strategy group. A liberal transfusion strategy, as compared with a restrictive strategy, did not reduce rates of death or inability to walk on a 60-day follow-up or reduce in-hospital morbidity in elderly patients at high cardiovascular risk [30]. In clinical practice, however, there is still discussion on the Hb concentration threshold at which postoperative RBC transfusion is warranted [31]. When the relationship between anemia, RBC transfusions and outcomes was retrospectively investigated in 5,925 surgical ICU patients, higher Hb concentrations and receipt of allogeneic RBCs were independently associated with a lower risk of in-hospital death, especially in patients aged between 66 and 80 years, in patients admitted to the ICU after non-cardiovascular surgery, in patients with higher severity scores, and in patients with severe sepsis. The authors concluded that randomized control studies are warranted to confirm the potential benefit of blood transfusions in these subpopulations [31]. Lelubre and Vincent [32] have recently proposed to personalize blood transfusion according to physiological endpoints rather than to use arbitrary thresholds. In daily clinical routine, however, guidelines providing threshold values are of practical usefulness.
Ischemic Myocardial Disease
The association between blood transfusion and mortality was investigated among patients with acute coronary syndromes (ACS) who develop bleeding, anemia, or both during their hospital course [33]. The analysis, which included 24,112 enrollees in 3 large international trials, revealed that patients who underwent transfusion were older and had more comorbid illness at presentation and also had a significantly higher unadjusted rate of 30-day death, MI, and death/MI compared with patients who did not undergo transfusion [33]. In patients with acute MI, the development of anemia during hospitalization was found to be associated with increased mortality [34]. In patients with non-ST-segment elevation ACS (NSTE ACS), the likelihood of cardiovascular death, MI, or recurrent ischemia increased when Hb concentrations fell below 110 g/l [35]. The need for RBC transfusion is a risk factor for mortality in such patients [36]. In patients with ST-segment elevation MI (STEMI), cardiovascular mortality increased when Hb concentrations fell below 140 g/l with an adjusted odds ratio (OR) of 1.21 for each 10 g/l decrement in Hb concentration [35]. Patients with STEMI and Hb concentration ≤ 120 g/l had improved outcomes when transfused. In a multicenter study of 5,065 patients who underwent coronary artery bypass grafting (CABG), preoperative anemia and intraoperative RBC transfusions were independently associated with adverse postoperative cerebral and renal outcomes [37]. In a study of over 6,000 patients undergoing percutaneous coronary intervention (PCI), anemia proved to be associated with increased 30-day major cardiac events and with decreased 1-year survival rates [38]. In a retrospective analysis of data on almost 79,000 Medicare beneficiaries 65 years old or older who were hospitalized with acute MI, blood transfusion was associated with a lower short-term mortality rate only if Hct was ≥30.0% on admission [39]. It has been concluded that the benefits of RBC transfusions exceed the risks, when the Hb concentration falls <70 g/l in this population [40].
Sepsis
With respect to sepsis, a TRICC trial [28] subgroup analysis of patients with severe infection and shock showed no difference in 30-day mortality between the restrictive and the liberal RBC transfusion strategy groups (threshold Hb concentration ≥70 g/l vs. ≥90 g/l). Based on the results of the single-center Early Goal-Directed Therapy (EGDT) trial on 263 patients with severe sepsis, Rivers et al. [41] developed a treatment algorithm suggesting a target Hb concentration of 100 g/l (Hct ∼30%) for patients during the early phase of severe sepsis and central venous O2 saturation (ScvO2) ≤ 70%. This procedure has become standard of care, although it is partly at odds with the implications of the TRICC trial. Sweet et al. [42] have pointed out that it is difficult to perform the full EGDT protocol in a busy emergency department because of the time needed to place the various invasive catheters and perform the complex resuscitation and because many departments are not set up to measure ScvO2. In fact, a recent prospective study has shown that RBC transfusions, despite increasing Hb concentration, do not lead to an improvement in tissue oxygenation in patients with systemic inflammatory response syndrome (SIRS)/sepsis and Hb concentration <90 g/l [43]. In view of these areas of scientific uncertainty RBC transfusion guidelines appear of use to the ICU clinical teams, particularly when dealing with anemia management in septic patients.
Transfusion Guidelines
There are guidelines for transfusion of critically ill patients [5, 6, 44, 45]. For example, the British Committee for Standards in Haematology (BCSH) [45] recommends a transfusion threshold Hb concentration of ≥70 g/l, with a target Hb concentration of 70–90 g/l, in critically ill patients, unless specific co-morbidities or acute illness-related factors modify clinical decision-making. In the early resuscitation of patients with severe sepsis, transfusion of RBCs to a target Hb concentration of 90–100 g/l should be considered, if there is clear evidence of inadequate systemic O2 delivery [45]. In patients with traumatic brain injury, the target Hb concentration should be >90 g/l in case of evidence of cerebral ischemia, otherwise it should be 70–90 g/l according to the BCSH guidelines [45]. In patients with subarachnoid hemorrhage, the target Hb concentration should be 80–100 g/l [45]. In patients with an acute ischemic stroke, Hb concentrations >90 g/l, and in patients with ACS >80 g/l are recommended. [45]. Of note, different guidelines may be applicable in individual countries or hospitals.
Basics of the Therapy with rhEpo and Its Analogues
Epo is essential for the production of RBCs in the bone marrow. Because Epo is mainly produced in the kidneys, patients with CKD (includes patients on dialysis and not on dialysis) are substituted with rhEpo (International Nonproprietary Name (INN): epoetin) or with an analogous ESA. Apart from CKD, ESAs can be indicated for the treatment of anemia in cancer patients on myelosuppressive chemotherapy. Depending on country and brand type, rhEpo can also be approved for anemia associated with zidovudine treatment in HIV infection, the support of an autologous blood collection program, elective surgery, and anemia in preterm infants. Common weekly doses are 2,000–8,000 IU rhEpo in anemic CKD patients (dose depending upon patient's weight, severity of anemia, and associated symptoms) and 30,000–40,000 IU rhEpo in cancer patients on chemotherapy.
Epo suppresses the programmed cell death (apoptosis) of the colony-forming units-erythroid (CFU-Es) and their offsprings, thereby promoting the generation of an increased number of normoblasts and, eventually, reticulocytes [46]. Importantly, the increase in the number of reticulocytes in blood becomes significant only after a lag of 3–4 days following the administration of rhEpo [47]. Maximum increases in Hb concentration by ∼1.5 g/l/day may become possible, when extremely high Epo levels are reached (e.g. following the application of 500 IU rhEpo per kg body weight (bw)) [48]. For comparison, the transfusion of one RBC unit will produce an immediate increase in the Hb concentration by ∼10 g/l [49].
Off-label uses of ESAs have been described with respect to the anemias of chronic diseases (i.e., rheumatoid arthritis, systemic lupus erythematosus, or inflammatory bowel disease), myelodysplastic disorders, and hepatitis C/ribavirin therapy. In patients with reduced iron availability, iron supplementation (to achieve transferrin saturation ≥ 20%) may increase the effectiveness of rhEpo. Infused, rather than oral, iron supplementation is advised because hepcidin inhibits the gastrointestinal uptake of iron [24, 25].
Biopharmaceuticals may be immunogenic, and cases of neutralizing antibody (Ab) formation against ESAs have been detected in CKD patients [50]. Neutralizing anti-Epo Abs may cause pure red cell aplasia (PRCA), which is characterized by severe normochromic normocytic anemia of sudden onset, reticulocytopenia, and the lack of erythrocytic precursors in the bone marrow. However, anti-Epo Ab formation is unlikely to occur in ESA-treated critically ill patients for several reasons: i) The incidence of anti-Epo Ab-induced PRCA is generally very low (0.26/10,000 patient years) [51]. ii) Based on current knowledge the period until anti-Epo Abs form exceeds three months of ESA therapy [51]. iii) ESAs can be administered via the intravenous (IV) route in ICU patients, while anti-Epo Abs occur almost exclusively on subcutaneous (SC) ESA administration [51]. There has been only one single case of anti-Epo Ab-induced PRCA, in which ESAs were solely administered via the IV route [52].
ESA Therapy in Critically Ill Patients
Corwin et al. [53] first performed a pilot study to determine whether rhEpo would reduce the need for RBC transfusions in ICU patients. A total of 160 patients were randomized to daily receive by SC injection either rhEpo (300 IU/kg bw) or placebo from ICU days 3 to 7 and then every other day. Reportedly, less RBC units were transfused in the rhEpo than in the placebo group, while rates of mortality and adverse events were similar [53]. A second study (EPO-2; 1,302 patients) in which lower rhEpo doses (40,000 IU/week, SC, for a total of 3 doses) were applied yielded similar results [54]. In a third study (EPO-3; 1,460 patients) the same rhEpo dosing (40,000 IU/week) did neither decrease the number of patients transfused nor the number of RBC units transfused [55]. The use of rhEpo was associated with an increased incidence of thrombovascular events (TVEs) in patients who did not receive heparin at baseline but not among those who received heparin at baseline [55].
Epo-induced increases in Hb concentration develop very slowly in critically ill patients due to the inflammatory processes. For example, in the study by van Iperen et al. [20] the administration of IV high-dose rhEpo (300 IU/kg bw) every other day for 9 days caused increases in reticulocytes, whereas Hb concentrations remained unchanged in the 9 ICU patients under study. The RBC zinc protoporphyrin level was elevated in the rhEpo-treated patients, indicating iron-deficient erythropoiesis despite daily IV administrations of 20 mg iron saccharate. These results are in line with findings by Vincent et al. [56] reporting that the change in Hb concentration from baseline through day 29 was not different when a rhEpo (SC 40,000 IU once weekly) and a placebo group were compared, although rhEpo-treated patients presented with a stronger reticulocyte response [56]. Another study showed a reduction in transfusion requirements along with an increase in Hct in ICU patients receiving SC 40,000 IU rhEpo once a week. There was little further improvement in patients receiving SC 40,000 IU rhEpo three times a week [57]. It should be remembered that such doses are about 10-fold higher than those commonly used for alleviation of anemia in CKD [58]. Only at extremely high concentration, Epo may overcome the inhibitory action of pro-inflammatory cytokines and stimulate the proliferation of erythrocytic progenitors in critically ill patients [59].
In order to investigate more precisely whether more frequent ESA administration raises efficacy, the pharmacokinetics and pharmacodynamics of six rhEpo dosing regimens were tested in a 28-day clinical trial on 60 ICU patients (Hb concentration ≥ 120 g/l) [60]. Alternative regimens were SC or IV 40,000 IU once weekly; SC or IV 15,000 IU every other day; or SC or IV 40,000 IU on days 1 and 3 followed by SC 15,000 IU once every other day on days 5–15; treatment duration in all groups 15 days. Peak serum Epo concentrations were 10–45 times higher on IV than on SC dosing. However, there was no increase in Hb concentration [60].
An earlier meta-analysis of controlled trials (9 studies, databases covering the period 1950–2007) has also pointed out that the use of rhEpo, compared with placebo or no intervention, had no significant effect on overall mortality, duration of mechanical ventilation, and length of stay in the ICU [61]. The mean number of RBC units transfused per patient was reduced by 0.41 in the rhEpo group but the authors recalled that most of the included studies were performed before the widespread adoption of a restrictive transfusion strategy [61].
Taken together, rhEpo treatment does not appear to be very effective in ICU patients. The possibility remains that the efficacy of ESA therapy differs depending on the individual pathology, and benefits could be detected in distinct groups of ICU patients.
ESA Use in Trauma
Since endogenous Epo levels were relatively low in multiply traumatized patients [21], ESA substitution therapy has been considered [62]. In fact, in the trauma subgroup of the above described trial EPO-2 a 29-day survival benefit was assessed in the rhEpo-treated patients (mortality 8.9 vs. 4.1%) [54]. The similar trial EPO-3 [55] confirmed this survival benefit (mortality 6.7 vs. 3.5%), but it also showed an increase in clinically relevant TVEs in the rhEpo-treated trauma group (16.4 vs. 12.5%) [62]. In particular, a trend was seen toward increased risk of venous TVEs in rhEpo-treated patients not receiving prophylaxis by use of heparin [62].
Neurological outcomes following traumatic brain injury will be worsened in anemic subjects, due to a reduction in cerebral O2 tension and hypoxia-induced cell death. Vascular and cellular mechanisms that may help to maintain cerebral O2 delivery in anemia have been discussed elsewhere [63]. Talving et al. [64] performed a prospective observational study on 566 patients with severe traumatic brain injury. Patients who received ESA (darbepoetin alfa 0.40 μg/kg bw; corresponding to ∼SC 80 IU rhEpo/kg bw weekly) experienced longer lengths of stay in the surgical ICU (on average 16.1 vs. 8.6 days) and comparable ICU-free days. In-hospital mortality was reduced for patients who received darbepoetin alfa compared with those who did not (9.3 vs. 25.3%).
In the Long Term Trauma Outcomes Study patients with major blunt trauma orthopedic injuries were administered rhEpo or placebo weekly both in hospital and after discharge for up to 12 weeks or until Hb concentration was >120 g/l [65]. Hb concentration increased from baseline to hospital discharge to a similar degree in the rhEpo (by 12 g/l blood) and the placebo (by 9 g/l blood) group. Transfusion requirements were also similar in both groups [65].
Silver et al. [66] assessed the efficacy of rhEpo therapy in decreasing the occurrence of RBC transfusions in critically ill patients admitted to a long-term acute care facility. The treatment with rhEpo (SC 40,000 IU weekly for up to 12 doses) was associated with a reduction in RBC transfusions and higher Hb concentration during the initial 42 days, with little additional benefit achieved with rhEpo therapy to 84 days. Mortality rate and serious adverse clinical events were not statistically different between the two groups.
Chui et al. [67] evaluated the cost-effectiveness of rhEpo in surgical trauma patients in an ICU setting. The authors constructed a decision analytic model to compare adjunctive use of rhEpo with standard care in trauma patients from the perspective of a Canadian payer. It was concluded that, although the cost per quality-adjusted life year (QALY) gained with rhEpo use may fall into an acceptable range, there is great uncertainty about its true cost-effectiveness [67].
ESA Use in Myocardial Disease
Pilot studies investigating effects of ESA administration in patients with acute MIs provided conflicting results with respect to the left ventricular ejection fraction (LVEF) and infarct size [for review see 68]. Larger trials on patients with acute STEMI treated with PCI have shown that the therapy with high-dose rhEpo does not improve LVEF or reduce MI size [69, 70, 71]. In the Reduction of Infarct Expansion and Ventricular Remodeling With Erythropoietin After Large Myocardial Infarction (REVEAL) trial on 222 patients with STEMI and PCI, the mean infarct size within the first week was even larger in the rhEpo group, compared to the placebo group, in patients aged 70 years or older [72]. In the safety cohort, of the 125 patients who received rhEpo, the composite outcome of death, MI, stroke, or stent thrombosis occurred in 5 patients but in none of the 97 who received placebo [72]. Two meta-analyses of randomized controlled trials have confirmed that there is no benefit of ESAs over conventional therapy in patients with acute MI [73, 74].
There is a single report proposing that extremely high doses of rhEpo (90,000 IU), given as a bolus early during cardiopulmonary resuscitation, can improve the hemodynamic efficacy of chest compression yielding higher rates of initial resuscitation and higher rates of survival to hospital discharge, compared with concurrent controls [75]. This interesting finding requires further investigation.
ESA Use in Stroke
Analogues and derivatives of rhEpo have been considered as a treatment means in stroke patients for two reasons: i) ESAs might be regularly used to stimulate erythropoiesis. Although Hb concentrations as low as 70 g/l are tolerated in most critically ill patients, such a severe degree of anemia could be harmful in brain-injured patients [76]. ii) Derivatives of rhEpo could be applied for neuroprotection [77] since Epo was assigned pleiotropic anti-apoptotic potential (see below). However, in a well-designed ischemic stroke trial (German Multicenter EPO Stroke Trial) the use of rhEpo for neuroprotection resulted in a higher death rate as compared with placebo, particularly in patients requiring thrombolytic therapy [78]. Serum biomarker profiles, as an outcome measure of brain damage, corroborated some advantageous effects of rhEpo in ischemic stroke [79]. With respect to current clinical practice, however, rhEpo and its analogues appear of little use in stroke. In anemic stroke patients ESAs are of little help because of the delay in RBC production [80]. In non-anemic stroke patients ESA administration may be harmful due to adverse events resulting from the stimulation of erythropoiesis, particularly the risk to promote thromboembolism [81].
ESA Use in Burn Injury
Still et al. [82] performed a prospective double-blind randomized study of 40 patients to evaluate the effects of rhEpo in preventing anemia in acutely burned patients (burns from 25 to 65% total body surface). RhEpo (100 IU/kg bw) or a placebo was begun within 72 h of admission and then daily for 1 week; thereafter, the dose was reduced. The administration of rhEpo in the acutely burned patients did neither prevent the development of postburn anemia nor decrease transfusion requirements [82]. Lundy et al. [83] examined retrospectively the effect of rhEpo (40,000 IU weekly) on mortality and transfusion in 25 burned patients (burns ≥ 30% total body surface, ICU stay ≥ 15 days). The patients were treated with 40,000 IU rhEpo over an 18-month period. No effect was seen for rhEPO treatment on mortality or RBC transfusion requirements in the severely burned, when compared to a group of matched historic controls [83]. Very recently a large, prospective, randomized, double-blind, multicenter study has been initiated to investigate the effects of rhEpo treatment (150 IU/kg bw every other day for 21 days) in severely burned patients [84]. However, anemia treatment is not the primary goal here, instead the study will investigate effects on wound healing [84].
ESA Use for Tissue Protection in the Perioperative Period
Preclinical observations triggered the hypothesis Epo is a pleiotropic survival factor with ubiquitous anti-apoptotic properties [85], leading to clinical trials of the use of rhEpo or its derivatives to protect tissues in the critically ill. However, recent research has shown that solely hematopoietic tissues have high levels of Epo receptor molecules with undetectable levels in non-hematopoietic tissues [86]. Accordingly, the enthusiastic preclinical tissue-protective effects assigned to Epo did not stand firm in well-controlled large clinical trials [87]. For example, while a small pilot study suggested that the prophylactic administration of rhEpo (300 IU/kg bw, IV) could prevent acute kidney injury (AKI) in patients undergoing CABG [88], another trial did not detect nephroprotective properties of rhEpo, when administered to patients on arrival to the ICU immediately after cardiac surgery [89]. Neurocognitive dysfunction can also complicate CABG surgery. In a small double-blind, placebo controlled, proof-of-concept trial, treatment with high doses of rhEpo (up to 1,500 IU/kg bw, divided in 3 daily doses, starting the day before surgery), the postoperative cognitive decline did not differ statistically between rhEpo-treated patients and controls, and there were no benefits with respect to the mortality rate [90]. Likewise, an interventional study in high-risk ICU patients revealed that rhEpo treatment (500 IU/kg bw. IV) will neither prevent AKI nor reduce the risk for mortality [91].
RhEpo Treatment in Jehova's Witnesses
In 2008, Ball et al. [92] summarized the reports of rhEpo therapy in critically ill Jehovah's Witnesses who refused blood transfusions or blood products for religious reasons. Among the cases (trauma, burns, general surgery, gastrointestinal hemorrhage), there was major variation with respect to time to the start of treatment, dosages, route of administration, and treatment duration. Reading leaves an impression that the administration of ESAs in combination with blood conservation techniques might have increased Hb concentration and survival in the patients. For example, there is a report on 4 severely anemic Jehovah's Witness patients (lowest Hb concentration was 27 g/l), who were discharged from the hospital in good condition after daily treatment with rhEpo (50–280 IU/kg bw) [27]. RhEpo treatment was followed by a rise in reticulocytes and Hb concentration. However, it is obvious that none of the studies was blinded or placebo-controlled. Hence, the administration of an ESA can be justified in the management of life-threatening anemia, although none but on a humanitarian basis, because there is no predictor for the possible spontaneous recovery [27]. Of note, high doses of both ESA and iron are required to stimulate erythropoiesis in these patients.
Conclusions
It has been proposed to treat ICU patients with high-dose rhEpo, or analogues or derivatives thereof. The primary goal of such therapy is achieving an increase in Hb concentration and, hence, to reduce the need for allogeneic RBC transfusions. Other parameters of interest include the length of stay in ICU or in hospital after ICU discharge and the functional outcome after hospital discharge.
In weighing the pros and cons, it is concluded that there is no convincing evidence in support of concepts for a common use of ESAs in ICU patients. The change in Hb concentration resulting from ESA therapy was generally small, and – if at all – the number of RBC units transfused was no better than moderately reduced. The use of rhEpo, compared with placebo or no intervention, had no significant effect on overall mortality, length of stay in hospital or ICU. ESAs act in a delayed way, causing an increase in blood reticulocytes only after a lag of 3–4 days. Many critically ill patients present with ESA resistance as inflammatory mediators impair iron availability and erythropoietic cell proliferation. The ESA doses used for treatment of ICU patients are very high, thus the therapy is less economic. Also, ESAs are not legally approved for general use in ICU patients. Therefore, ESA therapy is not recommended in ICU patients unless specific medical indications exist (e.g., renal disease). Solely in distinct cases, such as Jehovah's Witnesses who refuse allogeneic blood transfusions due to religious beliefs, ESAs may be considered an exceptional therapy.
Disclosure Statement
I.J. has nothing to declare. W.J. has had a compensated consultant/advisory role and received honoraria and research funding from pharmaceutical companies producing and/or marketing ESAs.
References
- 1.Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G, Peres-Bota D. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 2.Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, MacIntyre NR, Shabot MM, Duh MS, Shapiro MJ. The CRIT Study: anemia and blood transfusion in the critically ill – current clinical practice in the United States. Crit Care Med. 2004;32:39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- 3.Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113:3406–3417. doi: 10.1182/blood-2008-10-167643. [DOI] [PubMed] [Google Scholar]
- 4.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–2674. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 5.Napolitano LM, Kurek S, Luchette FA, Corwin HL, Barie PS, Tisherman SA, Hebert PC, Anderson GL, Bard MR, Bromberg W, Chiu WC, Cipolle MD, Clancy KD, Diebel L, Hoff WS, Hughes KM, Munshi I, Nayduch D, Sandhu R, Yelon JA. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med. 2009;37:3124–3157. doi: 10.1097/CCM.0b013e3181b39f1b. [DOI] [PubMed] [Google Scholar]
- 6.Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, Song HK, Clough ER, Shore-Lesserson LJ, Goodnough LT, Mazer CD, Shander A, Stafford-Smith M, Waters J, Baker RA, Dickinson TA, FitzGerald DJ, Likosky DS, Shann KG. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944–982. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 7.Stubbs JR. Alternatives to blood product transfusion in the critically ill: erythropoietin. Crit Care Med. 2006;34(5 suppl):S160–S169. doi: 10.1097/01.CCM.0000214290.11479.5C. [DOI] [PubMed] [Google Scholar]
- 8.Corwin HL. The role of erythropoietin therapy in the critically ill. Transfus Med Rev. 2006;20:27–33. doi: 10.1016/j.tmrv.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Afshar M, Netzer G. Update in critical care for the nephrologist: transfusion in nonhemorrhaging critically ill patients. Adv Chronic Kidney Dis. 2013;20:30–38. doi: 10.1053/j.ackd.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Jelkmann W. Physiology and pharmacology of erythropoietin. Transfus Med Hemother. 2013;40:302–309. doi: 10.1159/000356193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aher SM, Ohlsson A. Early versus late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2012;10:CD004865. doi: 10.1002/14651858.CD004865.pub3. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organisation : Nutritional anaemias. Report of a WHO scientific group; World Health Organ Tech Rep Ser, 1968, pp 5–37. [PubMed]
- 13.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009;12:444–454. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 14.Singh S, Gudzenko V, Fink MP. Pathophysiology of perioperative anaemia. Best Pract Res Clin Anaesthesiol. 2012;26:431–439. doi: 10.1016/j.bpa.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 15.von Ahsen N, Muller C, Serke S, Frei U, Eckardt KU. Important role of nondiagnostic blood loss and blunted erythropoietic response in the anemia of medical intensive care patients. Crit Care Med. 1999;27:2630–2639. doi: 10.1097/00003246-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Hayden SJ, Albert TJ, Watkins TR, Swenson ER. Anemia in critical illness: insights into etiology, consequences, and management. Am J Respir Crit Care Med. 2012;185:1049–1057. doi: 10.1164/rccm.201110-1915CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson Y, Hostmann A, Matenov A, Ertel W, Oberholzer A. Erythropoiesis in multiply injured patients. J Trauma. 2006;61:1285–1291. doi: 10.1097/01.ta.0000240969.13891.9b. [DOI] [PubMed] [Google Scholar]
- 18.Jelkmann W. Proinflammatory cytokines lowering erythropoietin production. J Interferon Cytokine Res. 1998;18:555–559. doi: 10.1089/jir.1998.18.555. [DOI] [PubMed] [Google Scholar]
- 19.Rogiers P, Zhang H, Leeman M, Nagler J, Neels H, Melot C, Vincent JL. Erythropoietin response is blunted in critically ill patients. Intensive Care Med. 1997;23:159–162. doi: 10.1007/s001340050310. [DOI] [PubMed] [Google Scholar]
- 20.van Iperen CE, Gaillard CA, Kraaijenhagen RJ, Braam BG, Marx JJ, van de Wiel A. Response of erythropoiesis and iron metabolism to recombinant human erythropoietin in intensive care unit patients. Crit Care Med. 2000;28:2773–2778. doi: 10.1097/00003246-200008000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Hobisch-Hagen P, Wiedermann F, Mayr A, Fries D, Jelkmann W, Fuchs D, Hasibeder W, Mutz N, Klingler A, Schobersberger W. Blunted erythropoietic response to anemia in multiply traumatized patients. Crit Care Med. 2001;29:743–747. doi: 10.1097/00003246-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Cooper AC, Mikhail A, Lethbridge MW, Kemeny DM, MacDougall IC. Increased expression of erythropoiesis inhibiting cytokines (IFN-gamma, TNF-alpha, IL-10, and IL-13) by T cells in patients exhibiting a poor response to erythropoietin therapy. J Am Soc Nephrol. 2003;14:1776–1784. doi: 10.1097/01.asn.0000071514.36428.61. [DOI] [PubMed] [Google Scholar]
- 23.Abel J, Spannbrucker N, Fandrey J, Jelkmann W. Serum erythropoietin levels in patients with sepsis and septic shock. Eur J Haematol. 1996;57:359–363. doi: 10.1111/j.1600-0609.1996.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 24.Finberg KE. Regulation of systemic iron homeostasis. Curr Opin Hematol. 2013;20:208–214. doi: 10.1097/MOH.0b013e32835f5a47. [DOI] [PubMed] [Google Scholar]
- 25.Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med. 2011;62:347–360. doi: 10.1146/annurev-med-050109-142444. [DOI] [PubMed] [Google Scholar]
- 26.Walsh TS, Saleh EE. Anaemia during critical illness. Br J Anaesth. 2006;97:278–291. doi: 10.1093/bja/ael189. [DOI] [PubMed] [Google Scholar]
- 27.Wolff M, Fandrey J, Hirner A, Jelkmann W. Perioperative use of recombinant human erythropoietin in patients refusing blood transfusions. Pathophysiological considerations based on 5 cases. Eur J Haematol. 1997;58:154–159. doi: 10.1111/j.1600-0609.1997.tb00941.x. [DOI] [PubMed] [Google Scholar]
- 28.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 29.Hebert PC, Yetisir E, Martin C, Blajchman MA, Wells G, Marshall J, Tweeddale M, Pagliarello G, Schweitzer I. Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases? Crit Care Med. 2001;29:227–234. doi: 10.1097/00003246-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, Nemo G, Dragert K, Beaupre L, Hildebrand K, Macaulay W, Lewis C, Cook DR, Dobbin G, Zakriya KJ, Apple FS, Horney RA, Magaziner J. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–2462. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakr Y, Lobo S, Knuepfer S, Esser E, Bauer M, Settmacher U, Barz D, Reinhart K. Anemia and blood transfusion in a surgical intensive care unit. Crit Care. 2010;14:R92. doi: 10.1186/cc9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lelubre C, Vincent JL. Red blood cell transfusion in the critically ill patient. Ann Intensive Care. 2011;1:43. doi: 10.1186/2110-5820-1-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao SV, Jollis JG, Harrington RA, Granger CB, Newby LK, Armstrong PW, Moliterno DJ, Lindblad L, Pieper K, Topol EJ, Stamler JS, Califf RM. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292:1555–1562. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 34.Aronson D, Suleiman M, Agmon Y, Suleiman A, Blich M, Kapeliovich M, Beyar R, Markiewicz W, Hammerman H. Changes in haemoglobin levels during hospital course and long-term outcome after acute myocardial infarction. Eur Heart J. 2007;28:1289–1296. doi: 10.1093/eurheartj/ehm013. [DOI] [PubMed] [Google Scholar]
- 35.Sabatine MS, Morrow DA, Giugliano RP, Burton PB, Murphy SA, McCabe CH, Gibson CM, Braunwald E. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042–2049. doi: 10.1161/01.CIR.0000162477.70955.5F. [DOI] [PubMed] [Google Scholar]
- 36.Yang X, Alexander KP, Chen AY, Roe MT, Brindis RG, Rao SV, Gibler WB, Ohman EM, Peterson ED. The implications of blood transfusions for patients with non-ST-segment elevation acute coronary syndromes: results from the CRUSADE National Quality Improvement Initiative. J Am Coll Cardiol. 2005;46:1490–1495. doi: 10.1016/j.jacc.2005.06.072. [DOI] [PubMed] [Google Scholar]
- 37.Kulier A, Levin J, Moser R, Rumpold-Seitlinger G, Tudor IC, Snyder-Ramos SA, Moehnle P, Mangano DT. Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation. 2007;116:471–479. doi: 10.1161/CIRCULATIONAHA.106.653501. [DOI] [PubMed] [Google Scholar]
- 38.Lee PC, Kini AS, Ahsan C, Fisher E, Sharma SK. Anemia is an independent predictor of mortality after percutaneous coronary intervention. J Am Coll Cardiol. 2004;44:541–546. doi: 10.1016/j.jacc.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 39.Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230–1236. doi: 10.1056/NEJMoa010615. [DOI] [PubMed] [Google Scholar]
- 40.Hebert PC, Fergusson DA. Do transfusions get to the heart of the matter? JAMA. 2004;292:1610–1612. doi: 10.1001/jama.292.13.1610. [DOI] [PubMed] [Google Scholar]
- 41.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 42.Sweet D, Marsden J, Ho K, Krause C, Russell JA. Emergency management of sepsis: the simple stuff saves lives. BC Med J. 2012;54:176–182. [Google Scholar]
- 43.Mazza BF, Machado FR, Mazza DD, Hassmann V. Evaluation of blood transfusion effects on mixed venous oxygen saturation and lactate levels in patients with SIRS/sepsis. Clinics (Sao Paulo) 2005;60:311–316. doi: 10.1590/s1807-59322005000400009. [DOI] [PubMed] [Google Scholar]
- 44.Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, Holcomb JB, Illoh O, Kaplan LJ, Katz LM, Rao SV, Roback JD, Shander A, Tobian AA, Weinstein R, Swinton McLaughlin LG, Djulbegovic B. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 45.Retter A, Wyncoll D, Pearse R, Carson D, Mc-Kechnie S, Stanworth S, Allard S, Thomas D, Walsh T. Guidelines on the management of anaemia and red cell transfusion in adult critically ill patients. Br J Haematol. 2013;160:445–464. doi: 10.1111/bjh.12143. [DOI] [PubMed] [Google Scholar]
- 46.Jelkmann W. Regulation of erythropoietin production. J Physiol. 2011;589:1251–1258. doi: 10.1113/jphysiol.2010.195057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Major A, Mathez-Loic F, Rohling R, Gautschi K, Brugnara C. The effect of intravenous iron on the reticulocyte response to recombinant human erythropoietin. Br J Haematol. 1997;98:292–294. doi: 10.1046/j.1365-2141.1997.2123031.x. [DOI] [PubMed] [Google Scholar]
- 48.Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med. 1987;316:73–78. doi: 10.1056/NEJM198701083160203. [DOI] [PubMed] [Google Scholar]
- 49.Naidech AM, Kahn MJ, Soong W, Green D, Batjer HH, Bleck TP. Packed red blood cell transfusion causes greater hemoglobin rise at a lower starting hemoglobin in patients with subarachnoid hemorrhage. Neurocrit Care. 2008;9:198–203. doi: 10.1007/s12028-008-9113-8. [DOI] [PubMed] [Google Scholar]
- 50.Boven K, Stryker S, Knight J, Thomas A, van Regenmortel M, Kemeny DM, Power D, Rossert J, Casadevall N. The increased incidence of pure red cell aplasia with an Eprex formulation in uncoated rubber stopper syringes. Kidney Int. 2005;67:2346–2353. doi: 10.1111/j.1523-1755.2005.00340.x. [DOI] [PubMed] [Google Scholar]
- 51.Macdougall IC, Roger SD, De Francisco A, Goldsmith D, Schellekens H, Ebbers H, Jelkmann W, London G, Casadevall N, Hörl WH, Kemeny M, Pollock C. Antibody-mediated pure red cell aplasia in chronic kidney disease patients receiving erythropoiesis stimulating agents: new insights. Kidney Int. 2012;81:727–732. doi: 10.1038/ki.2011.500. [DOI] [PubMed] [Google Scholar]
- 52.Shimizu H, Saitoh T, Ota F, Jimbo T, Tsukada Y, Murakami H, Nojima Y. Pure red cell aplasia induced only by intravenous administration of recombinant human erythropoietin. Acta Haematol. 2011;126:114–118. doi: 10.1159/000328041. [DOI] [PubMed] [Google Scholar]
- 53.Corwin HL, Gettinger A, Rodriguez RM, Pearl RG, Gubler KD, Enny C, Colton T, Corwin MJ. Efficacy of recombinant human erythropoietin in the critically ill patient: a randomized, double-blind, placebo-controlled trial. Crit Care Med. 1999;27:2346–2350. doi: 10.1097/00003246-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Shapiro MJ, Corwin MJ, Colton T. Efficacy of recombinant human erythropoietin in critically ill patients: a randomized controlled trial. JAMA. 2002;288:2827–2835. doi: 10.1001/jama.288.22.2827. [DOI] [PubMed] [Google Scholar]
- 55.Corwin HL, Gettinger A, Fabian TC, May A, Pearl RG, Heard S, An R, Bowers PJ, Burton P, Klausner MA, Corwin MJ. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med. 2007;357:965–976. doi: 10.1056/NEJMoa071533. [DOI] [PubMed] [Google Scholar]
- 56.Vincent JL, Spapen HD, Creteur J, Piagnerelli M, Hubloue I, Diltoer M, Roman A, Stevens E, Vercammen E, Beaver JS. Pharmacokinetics and pharmacodynamics of once-weekly subcutaneous epoetin alfa in critically ill patients: results of a randomized, double-blind, placebo-controlled trial. Crit Care Med. 2006;34:1661–1667. doi: 10.1097/01.CCM.0000217919.22155.85. [DOI] [PubMed] [Google Scholar]
- 57.Georgopoulos D, Matamis D, Routsi C, Michalopoulos A, Maggina N, Dimopoulos G, Zakynthinos E, Nakos G, Thomopoulos G, Mandragos K, Maniatis A. Recombinant human erythropoietin therapy in critically ill patients: a dose-response study [ISRCTN48523317] Crit Care. 2005;9:R508–R515. doi: 10.1186/cc3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.KDIGO KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl. 2012;2:272–335. [Google Scholar]
- 59.Gabriel A, Kozek S, Chiari A, Fitzgerald R, Grabner C, Geissler K, Zimpfer M, Stockenhuber F, Bircher NG. High-dose recombinant human erythropoietin stimulates reticulocyte production in patients with multiple organ dysfunction syndrome. J Trauma. 1998;44:361–367. doi: 10.1097/00005373-199802000-00023. [DOI] [PubMed] [Google Scholar]
- 60.Arroliga AC, Guntupalli KK, Beaver JS, Langholff W, Marino K, Kelly K. Pharmacokinetics and pharmacodynamics of six epoetin alfa dosing regimens in anemic critically ill patients without acute blood loss. Crit Care Med. 2009;37:1299–1307. doi: 10.1097/CCM.0b013e31819cec94. [DOI] [PubMed] [Google Scholar]
- 61.Zarychanski R, Turgeon AF, McIntyre L, Fergusson DA. Erythropoietin-receptor agonists in critically ill patients: a meta-analysis of randomized controlled trials. CMAJ. 2007;177:725–734. doi: 10.1503/cmaj.071055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Napolitano LM, Fabian TC, Kelly KM, Bailey JA, Block EF, Langholff W, Enny C, Corwin HL. Improved survival of critically ill trauma patients treated with recombinant human erythropoietin. J Trauma. 2008;65:285–297. doi: 10.1097/TA.0b013e31817f2c6e. [DOI] [PubMed] [Google Scholar]
- 63.Hare GM, Tsui AK, McLaren AT, Ragoonanan TE, Yu J, Mazer CD. Anemia and cerebral outcomes: many questions, fewer answers. Anesth Analg. 2008;107:1356–1370. doi: 10.1213/ane.0b013e318184cfe9. [DOI] [PubMed] [Google Scholar]
- 64.Talving P, Lustenberger T, Kobayashi L, Inaba K, Barmparas G, Schnuriger B, Lam L, Chan LS, Demetriades D. Erythropoiesis stimulating agent administration improves survival after severe traumatic brain injury: a matched case control study. Ann Surg. 2010;251:1–4. doi: 10.1097/SLA.0b013e3181b844fa. [DOI] [PubMed] [Google Scholar]
- 65.Luchette FA, Pasquale MD, Fabian TC, Langholff WK, Wolfson M. A randomized, double-blind, placebo-controlled study to assess the effect of recombinant human erythropoietin on functional outcomes in anemic, critically ill, trauma subjects: the Long Term Trauma Outcomes Study. Am J Surg. 2012;203:508–516. doi: 10.1016/j.amjsurg.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 66.Silver M, Corwin MJ, Bazan A, Gettinger A, Enny C, Corwin HL. Efficacy of recombinant human erythropoietin in critically ill patients admitted to a long-term acute care facility: a randomized, double-blind, placebo-controlled trial. Crit Care Med. 2006;34:2310–2316. doi: 10.1097/01.CCM.0000233873.17954.42. [DOI] [PubMed] [Google Scholar]
- 67.Chui BK, Pannu N, Hazel M, Dong J, Tonelli M, Klarenbach SW. Economic analysis of epoetin alfa in critically ill trauma patients. J Trauma Acute Care Surg. 2012;73:195–201. doi: 10.1097/TA.0b013e31824ba1da. [DOI] [PubMed] [Google Scholar]
- 68.Jelkmann W, Elliott S: Erythropoietin and the vascular wall: the controversy continues. Nutr Metabol Cardiovasc Dis Nutr Metab Cardiovasc Dis2012; 10.1016/j.numecd.2012.04.002. [DOI] [PubMed]
- 69.Ludman AJ, Yellon DM, Hasleton J, Ariti C, Babu GG, Boston-Griffiths E, Venugopal V, Walker M, Holdright D, Swanton H, Crake T, Brull D, Moon JC, Puranik R, Mutharangu V, Taylor A, Hausenloy DJ. Effect of erythropoietin as an adjunct to primary percutaneous coronary intervention: a randomised controlled clinical trial. Heart. 2011;97:1560–1565. doi: 10.1136/hrt.2011.223867. [DOI] [PubMed] [Google Scholar]
- 70.Ott I, Schulz S, Mehilli J, Fichtner S, Hadamitzky M, Hoppe K, Ibrahim T, Martinoff S, Massberg S, Laugwitz KL, Dirschinger J, Schwaiger M, Kastrati A, Schmig A. Erythropoietin in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: a randomized, double-blind trial. Circ Cardiovasc Interv. 2010;3:408–413. doi: 10.1161/CIRCINTERVENTIONS.109.904425. [DOI] [PubMed] [Google Scholar]
- 71.Voors AA, Belonje AM, Zijlstra F, Hillege HL, Anker SD, Slart RH, Tio RA, van ‘t Hof A, Jukema JW, Peels HO, Henriques JP, Ten Berg JM, Vos J, van Gilst WH, van Veldhuisen DJ, HEBE III Investigators A single dose of erythropoietin in ST-elevation myocardial infarction. Eur Heart J. 2010;31:2593–2600. doi: 10.1093/eurheartj/ehq304. [DOI] [PubMed] [Google Scholar]
- 72.Najjar SS, Rao SV, Melloni C, Raman SV, Povsic TJ, Melton L, Barsness GW, Prather K, Heitner JF, Kilaru R, Gruberg L, Hasselblad V, Greenbaum AB, Patel M, Kim RJ, Talan M, Ferrucci L, Longo DL, Lakatta EG, Harrington RA. Intravenous erythropoietin in patients with ST-segment elevation myocardial infarction: REVEAL: a randomized controlled trial. JAMA. 2011;305:1863–1872. doi: 10.1001/jama.2011.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li J, Xu H, Gao Q, Wen Y. Effect of erythropoiesis-stimulating agents in acute ST-segment elevation myocardial infarction: a systematic review. Eur J Clin Pharmacol. 2011;68:469–477. doi: 10.1007/s00228-011-1160-y. [DOI] [PubMed] [Google Scholar]
- 74.Gao D, Ning N, Niu X, Dang Y, Dong X, Wei J, Zhu C. Erythropoietin treatment in patients with acute myocardial infarction: a meta-analysis of randomized controlled trials. Am Heart J. 2012;164:715–727. doi: 10.1016/j.ahj.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 75.Grmec S, Strnad M, Kupnik D, Sinkovic A, Gazmuri RJ. Erythropoietin facilitates the return of spontaneous circulation and survival in victims of out-of-hospital cardiac arrest. Resuscitation. 2009;80:631–637. doi: 10.1016/j.resuscitation.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 76.Kramer AH, Zygun DA. Anemia and red blood cell transfusion in neurocritical care. Crit Care. 2009;13:R89. doi: 10.1186/cc7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Siren AL, Fasshauer T, Bartels C, Ehrenreich H. Therapeutic potential of erythropoietin and its structural or functional variants in the nervous system. Neurotherapeutics. 2009;6:108–127. doi: 10.1016/j.nurt.2008.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, Schellinger PD, Bohn M, Becker H, Wegrzyn M, Jähnig P, Herrmann M, Knauth M, Bähr M, Heide W, Wagner A, Schwab S, Reichmann H, Schwendemann G, Dengler R, Kastrup A, Bartels C. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40:647–656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- 79.Ehrenreich H, Kastner A, Weissenborn K, Streeter J, Sperling S, Wang KK, Worthmann H, Hayes RL, von AN, Kastrup A, Jeromin A, Herrmann M. Circulating damage marker profiles support a neuroprotective effect of erythropoietin in ischemic stroke patients. Mol Med. 2011;17:1306–1310. doi: 10.2119/molmed.2011.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robertson C, Sadrameli S. Erythropoietin in the neurology ICU. Curr Treat Options Neurol. 2013;15:104–112. doi: 10.1007/s11940-013-0222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patel NS, Nandra KK, Thiemermann C. Bench-to-bedside review: erythropoietin and its derivatives as therapies in critical care. Crit Care. 2012;16:229. doi: 10.1186/cc11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Still JM, Jr, Belcher K, Law EJ, Thompson W, Jordan M, Lewis M, Saffle J, Hunt J, Purdue GF, Waymack JP, et al. A double-blinded prospective evaluation of recombinant human erythropoietin in acutely burned patients. J Trauma. 1995;38:233–236. doi: 10.1097/00005373-199502000-00015. [DOI] [PubMed] [Google Scholar]
- 83.Lundy JB, Hetz K, Chung KK, Renz EM, White CE, King BT, Huzar T, Wolf SE, Blackbourne LH. Outcomes with the use of recombinant human erythropoietin in critically ill burn patients. Am Surg. 2010;76:951–956. [PubMed] [Google Scholar]
- 84.Günter CI, Bader A, Dornseifer U, Egert S, Sebastian D, Grieb G, Wolter T, Pallua N, von WT, Siemers F, Mailander P, Thamm O, Ernert C, Steen M, Sievers R, Reichert B, Rahmanian-Schwarz A, Schaller H, Hartmann B, Otte M, Kehl V, Ohmann C, Jelkmann W, Machens HG. A multi-center study on the regenerative effects of erythropoietin in burn and scalding injuries: study protocol for a randomized controlled trial. Trials. 2013;14:124. doi: 10.1186/1745-6215-14-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dumont F, Bischoff P. Non-erythropoietic tissue-protective peptides derived from erythropoietin: WO2009094172. Expert Opin Ther Pat. 2010;20:715–723. doi: 10.1517/13543771003627464. [DOI] [PubMed] [Google Scholar]
- 86.Elliott S, Sinclair A. The effect of erythropoietin on normal and neoplastic cells. Biologics. 2012;6:163–189. doi: 10.2147/BTT.S32281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Solling C. Organ-protective and immunomodulatory effects of erythropoietin – an update on recent clinical trials. Basic Clin Pharmacol Toxicol. 2011;110:113–121. doi: 10.1111/j.1742-7843.2011.00820.x. [DOI] [PubMed] [Google Scholar]
- 88.Song YR, Lee T, You SJ, Chin HJ, Chae DW, Lim C, Park KH, Han S, Kim JH, Na KY. Prevention of acute kidney injury by erythropoietin in patients undergoing coronary artery bypass grafting: a pilot study. Am J Nephrol. 2009;30:253–260. doi: 10.1159/000223229. [DOI] [PubMed] [Google Scholar]
- 89.de Seigneux S, Ponte B, Weiss L, Pugin J, Romand JA, Martin PY, Saudan P. Epoetin administrated after cardiac surgery: effects on renal function and inflammation in a randomized controlled study. BMC Nephrol. 2012;13:132. doi: 10.1186/1471-2369-13-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haljan G, Maitland A, Buchan A, Arora RC, King M, Haigh J, Culleton B, Faris P, Zygun D. The erythropoietin neuroprotective effect: assessment in CABG surgery (TENPEAKS): a randomized, double-blind, placebo controlled, proof-of-concept clinical trial. Stroke. 2009;40:2769–2775. doi: 10.1161/STROKEAHA.109.549436. [DOI] [PubMed] [Google Scholar]
- 91.Endre ZH, Walker RJ, Pickering JW, Shaw GM, Frampton CM, Henderson SJ, Hutchison R, Mehrtens JE, Robinson JM, Schollum JB, Westhuyzen J, Celi LA, McGinley RJ, Campbell IJ, George PM. Early intervention with erythropoietin does not affect the outcome of acute kidney injury (the EARLYARF trial) Kidney Int. 2010;77:1020–1030. doi: 10.1038/ki.2010.25. [DOI] [PubMed] [Google Scholar]
- 92.Ball AM, Winstead PS. Recombinant human erythropoietin therapy in critically ill Jehovah's Witnesses. Pharmacotherapy. 2008;28:1383–1390. doi: 10.1592/phco.28.11.1383. [DOI] [PubMed] [Google Scholar]