Summary
Almost 3 decades have passed since the discovery and cloning of IL-6, and a tremendous amount of work has contributed to the current knowledge of the biological functions of this cytokine, its receptor, and the signaling pathways that are activated. The understanding of the role of IL-6 in human disease has led to the development of novel therapeutic strategies that block the biological functions of IL-6. In clinical studies, IL-6 and IL-6 receptor antibodies have proven efficacy in rheumatoid arthritis, systemic juvenile idiopathic arthritis, and Castleman's disease, conditions that are known to be driven by IL-6. The focus of this overview is the role of IL-6 in the pathophysiology of hematological malignancies.
KeyWords: Interleukin-6, gp130, Leukemia, Lymphoma, Multiple myeloma
Introduction
Almost 3 decades have passed since the discovery and cloning of IL-6, and a tremendous amount of work has contributed to the current knowledge of the biological functions of this cytokine, its receptor, and the signaling pathways that are activated. The understanding about the role of IL-6 in human disease has led to the development of novel therapeutic strategies that block the biological functions of IL-6. In clinical studies, IL-6 and IL-6 receptor antibodies have proven efficacy in rheumatoid arthritis, systemic juvenile idiopathic arthritis, and Castleman's disease (CD), conditions that are known to be driven by IL-6. This review focusses on the role of IL-6 in the pathophysiology of hematological malignancies.
Biology of Interleukin-6
Properties of IL-6 and Gene Regulation
Human IL-6 is a glycoprotein composed of 184 amino acids with a molecular weight of 21–28 kDa, depending on its degree of glycosylation. It has a 4-helix bundle structure made up of 4 long α-helices arranged in an up-up-down-down topology. The gene for IL-6 is located at chromosome 7p21, and consists of 5 exons and 4 introns. The promoter region contains several transcription start sites with response elements for nuclear factor (NF)-κB, CCAT/enhancer-binding protein beta (C/EPBβ; formerly NF-IL6), and activator protein (AP)-1 [1, 2]. IL-6 is produced in many tissues and various cell types including monocytes, macrophages, T and B lymphocytes, neutrophils, fibroblasts, epithelial and endothelial cells, keratinocytes, mesangial cells, adipocytes, chondrocytes, osteoblasts, and more. Under normal physiological conditions, IL-6 gene expression is induced mostly by stimuli that cause an inflammatory response, such as TNF-α and -β, IL-1, bacterial endotoxin and lipopolysaccharide, virus infection, and interferons [3, 4].
The IL-6 Receptor: Classical Signaling and Transsignaling
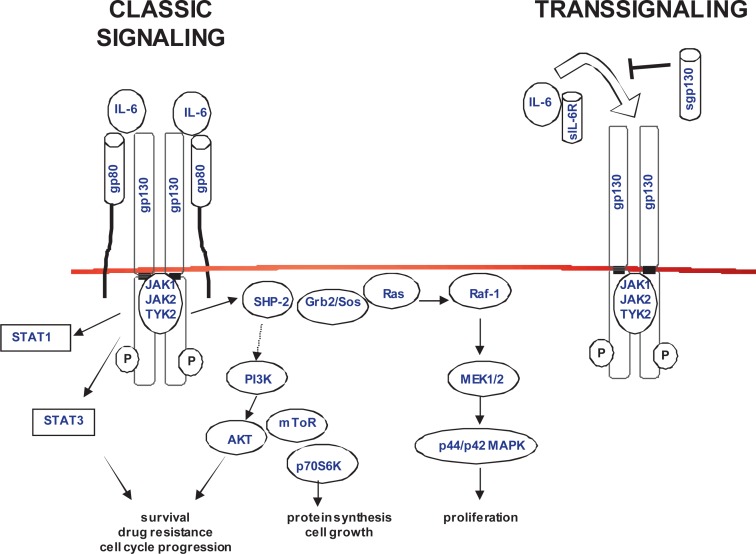
On target cells, IL-6 exerts its functions by first binding to its ligand-specific α-receptor (IL-6R/CD126), a glycoprotein (gp) of 80 kDa, and then recruiting the signaling chain gp130 (CD130) (fig. 1). The gp130 signal transducer is also used by other cytokines that comprise one family with partly redundant activities [5]. The complete signaling competent receptor complex is a hexamer consisting of 2 molecules of IL-6, IL-6R, and gp130 [6]. Upon gp130 dimerization, associated protein kinases, the so-called Janus kinases (JAK1, JAK2, and TYK2), become activated and phosphorylate specific tyrosine residues on gp130, which serve as docking sites for transcription factors and adapter proteins. The main signaling pathways induced by gp130 are the activation of signal transducer and activator of transcription (STAT)-1 and STAT3, the Ras/Raf/mitogen-activated protein kinase (MAPK) cascade, and the phosphatidylinositol-3 kinase (PI3K)/protein kinase B (AKT) pathway [6]. The resulting changes in gene expression patterns are dependent on the cell type, and involve proteins that regulate acute-phase and immune responses, proliferation, survival, cell cycle and differentiation, migration, angiogenesis, neutrophil trafficking, bone metabolism, as well as cartilage and lipid metabolism [4].
Fig. 1.
The IL-6/IL-6R/gp130 complex and main signaling pathways. IL-6 classical signaling and transsignaling induce receptor oligomerization, activation of JAKs, and phosphorylation of gp130. The signaling pathways lead to changes in gene transcription (gp = glycoprotein; JAK = Janus kinase; STAT = signal transducer and activator of transcription; SHP-2 = src homology 2 domain-containing phosphatase; Grb2 = growth factor receptor-bound protein; Sos = son-of-sevenless; MAPK = mitogen-activated protein kinase; MEK = MAPK kinase; PI3K = phosphatidyl-inositol-3 kinase; mToR = mammalian target of rapamycin; p70S6K = p70 S6 kinase).
In addition to the classical signaling through membrane-bound IL-6R, a soluble form of the IL-6R (sIL-6R) is generated primarily through proteolytic cleavage (shedding). The sIL-6R can bind to free IL-6, and this complex acts as an agonist and is capable of directly activating cells through membrane-bound gp130, a process called transsignaling (fig. 1). A soluble gp130 increases the complexity of IL-6 signaling, it is generated by alternative mRNA splicing and acts as a natural antagonist [7]. While gp130 is ubiquitously expressed on almost all cells in the body, expression of the ligand-specific IL-6R is more restricted and mainly occurs on hepatocytes and certain leukocytes, in particular activated B cells, T cells, megakaryocytes, monocytes and neutrophils. Therefore, during IL-6-mediated responses, the contribution of classic signaling through the membrane IL-6R versus transsignaling involving IL-6/sIL-6R depends on the physiological context and could have therapeutic implications [8, 9].
Biological Functions of IL-6
Originally, IL-6 was identified as a T cell-derived factor which induces the terminal differentiation of activated B cells into antibody-producing plasma cells, and was therefore given the designation B cell stimulatory factor-2. Shortly after its cloning by the group of Kishimoto in 1986 [1], it became clear that a number of other factors which had been well characterized at that time were identical to IL-6 (table 1). Today, IL-6 is known to be a cytokine with many multiple functions not limited to B cells but also involved in T cell immune responses, the regulation of hematopoiesis, induction of acute-phase reactions and inflammation, as well as bone, cartilage and lipid metabolism [4, 5] (table 1).
Table 1.
Cellular origin and biological activities of IL-6 [reviewed in 3, 4]
| Synonyms | Cells that produce IL-6 | Biological activities |
|---|---|---|
| 26-kDa protein | monocytes/macrophages | B cells: induction of immunoglobulin synthesis and differentiation |
| B cell differentiation factor (BCDF) | Kupffer cells | plasma cells: proliferation |
| B cell stimulatory factor-2 (BSF-2) | fibroblasts | hepatocytes: induction of acute phase proteins |
| Hepatocyte stimulating factor (HSF) | epithelial cells | T cells: growth and differentiation |
| Hybridoma growth factor (HGF) | endothelial cells | natural killer cells: augments activity |
| Interferon beta-2 (IFN-β2) | T and B cells | megakaryocytes: maturation |
| Cytotoxic T cell differentiation factor (CDF) | neutrophils | neuronal cells: adrenocorticotropic hormone production |
| T cell-replacing factor (TRF) | chondrocytes | mesangial cells and keratinocytes: proliferation |
| Macrophage-granulocyte inducer type 2 (MGI-2) | synoviocytes | bone metabolism: activation of osteoclasts |
| Thrombopoietin | adipocytes | endothelial cells: chemokine production |
| osteoblasts | chondrocytes: decreases collagen production | |
| keratinocytes | ||
| mesangial cells | ||
| smooth muscle cells | ||
| some tumor cells |
Genetic studies in mice have contributed to the current understanding of the role of IL-6 in vivo. The phenotype of IL-6-deficient mice is less severe than the pleiotropic functions would suggest. This can be explained by a certain degree of functional redundancy among the cytokines of the gp130 family. IL-6 knockout mice are viable with normal embryogenesis and development, but they exhibit defects in antigen-specific antibody production and anti-viral response leading to increased susceptibility to infections. They have defects in hematopoiesis, acute-phase protein synthesis, chemokine induction and leukocyte recruitment, and hepatocyte regeneration [10, 11]. In experimental disease models, IL-6 knockout mice show limited susceptibility to certain autoimmune and inflammatory conditions, CD, and plasmacytomas [8, 12]. Mice deficient in IL-6R display similar phenotypic characteristics to those of IL-6-deficient mice with subtle differences, for example in wound healing. In contrast, targeted deletion of the gp130 gene results in embryonic lethality demonstrating the fundamental role of gp130 in development and hematopoiesis [13].
Viral IL-6
Viral IL-6 (vIL-6) is a variant of IL-6 encoded by Kaposi's sarcoma-associated herpesvirus (KSHV) / human herpesvirus (HHV)-8 [14]. The gamma-herpesvirus is associated with Kaposi's sarcoma and 2 B lymphoproliferative disorders, pleural effusion lymphoma and a subset of CD [15]. The protein has approximately 25% homology with human IL-6 and signals through gp130 to activate the JAK-STAT pathway similar to cellular IL-6 [16, 17]. The first evidence that vIL-6 is functionally active on human cells came from our own work using a newly established IL-6-dependent plasmacytoma cell line [18]. In these cells, vIL-6 stimulated proliferation which could be inhibited with IL-6R and gp130 antibodies. Others have shown that gp130 is sufficient for vIL-6 binding and signal transduction [19].
Role of IL-6 in the Pathophysiology of Hematological Tumors
IL-6 is an important factor in a variety of human disease states including cardiovascular disease, sepsis, fever, cachexia, insulin resistance, osteoporosis, and neurologic disorders. Most notably, increased production of IL-6 contributes to the pathogenesis of many chronic inflammatory and autoimmune diseases [1]. There is growing evidence that there is also a link between chronic inflammation and tumor growth, shown for inflammatory bowel diseases and colitis-associated colon cancer. By virtue of its function as a cell growth modulator, IL-6 plays a role in many types of cancer comprising solid as well as hematological tumors, acting in an autocrine or paracrine manner. Elevated serum levels of IL-6 are often correlated with adverse prognosis, and probably contribute to weight loss, night sweats, fever, and other paraneoplastic symptoms [3, 4].
In the hematopoietic system, a growth-regulatory role of IL-6 is predominantly found in tumors arising from the B cell compartment. IL-6 transgenic mice where the human IL-6 gene was linked to the human Ig heavy chain enhancer, developed polyclonal hypergammaglobulinemia and diffuse plasmacytosis with infiltration of the spleen and lymph nodes. By introduction of a Balb/c genetic background, monoclonal and transplantable plasmacytomas were induced [20]. IL-6-deficient mice with Balb/c genetic background are completely resistant to the development of plasmacytomas induced by pristane oil demonstrating that IL-6 also plays a crucial pathogenic role in the onset of plasma cell tumors in vivo. In a transgenic mouse model with human IL-6 driven by a major histocompatibility complex promoter, plasmacytoma incidence and latency could be improved in the presence of c-myc [21]. In a different approach using retroviral-mediated transfer of the IL-6 gene into hematopoietic cells, sustained IL-6 production resulted in a syndrome in mice that resembled CD, a benign lymphoproliferative disease [22].
Castleman's Disease
CD is a lymphoproliferative disease with benign hyperplastic lymph nodes. In multicentric CD, a rare condition, polyclonal plasmablasts accumulate in multiple lymph nodes with a propensity toward development of lymphoma. Constitutive production of IL-6 by B cells in the germinal centers of the affected lymph nodes is associated with increased IL-6 serum levels [23]. High amounts of IL-6 may also be released by peripheral blood cells, providing an additional paracrine loop [24]. IL-6R polymorphism may also contribute to disease pathophysiology by being correlated to increased sIL-6R levels in patients who carry at least 1 copy of the minor allele [25]. The patients suffer from severe inflammatory symptoms, such as fever, weight loss, fatigue, anemia, increased levels of acute-phase proteins, and hypergammaglobulinemia. Treatment with an antibody against the IL-6R resulted in alleviation of the systemic manifestations [26], and high response rates were recently reported from a phase I study with the anti-IL-6 antibody siltuximab [27].
Multiple Myeloma
The role of IL-6 as a growth and survival factor in multiple myeloma (MM), a neoplasm of terminally differentiated B cells, is well established [28]. The disease is characterized by the accumulation of monoclonal plasma cells in the bone marrow (BM) that leads to serum monoclonal gammopathy, immune suppression, and skeletal destruction [29]. In contrast to the differentiating activity on normal B cells, myeloma cells are stimulated to proliferate in response to IL-6 [30, 31]. Some myeloma cells can produce their own IL-6 [32], but BM stromal cells are the main source, establishing a strong paracrine growth stimulation for the malignant plasma cells [33]. The production of IL-6 is upregulated by cytokines released into the surrounding tumor environment and by direct cellular contact between MM cells and stromal cells [34]. The orchestration of IL-6-induced signaling pathways contributes to the pleiotropic effects of IL-6 with regard to proliferation, survival, drug resistance, and migration of MM cells, thereby facilitating disease progression. Important survival signals are provided by activation of STAT3 which regulates the transcription of proteins of the Bcl-2 family leading to protection from apoptotic cell death induced by various agents including corticosteroids [35, 36]. IL-6 also induces the Ras/Raf/MAPK and the PI3K/AKT pathway in MM cells [37]. In the presence of IL-6, cytokine-dependent human plasma cell lines could be obtained, but only from patients with terminal and extramedullary disease [38, 39]. It is not entirely clear at which stage in the development of the disease or exactly which cell populations within the malignant clone are dependent on IL-6 or need additional signals. A recent study suggests that autonomous growth, as assessed by spontaneous colony formation, is regulated by insulin-like growth factor (IGF)-1 and stem cell factor (SCF) rather than IL-6 [40]. IL-6 is also a potent osteoclast-activating factor, and contributes to the development of bone lesions. Elevated levels of IL-6 and sIL-6 receptor are frequently present in the BM, plasma, and serum of MM patients, and are considered as an indicator of poor prognosis. Similar to CD, IL-6R polymorphism has been shown to be significantly correlated with increased sIL-6R levels [41]. MM accounts for approximately 10% of newly diagnosed hematological malignancies, affecting more than 20,000 people in the EU. It is still an incurable disease, and novel treatment strategies are needed. Targeting IL-6 could be a promising approach, probably in combination with other treatments to overcome drug resistance. The anti-IL-6 antibody siltuximab is currently tested in multiple clinical studies (see also below).
Pleural Effusion Lymphoma (PEL)
PEL or body cavity based lymphoma (BCBL) is an aggressive immunoblastic B cell malignancy which usually presents as an effusion in the body cavities of patients with acquired immunodeficiency syndrome. It is almost always infected with KSHV and co-infected with Epstein-Barr virus (EBV) in up to 50% of cases. PEL cells constitutively produce IL-6 and express the IL-6R, and cell growth was inhibited by human IL-6 antisense oligonucleotides [42]. In vivo growth of a PEL cell line in SCID mice could be delayed with a neutralizing anti-IL-6-antibody which does not detect vIL-6 [43]. Both human and vIL-6 may contribute to PEL growth (see below).
Other Hematological Tumors
An implication of IL-6 in the pathophysiology of a variety of B-cell leukemias and lymphomas as well as some non-B cell malignancies has been suggested. In many cases, serum IL-6 or sIL-6R levels are elevated, as shown for low and high-grade non-Hodgkin's lymphomas (NHL), Hodgkin's disease (HD), and in adult T cell leukemia/lymphoma (reviewed in [3, 44]).
In B cell chronic lymphocytic leukemia (B-CLL), the most common leukemia, the leukemic cells can produce IL-6, and in a subset of patients, IL-6 serum levels are elevated and correlate with disease stage and shorter survival rates. Serum sIL-6R levels also have prognostic value. In diffuse large B cell lymphoma (DLBCL), serum IL-6 levels correlate with prognosis and autocrine IL-6 production may provide proliferative and anti-apoptotic signals. In an analysis of IL-6 expression in high-grade B cell lymphomas comprising Burkitt's lymphomas (BL), DLBCL and immunoblastic lymphomas, IL-6 was mainly produced in tumor samples of non-BLs, but not in BLs. Immunohistochemical studies revealed that IL-6 was expressed in the nonmalignant cells, while the tumor cells were positive for the IL-6R. Moreover, a correlation was found between IL-6 expression and the presence of immunoblasts within the malignant clone [45]. Others have found IL-6 in the tumor cells of NHL samples [46]. An analysis of 2 EBV-positive B cell lines from immunocompromized patients revealed heterogeneity with regard to IL-6 requirement for cell growth; however, in cases that are responsive to IL-6, neutralizing antibodies were able to control tumor growth in SCID mice [47, 48]. In mantle cell lymphoma, another aggressive B cell NHL, IL-6 was identified as a key growth and survival factor acting in an autocrine fashion [49].
Serum IL-6 levels are also elevated in advanced HD and correlate with survival and B symptoms. HD-derived cell lines express both IL-6 and IL-6R. Moreover, IL-6 expression in Reed-Sternberg cells was detected in the majority of patients examined. IL-6 and IL-6R are expressed by blast cells in acute myeloblastic leukemia; however, both stimulatory as well as inhibitory effects on clonogenic blast growth have been described, reflecting the heterogenous biology of this disease [50, 51]. Furthermore, IL-6 could have a differentiating function on acute promyelocytic leukemia cells [52].
Role of Viral IL-6
HHV-8/KSHV infection is associated with 2 B-lymphoproliferative disorders, PEL or BCBL and multicentric CD [15]. In multicentric CD, HHV-8 infection is very common in HIV-positive patients. Here, a proportion of the plasmablastic cells in the lymph nodes are infected with HHV-8 and express vIL-6 which is also present in high amounts in the circulation [53]. Interestingly, vIL-6 can induce human IL-6 in patient-derived cells, but the biologic significance of the interplay between vIL-6 and cellular IL-6 remains unknown. Mice transgenic for vIL-6 spontaneously develop syndromes comparable to CD, inducing splenomegaly, multifocal lymphadenopathy, and plasmacytosis [54]. This phenotype was abrogated in mice with an IL-6-deficient genetic background, suggesting that IL-6 may play an important role in the pathogenesis of HHV-8-associated multicentric CD. In PEL, the pleural and peritoneal effusions contain vIL-6 at high concentrations. IL-6 encoded by HHV-8 promotes autocrine growth of PEL cells, but seems to be expressed at only low levels in PEL cell lines. The regulation of vIL-6 expression is complex and dependent on virus reactivation. The relative contribution of vIL-6 versus cellular IL-6 in the pathophysiology of PEL has remained open.
A causative role for vIL-6 in the pathophysiology of MM had been suggested based on the initial finding of the presence of HHV-8 in BM dendritic cells of MM patients [55]. However, lack of serological evidence and contradictory results from polymerase chain reaction (PCR) studies have not clearly confirmed such an association. In summary, cellular and viral IL-6 often co-exist in HHV-8 associated diseases; therefore, their relationship in terms of regulation of expression and their specific contribution to disease remain to be defined.
Therapeutic Implications
Preclinical and translational findings support a role for IL-6 in the pathophysiology of many diseases, and different strategies for blocking its activity have been developed. Conventional agents like interferons or novel immunomodulatory drugs such as lenalidomide (Revlimid®, Celgene, Summit, NJ, USA) indirectly inhibit the effects of IL-6, for example by suppressing IL-6 expression. In contrast, specific inhibition of IL-6 can be achieved with monoclonal antibodies (mAb) against IL-6 or the IL-6R, with IL-6R antagonists and specially designed recombinant proteins (table 2). The increasing knowledge of the intracellular signaling pathways induced by IL-6 builds the molecular basis for targeted therapeutic approaches with small molecules [3, 8, 56].
Table 2.
| Agent | Company | Target/molecule | Disease | Clinical status |
|---|---|---|---|---|
| Tocilizumab (Actemra, RoActemra) | Chugai, Roche | IL-6R/humanized mAb | rheumatoid arthritis, systemic juvenile idiopathic arthritis, CD | approved |
| other | clinical trials | |||
| REGN-88 (SAR153191) | Regeneron/Sanofi-Aventis | IL-6R/human mAb | rheumatoid arthritis | clinical trials |
| Siltuximab (CNTO 328) | Centocor | IL-6/chimeric mAb | multiple myeloma, B cell lymphoma, solid tumors | clinical trials |
| Sirukumab (CNTO 136) | Centocor | IL-6/human mAb | rheumatoid arthritis | clinical trials |
| B-E8/mAb 1339 | Diaclone/GlaxoSmithKline | IL-6/mouse mAb / human mAb | multiple myeloma/lymphoma | clinical trials/preclinical |
| FE999301 | CONARIS/Ferring | IL-6/sIL-6R complex / soluble gp130-Fc fusion protein | Crohn's disease | preclinical |
| Ruxolitinib (Jakafi/Jakavi, INCB018424/INC424) | Incyte/Novartis | JAK1/JAK2 | myelofibrosis; myeloproliferative diseases, leukemia, psoriasis | approved clinical trials |
Monoclonal Antibodies
Most of the clinical studies with mAb targeting IL-6 or IL-6R have been conducted in chronic inflammatory and autoimmune diseases where good responses can be achieved. There is yet no evidence showing which strategy is better, inhibiting the IL-6 ligand or blocking the IL-6R. At this time, only 1 antibody, tocilizumab (Actemra®/RoActemra® in the EU, Chugai, Tokyo, Japan/Roche, Basel, Switzerland), has been approved, but not for cancer treatment. Tocilizumab is a humanized anti-IL-6R antibody which binds to the IL-6 binding site of human IL-6R and competitively inhibits IL-6 signaling [1]. The antibody binds to both membrane-bound and soluble IL-6R, and therefore is able to block classical IL-6 signaling as well as transsignaling [57]. Tocilizumab was proven to be therapeutically effective in rheumatoid arthritis, systemic juvenile idiopathic arthritis, and CD. It may be effective in the treatment of HHV-8-associated diseases, and is currently in a phase II study in patients with symptomatic KSHV-associated multicentric CD (NCT01441063).
Several antibodies that neutralize IL-6 have been developed, but most of the clinical experience comes from studies with B-E8 (elsilimomab, Diaclone, Besançon, France) and CNTO 328 (siltuximab, Centocor Ortho Biotech Inc., Horsham, PA, USA; formerly CLB IL6/8). B-E8 is a murine mAb that was used in early clinical trials in patients with MM and HIV-associated lymphoma, CNTO 328 is a chimeric human-mouse antibody and therefore less immunogenic and with a prolonged half-life. Both antibodies were well tolerated and showed promising activity in MM, lymphoma, and B-lymphoproliferative disorders [44]. One of the remarkable effects of antibody treatment was the alleviation of inflammatory symptoms such as fever and reduction of C-reactive protein which serves as a surrogate marker for IL-6 inhibition. Currently, there are multiple studies with siltuximab, completed or ongoing, in patients with MM, B cell NHL, and CD. Results from a phase I study show high response rates in CD [27], and in combination with dexamethasone, clinical activity was seen even in dexamethasone-refractory MM [58].
A high-affinity, fully human derivative of B-E8, mAb 1339 (licensed to GlaxoSmithKline, Brentford, Middlesex, UK), was developed which showed significant in vitro and in vivo activity in a preclinical SCID-hu model of myeloma with the IL-6-dependent cell line INA-6 [59].
Recombinant Proteins
Recombinant proteins have been designed that use very different mechanisms to block IL-6. None of these reagents have yet reached the clinical stage. A very promising approach was the development of IL-6R antagonists. These molecules are IL-6 variants generated by site-directed mutagenesis. They bind to the membrane IL-6R with much higher affinity than natural IL-6 does, while the interaction with gp130 is abolished [60]. The superantagonist Sant-7 has shown promising activity on myeloma cells in vitro and in vivo, but has not been further developed for clinical use [18, 61, 62].
The development of a recombinant soluble gp130 (sgp130) protein was based on the finding that sgp130 is a natural inhibitor of IL-6/sIL-6R [63]. The protein is composed of sgp130 linked to the Fc-region of human IgG. It mostly blocks IL-6 transsignaling which primarily drives the inflammatory activities of IL-6. The molecule has shown effects in several in vivo models of arthritis, colitis, infection, allergy, and inflammation-induced cancer [64]. First clinical studies with FE999301 (CONARIS Research Institute AG, Kiel, Germany/Ferring Pharmaceuticals, Saint-Prex, Switzerland) are planned for 2013. It will need to be determined whether this inhibitor offers a clinical advantage over mAb.
IL6(T23)-PE38KDEL is a fusion protein composed of IL-6 and a truncated mutant form of Pseudomonas exotoxin. Upon receptor binding, IL-6 is internalized and transports the toxin into the cell. In mouse models of MM, the immunotoxin has demonstrated antitumor effects [65].
Small Molecule Inhibitors of IL-6 Signaling Pathways
The signaling pathways induced by IL-6R/gp130 activation together contribute to the proliferative and anti-apoptotic activities of IL-6. Targeting key components of these pathways, such as protein kinases, has become a novel strategy for therapeutic invention in cancer and other indications. Central mediators of IL-6 signaling are the tyrosine kinases of the JAK family by being directly associated with the gp130 membrane receptor. Inhibition of JAKs directly suppresses STAT activation, which in turn results in downregulation of important survival and angiogenic factors [66]. For example, JAK inhibition in an IL-6-dependent human plasma cell line resulted in growth inhibition in vitro and in vivo [67]. One advantage of signaling inhibition would be to overcome the redundancy that may occur among the gp130 family of cytokines [38]. Despite the adverse effects of JAK inhibitors due to the important role of JAK1 and JAK2 in normal hematopoiesis, the first JAK inhibitor has been approved for treatment of patients with myelofibrosis, a myeloproliferative disorder carrying an activating point mutation in the JAK2 gene [68]. Ruxolitinib (Jakafi®/Jakavi®, formerly INCB018424/NVP-INC424, Incyte, Wilmington, DE, USA/Novartis Oncology, East Hanover, NJ, USA) is currently in several clinical studies for advanced hematological malignancies which may also harbor activation of the JAK/STAT pathway independent of cytokine stimulation [69].
Conclusion
IL-6 is a pleiotropic cytokine, and its dysregulation is involved in many diseases including chronic inflammatory and autoimmune disorders, coronary artery and neurologic disease, and in cancer. Innovative therapeutic strategies that block the biologic functions of IL-6 are being developed and are emerging in the clinic. The best responses with IL-6 and IL-6R antibodies were seen in rheumatoid arthritis, systemic juvenile idiopathic arthritis, and CD, conditions that are known to be driven by IL-6. There is clear evidence that overproduction of IL-6 is also involved in hematological malignancies, particularly in B cell-derived and plasma cell tumors. The challenge is to identify patients that may benefit most from IL-6-blocking therapies, especially in heterogeneous diseases such as myeloma [70].
Disclosure Statement
There are no relevant conflicts of interest to disclose.
References
- 1.Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22:347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 2.Yasukawa K, Hirano T, Watanabe Y, Muratani K, Matsuda T, Nakai S, Kishimoto T. Structure and expression of human B cell stimulatory factor-2 (BSF-2/IL-6) gene. EMBO J. 1987;6:2939–2945. doi: 10.1002/j.1460-2075.1987.tb02598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer. 2007;110:1911–1928. doi: 10.1002/cncr.22999. [DOI] [PubMed] [Google Scholar]
- 4.Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2012;122:143–159. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- 5.Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood. 1995;86:1243–1254. [PubMed] [Google Scholar]
- 6.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80:227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 8.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011;121:3375–3383. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 10.Fasnacht N, Muller W. Conditional gp130 deficient mouse mutants. Semin Cell Dev Biol. 2008;19:379–384. doi: 10.1016/j.semcdb.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 12.Hilbert DM, Kopf M, Mock BA, Kohler G, Rudikoff S. Interleukin 6 is essential for in vivo development of b lineage neoplasms. J Exp Med. 1995;182:243–248. doi: 10.1084/jem.182.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida K, Taga T, Saito M, Suematsu S, Kumanogoh A, Tanaka T, Fujiwara H, Hirata M, Yamagami T, Nakahata T, Hirabayashi T, Yoneda Y, Tanaka K, Wang WZ, Mori C, Shiota K, Yoshida N, Kishimoto T. Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc Natl Acad Sci U S A. 1996;93:407–411. doi: 10.1073/pnas.93.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neipel F, Albrecht JC, Ensser A, Huang YQ, Li JJ, Friedman-Kien AE, Fleckenstein B. Human herpesvirus 8 encodes a homolog of interleukin-6. J Virol. 1997;71:839–842. doi: 10.1128/jvi.71.1.839-842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakakibara S, Tosato G. Viral interleukin-6: Role in Kaposi's sarcoma-associated herpesvirus: associated malignancies. J Interferon Cytokine Res. 2011;31:791–801. doi: 10.1089/jir.2011.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hideshima T, Chauhan D, Teoh G, Raje N, Treon SP, Tai YT, Shima Y, Anderson KC. Characterization of signaling cascades triggered by human interleukin-6 versus Kaposi's sarcoma-associated herpes virus-encoded viral interleukin 6. Clin Cancer Res. 2000;6:1180–1189. [PubMed] [Google Scholar]
- 17.Suthaus J, Adam N, Grotzinger J, Scheller J, Rose-John S. Viral interleukin-6: structure, pathophysiology and strategies of neutralization. Eur J Cell Biol. 2011;90:495–504. doi: 10.1016/j.ejcb.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Burger R, Neipel F, Fleckenstein B, Savino R, Ciliberto G, Kalden JR, Gramatzki M. Human herpesvirus type 8 interleukin-6 homologue is functionally active on human myeloma cells. Blood. 1998;91:1858–1863. [PubMed] [Google Scholar]
- 19.Mullberg J, Geib T, Jostock T, Hoischen SH, Vollmer P, Voltz N, Heinz D, Galle PR, Klouche M, Rose-John S. IL-6 receptor independent stimulation of human gp130 by viral IL-6. J Immunol. 2000;164:4672–4677. doi: 10.4049/jimmunol.164.9.4672. [DOI] [PubMed] [Google Scholar]
- 20.Suematsu S, Matsusaka T, Matsuda T, Ohno S, Miyazaki J, Yamamura K, Hirano T, Kishimoto T. Generation of plasmacytomas with the chromosomal translocation t(12;15) in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A. 1992;89:232–235. doi: 10.1073/pnas.89.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutsch S, Neppalli VT, Shin DM, DuBois W, Morse HC, 3rd, Goldschmidt H, Janz S. IL-6 and myc collaborate in plasma cell tumor formation in mice. Blood. 2010;115:1746–1754. doi: 10.1182/blood-2009-08-237941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandt SJ, Bodine DM, Dunbar CE, Nienhuis AW. Dysregulated interleukin 6 expression produces a syndrome resembling Castleman's disease in mice. J Clin Invest. 1990;86:592–599. doi: 10.1172/JCI114749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshizaki K, Matsuda T, Nishimoto N, Kuritani T, Taeho L, Aozasa K, Nakahata T, Kawai H, Tagoh H, Komori T, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman's disease. Blood. 1989;74:1360–1367. [PubMed] [Google Scholar]
- 24.Burger R, Wendler J, Antoni K, Helm G, Kalden JR, Gramatzki M. Interleukin-6 production in B-cell neoplasias and Castleman's disease: evidence for an additional paracrine loop. Ann Hematol. 1994;69:25–31. doi: 10.1007/BF01757344. [DOI] [PubMed] [Google Scholar]
- 25.Stone K, Woods E, Szmania SM, Stephens OW, Garg TK, Barlogie B, Shaughnessy JD, Jr, Hall B, Reddy M, Hoering A, Hansen E, van Rhee F. Interleukin-6 receptor polymorphism is prevalent in HIV-negative Castleman disease and is associated with increased soluble interleukin-6 receptor levels. PLoS One. 2013;8:e54610. doi: 10.1371/journal.pone.0054610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishimoto N, Kanakura Y, Aozasa K, Johkoh T, Nakamura M, Nakano S, Nakano N, Ikeda Y, Sasaki T, Nishioka K, Hara M, Taguchi H, Kimura Y, Kato Y, Asaoku H, Kumagai S, Kodama F, Nakahara H, Hagihara K, Yoshizaki K, Kishimoto T. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106:2627–2632. doi: 10.1182/blood-2004-12-4602. [DOI] [PubMed] [Google Scholar]
- 27.Kurzrock R, Voorhees PM, Casper C, Furman RR, Fayad L, Lonial S, Borghaei H, Jagannath S, Sokol L, Usmani S, van de Velde H, Qin X, Puchalski TA, Hall B, Reddy M, Qi M, Van Rhee F. A phase I, open-label study of siltuximab, an anti-IL-6 monoclonal antibody, in patients with B-cell non-Hodgkin's lymphoma, multiple myeloma, or Castleman's disease. Clin Cancer Res. 2013;19:3659–3670. doi: 10.1158/1078-0432.CCR-12-3349. [DOI] [PubMed] [Google Scholar]
- 28.Klein B, Zhang XG, Lu ZY, Bataille R. Interleukin-6 in human multiple myeloma. Blood. 1995;85:863–872. [PubMed] [Google Scholar]
- 29.Anderson KC, Carrasco RD. Pathogenesis of myeloma. Annu Rev Pathol Mech Dis. 2011;6:249–274. doi: 10.1146/annurev-pathol-011110-130249. [DOI] [PubMed] [Google Scholar]
- 30.Anderson KC, Jones RM, Morimoto C, Leavitt P, Barut BA. Response patterns of purified myeloma cells to hematopoietic growth factors. Blood. 1989;73:1915–1924. [PubMed] [Google Scholar]
- 31.Tanabe O, Kawano M, Tanaka H, Iwato K, Asaoku H, Ishikawa H, Nobuyoshi M, Hirano T, Kishimoto T, Kuramoto A. BSF-2/IL-6 does not augment Ig secretion but stimulates proliferation in myeloma cells. Am J Hematol. 1989;31:258–262. doi: 10.1002/ajh.2830310408. [DOI] [PubMed] [Google Scholar]
- 32.Kawano M, Hirano T, Matsuda T, Taga T, Horii Y, Iwato K, Asaoku H, Tang B, Tanabe O, Tanaka H, et al. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988;332:83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- 33.Klein B, Zhang XG, Jourdan M, Content J, Houssiau F, Aarden L, Piechaczyk M, Bataille R. Paracrine rather than autocrine regulation of myelomacell growth and differentiation by interleukin-6. Blood. 1989;73:517–526. [PubMed] [Google Scholar]
- 34.Uchiyama H, Barut BA, Mohrbacher AF, Chauhan D, Anderson KC. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. Blood. 1993;82:3712–3720. [PubMed] [Google Scholar]
- 35.Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernandez-Luna JL, Nunez G, Dalton WS, Jove R. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 36.Puthier D, Derenne S, Barille S, Moreau P, Harousseau JL, Bataille R, Amiot M. Mcl-1 and Bcl-xL are co-regulated by IL-6 in human myeloma cells. Br J Haematol. 1999;107:392–395. doi: 10.1046/j.1365-2141.1999.01705.x. [DOI] [PubMed] [Google Scholar]
- 37.Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20:5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- 38.Burger R, Guenther A, Bakker F, Schmalzing M, Bernand S, Baum W, Duerr B, Hocke GM, Steininger H, Gebhart E, Gramatzki M. Gp130 and ras mediated signaling in human plasma cell line INA-6: a cytokine-regulated tumor model for plasmacytoma. Hematol J. 2001;2:42–53. doi: 10.1038/sj.thj.6200075. [DOI] [PubMed] [Google Scholar]
- 39.Zhang XG, Gaillard JP, Robillard N, Lu ZY, Gu ZJ, Jourdan M, Boiron JM, Bataille R, Klein B. Reproducible obtaining of human myeloma cell lines as a model for tumor stem cell study in human multiple myeloma. Blood. 1994;83:3654–3663. [PubMed] [Google Scholar]
- 40.Chiron D, Maiga S, Surget S, Descamps G, Gomez-Bougie P, Traore S, Robillard N, Moreau P, Le Gouill S, Bataille R, Amiot M, Pellat-Deceunynck C. Autocrine insulin-like growth factor 1 and stem cell factor but not interleukin 6 support self-renewal of human myeloma cells. Blood Cancer J. 2013;3:e120. doi: 10.1038/bcj.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stephens OW, Zhang Q, Qu P, Zhou Y, Chavan S, Tian E, Williams DR, Epstein J, Barlogie B, Shaughnessy JD., Jr An intermediate-risk multiple myeloma subgroup is defined by sIL-6r: levels synergistically increase with incidence of SNP rs2228145 and 1q21 amplification. Blood. 2012;119:503–512. doi: 10.1182/blood-2011-07-367052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asou H, Said JW, Yang R, Munker R, Park DJ, Kamada N, Koeffler HP. Mechanisms of growth control of Kaposi's sarcoma-associated herpes virus-associated primary effusion lymphoma cells. Blood. 1998;91:2475–2481. [PubMed] [Google Scholar]
- 43.Foussat A, Wijdenes J, Bouchet L, Gaidano G, Neipel F, Balabanian K, Galanaud P, Couderc J, Emilie D. Human interleukin-6 is in vivo an autocrine growth factor for human herpesvirus-8-infected malignant B lymphocytes. Eur Cytokine Netw. 1999;10:501–508. [PubMed] [Google Scholar]
- 44.Trikha M, Corringham R, Klein B, Rossi JF. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res. 2003;9:4653–4665. [PMC free article] [PubMed] [Google Scholar]
- 45.Emilie D, Coumbaras J, Raphael M, Devergne O, Delecluse HJ, Gisselbrecht C, Michiels JF, Van Damme J, Taga T, Kishimoto T, et al. Interleukin-6 production in high-grade β lymphomas: correlation with the presence of malignant immunoblasts in acquired immunodeficiency syndrome and in human immunodeficiency virus-seronegative patients. Blood. 1992;80:498–504. [PubMed] [Google Scholar]
- 46.Voorzanger N, Touitou R, Garcia E, Delecluse HJ, Rousset F, Joab I, Favrot MC, Blay JY. Interleukin (IL)-10 and IL-6 are produced in vivo by non-Hodgkin's lymphoma cells and act as cooperative growth factors. Cancer Res. 1996;56:5499–5505. [PubMed] [Google Scholar]
- 47.Durandy A, Emilie D, Peuchmaur M, Forveille M, Clement C, Wijdenes J, Fischer A. Role of IL-6 in promoting growth of human EBV-induced B-cell tumors in severe combined immunodeficient mice. J Immunol. 1994;152:5361–5367. [PubMed] [Google Scholar]
- 48.Mauray S, Fuzzati-Armentero MT, Trouillet P, Ruegg M, Nicoloso G, Hart M, Aarden L, Schapira M, Duchosal MA. Epstein-Barr virus-dependent lymphoproliferative disease: critical role of IL-6. Eur J Immunol. 2000;30:2065–2073. doi: 10.1002/1521-4141(200007)30:7<2065::AID-IMMU2065>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 49.Zhang L, Yang J, Qian J, Li H, Romaguera JE, Kwak LW, Wang M, Yi Q. Role of the microenvironment in mantle cell lymphoma: IL-6 is an important survival factor for the tumor cells. Blood. 2012;120:3783–3792. doi: 10.1182/blood-2012-04-424630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoang T, Haman A, Goncalves O, Wong GG, Clark SC. Interleukin-6 enhances growth factor-dependent proliferation of the blast cells of acute myeloblastic leukemia. Blood. 1988;72:823–826. [PubMed] [Google Scholar]
- 51.Sugiyama H, Inoue K, Ogawa H, Yamagami T, Soma T, Miyake S, Hirata M, Kishimoto T. The expression of IL-6 and its related genes in acute leukemia. Leuk Lymphoma. 1996;21:49–52. doi: 10.3109/10428199609067579. [DOI] [PubMed] [Google Scholar]
- 52.Xie P, Chan FS, Ip NY, Leung MF. IL-6 enhanced the retinoic acid-induced differentiation of human acute promyelocytic leukemia cells. Cancer Lett. 2000;148:207–213. doi: 10.1016/s0304-3835(99)00339-0. [DOI] [PubMed] [Google Scholar]
- 53.Aoki Y, Yarchoan R, Wyvill K, Okamoto S, Little RF, Tosato G. Detection of viral interleukin-6 in Kaposi sarcoma-associated herpesvirus-linked disorders. Blood. 2001;97:2173–2176. doi: 10.1182/blood.v97.7.2173. [DOI] [PubMed] [Google Scholar]
- 54.Suthaus J, Stuhlmann-Laeisz C, Tompkins VS, Rosean TR, Klapper W, Tosato G, Janz S, Scheller J, Rose-John S. HHV-8-encoded viral IL-6 collaborates with mouse IL-6 in the development of multicentric Castleman disease in mice. Blood. 2012;119:5173–5181. doi: 10.1182/blood-2011-09-377705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rettig MB, Ma HJ, Vescio RA, Pold M, Schiller G, Belson D, Savage A, Nishikubo C, Wu C, Fraser J, Said JW, Berenson JR. Kaposi's sarcoma-associated herpesvirus infection of bone marrow dendritic cells from multiple myeloma patients. Science. 1997;276:1851–1854. doi: 10.1126/science.276.5320.1851. [DOI] [PubMed] [Google Scholar]
- 56.Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev. 2012;38:904–910. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 57.Mihara M, Kasutani K, Okazaki M, Nakamura A, Kawai S, Sugimoto M, Matsumoto Y, Ohsugi Y. Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int Immunopharmacol. 2005;5:1731–1740. doi: 10.1016/j.intimp.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 58.Voorhees PM, Manges RF, Sonneveld P, Jagannath S, Somlo G, Krishnan A, Lentzsch S, Frank RC, Zweegman S, Wijermans PW, Orlowski RZ, Kranenburg B, Hall B, Casneuf T, Qin X, van de Velde H, Xie H, Thomas SK. A phase 2 multicentre study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with relapsed or refractory multiple myeloma. Br J Haematol. 2013;161:357–366. doi: 10.1111/bjh.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fulciniti M, Hideshima T, Vermot-Desroches C, Pozzi S, Nanjappa P, Shen Z, Patel N, Smith ES, Wang W, Prabhala R, Tai YT, Tassone P, Anderson KC, Munshi NC. A high-affinity fully human anti-IL-6 mab, 1339, for the treatment of multiple myeloma. Clin Cancer Res. 2009;15:7144–7152. doi: 10.1158/1078-0432.CCR-09-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sporeno E, Savino R, Ciapponi L, Paonessa G, Cabibbo A, Lahm A, Pulkki K, Sun RX, Toniatti C, Klein B, Ciliberto G. Human interleukin-6 receptor super-antagonists with high potency and wide spectrum on multiple myeloma cells. Blood. 1996;87:4510–4519. [PubMed] [Google Scholar]
- 61.Honemann D, Chatterjee M, Savino R, Bommert K, Burger R, Gramatzki M, Dorken B, Bargou RC. The IL-6 receptor antagonist SANT-7 overcomes bone marrow stromal cell-mediated drug resistance of multiple myeloma cells. Int J Cancer. 2001;93:674–680. doi: 10.1002/ijc.1388. [DOI] [PubMed] [Google Scholar]
- 62.Tassone P, Neri P, Burger R, Savino R, Shammas M, Catley L, Podar K, Chauhan D, Masciari S, Gozzini A, Tagliaferri P, Venuta S, Munshi NC, Anderson KC. Combination therapy with interleukin-6 receptor superantagonist Sant7 and dexamethasone induces antitumor effects in a novel SCID-hu in vivo model of human multiple myeloma. Clin Cancer Res. 2005;11:4251–4258. doi: 10.1158/1078-0432.CCR-04-2611. [DOI] [PubMed] [Google Scholar]
- 63.Jostock T, Mullberg J, Ozbek S, Atreya R, Blinn G, Voltz N, Fischer M, Neurath MF, Rose-John S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268:160–167. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 64.Rabe B, Chalaris A, May U, Waetzig GH, Seegert D, Williams AS, Jones SA, Rose-John S, Scheller J. Transgenic blockade of interleukin 6 transsignaling abrogates inflammation. Blood. 2008;111:1021–1028. doi: 10.1182/blood-2007-07-102137. [DOI] [PubMed] [Google Scholar]
- 65.Guo DJ, Han JS, Li YS, Liu ZS, Lu SY, Ren HL. In vitro and in vivo antitumor effects of the recombinant immunotoxin IL6(T23)-PE38KDEL in multiple myeloma. Oncol Lett. 2012;4:311–318. doi: 10.3892/ol.2012.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sansone P, Bromberg J. Targeting the interleukin-6/Jak/Stat pathway in human malignancies. J Clin Oncol. 2012;30:1005–1014. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burger R, Le Gouill S, Tai YT, Shringarpure R, Tassone P, Neri P, Podar K, Catley L, Hideshima T, Chauhan D, Caulder E, Neilan CL, Vaddi K, Li J, Gramatzki M, Fridman JS, Anderson KC. Janus kinase inhibitor INCB20 has antiproliferative and apoptotic effects on human myeloma cells in vitro and in vivo. Mol Cancer Ther. 2009;8:26–35. doi: 10.1158/1535-7163.MCT-08-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mascarenhas J, Hoffman R. Ruxolitinib: the first FDA approved therapy for the treatment of myelofibrosis. Clin Cancer Res. 2012;18:3008–3014. doi: 10.1158/1078-0432.CCR-11-3145. [DOI] [PubMed] [Google Scholar]
- 69.Burger R, Gramatzki M. JAK kinases in leukemias, lymphomas, and multiple myeloma. In: Fabbro D, McCormick F, editors. Protein Tyrosine Kinases: From Inhibitors to Useful Drugs (Cancer Drug Discovery and Development) Totowa, NJ: Humana Press; 2006. pp. 115–144. [Google Scholar]
- 70.Chari A, Pri-Chen H, Jagannath S. Complete remission achieved with single agent CNTO 328, an anti-IL-6 monoclonal antibody, in relapsed and refractory myeloma. Clin Lymphoma Myeloma Leuk. 2013;13:333–337. doi: 10.1016/j.clml.2012.12.010. [DOI] [PubMed] [Google Scholar]