Abstract
Rationale
The Dorsal Mesenchymal Protrusion (DMP) is a prong of mesenchyme derived from the Second Heart Field (SHF) located at the venous pole of the developing heart. Recent studies have shown that perturbation of its development is associated with the pathogenesis of atrioventricular septal defect (AVSD). Although the importance of the DMP to AV septation is now established, the molecular and cellular mechanisms underlying its development are far from fully understood. Prior studies have demonstrated that bone morphogenetic protein (BMP) signaling is essential for proper formation of the AV endocardial cushions and the cardiac outflow tract. A role for BMP signaling in regulation of DMP development remained to be elucidated.
Objective
To determine the role of BMP signaling in DMP development.
Methods and Results
Conditional deletion of the BMP receptor Alk3 from venous pole SHF cells leads to impaired formation of the DMP and a completely penetrant phenotype of ostium primum defect, a hallmark feature of AVSDs. Analysis of mutants revealed decreased proliferative index of SHF cells and, consequently, reduced number of SHF cells at the cardiac venous pole. In contrast, volume and expression of markers associated with proliferation and active BMP/TGFβ signaling was not significantly altered in the AV cushions of SHF-Alk3 mutants.
Conclusions
BMP signaling is required for expansion of the SHF-derived DMP progenitor population at the cardiac venous pole. Perturbation of Alk3-mediated BMP signaling from the SHF results in impaired development of the DMP and ostium primum defects.
Keywords: DMP, AVSD, Alk3, BMP, ASD
INTRODUCTION
Proper formation of the septal structures at the atrioventricular (AV) junction is crucial to the development of the four-chambered heart. Perturbation of this process can result in cardiac malformations, including atrioventricular septal defect (AVSD). AVSDs, which comprise approximately 5% of all congenital heart defects, are associated with genetic disorders such as Down’s syndrome and visceral heterotaxy syndrome1. While all AVSDs are characterized by the presence of a common AV junction, two subtypes can be distinguished based on the potential for shunting. In incomplete (or partial) AVSDs, this potential is restricted to the atrial level via the so-called ostium primum defect, whereas in complete AVSDs shunting can occur at both atrial and ventricular levels2.
In the past, AVSDs were thought to arise solely from perturbed development and/or fusion of the AV endocardial cushions3–5. A series of recently published studies, however, indicate that abnormal development of tissues derived from the Second Heart Field (SHF), including the primary atrial septum and the Dorsal Mesenchymal Protrusion (DMP), may also play a role in the pathogenesis of these defects6–14.
The SHF-derived DMP is a body of mesenchyme found at the venous pole that resembles a structure originally described by His as the spina vestibule or, vestibular spine9, 10, 15. The development of the DMP is intrinsically related to remodeling events involving the dorsal mesocardium9, 10, 16. The walls of the dorsal mesocardium are formed by the mesocardial reflections16–18, two structures that symmetrically flank the mid-pharyngeal endothelial strand, the precursor of the pulmonary vein19, 20, 21. The development of the DMP is preceded by the accumulation of SHF cells in the region sandwiched between the foregut and the dorsal mesocardium. Around murine ED10.5, this cell population expands between the right mesocardial reflection and the developing pulmonary vein and projects into the cavity of the common atrium, thereby forming the DMP10, 9. This results in a leftward displacement of the orifice of the pulmonary vein, a process crucial to its eventual incorporation into the left atrium22.
Fusion of the DMP with the mesenchymal cap of the primary atrial septum (PAS) and the AV cushions results in closure of the ostium primum and formation of the AV mesenchymal complex10, 20. Failure of these tissues to properly develop and/or interact with one another can result in an ostium primum defect as well as other malformations characteristically associated with AVSDs. Although compromised development of the endocardially-derived AV cushions has long been implicated in the pathogenesis of AVSD, only relatively recently has perturbation of SHF-derived tissues at the venous pole been linked to these defects7, 9, 11, 21, 23. Here, we contribute to the growing body of evidence that proper DMP development is important, if not crucial, to AV septation. Specifically, we demonstrate that Bone Morphogenetic Protein (BMP) signaling through the receptor Alk3 is of critical importance for DMP development as its deletion from the SHF results in impaired DMP formation and ostium primum defects. As the molecular mechanisms involved in expansion and differentiation of SHF progenitors at the venous pole become more clearly defined, so may understanding of the morphological and molecular basis of AVSD.
METHODS
Mice
Mef2c-AHF-cre and floxed Alk3 mice24, 25 were used to generate Mef2c-AHF-cre;Alk3f/f (SHF-Alk3) ckos, and control embryos. For cell fate studies, Mef2c-AHF-cre mice were crossed with B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato, dTomatLuo/J reporter mice26. Mef2cCRE;Alk3f/+ and Alk3f/f;R26mT mice were mated to generate SHF-Alk3 ckos that also possess a GFP reporter allele (Mef2cCRE;Alk3f/f;R26mG).
Proliferative index of dorsal mesocardium
Eight evenly-spaced 5μm sections from three Mef2cCRE;Alk3f/f;R26mG and four Mef2cCRE;Alk3f/+;R26mG littermates at ED10 were assessed using GFP, Ki67 and Isl1 to determine the proportion of actively dividing SHF cells. Average SHF proliferation was calculated by dividing the number of Isl1-positive;Ki67-positive or GFP-positive;Ki67-positive cells by, respectively, the total number of Isl1-positive or GFP-positive cells. Overall proliferation was determined by dividing number of Ki67-positive cells by total cell number. Number of GFP-positive cells expressing Isl1 was determined by dividing by number of GFP-positive;Isl1-positive cells by total number of GFP-positive cells. Significance was determined using a two-tailed student’s t-test.
AMIRA 3D reconstruction
Twenty 5 μm serial sections of the dorsal mesocardium between the primary atrial septum and the bronchi were stained for Isl1 and GFP in Mef2cCRE;Alk3f/f;R26mG and Mef2cCRE;Alk3f/+;R26mG specimens. Isl1 and GFP expression was recorded using a set exposure time, after which AMIRA software was used to create threshold segmentation and generate 3D-renderings.
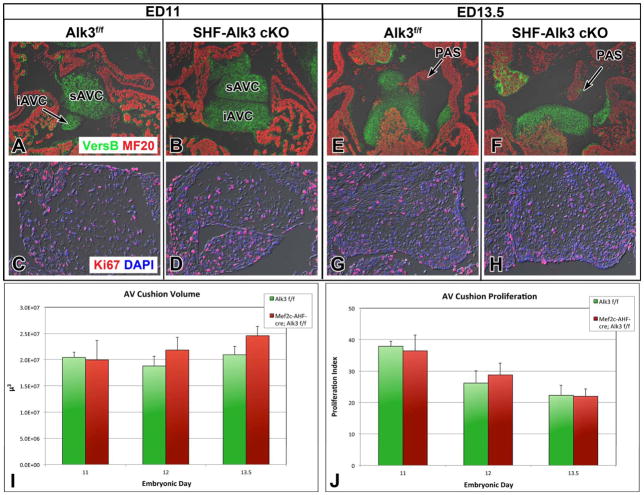
AV cushion volume and proliferation
The AV cushions were assessed using images from the entire AV junction of three SHF-Alk3 cko and three control specimens at ED11, 12 and 13.5. AMIRA was used to create 3D-renderings and determine volume. Proliferation was assessed using six 5 μm sections from the major AV cushions of three SHF-Alk3 cko and 3 control specimens at ED11, 12 and 13.5. Number of cells expressing Ki67 was divided by total number of cells and significance determined by two-tailed student’s t-test.
RESULTS
Expression of BMP signaling pathway components at the venous pole of the heart
While prior studies have unequivocally demonstrated the importance of BMPs to outflow tract accretion and septation27, 28, the role of BMP signaling in the development of the venous pole has thus far garnered relatively little attention.
At ED9.5, two SHF-derived mesocardial reflections symmetrically flank the mid-pharyngeal endothelial strand at the cardiac venous pole19, 20, 21. At this stage, the dorsal mesocardium is sparsely populated and expression of Isl1, a transcription factor characteristically expressed in the SHF29, is largely confined to the reflections (Fig. 1A, B). To define the spatiotemporal expression patterns of BMP isoforms during this critical stage of venous pole development, in situ hybridizations were performed on serial sections of ED9.5 embryos with probes specific to BMP2, BMP4, and BMP7. This analysis showed that BMP7 is expressed throughout the entire myocardium, including the myocardial mesocardial reflections and the AV myocardium (data not shown). BMP2 is not expressed in the mesocardial reflections (Fig. 1D) but, consistent with prior reports, is expressed within the AV myocardium underlying the developing AV cushions30 (Fig. 1D′). BMP4 expression, in contrast, is largely confined to the mesocardial reflections (Fig. 1C, C′).
Figure 1. BMP expression at the venous pole of the developing heart.
Transverse sections of ED9.5 hearts at the level of the dorsal mesocardium were H&E stained (A) and processed for immunofluorescent detection of the SHF-marker Isl1 (B) to demonstrate SHF contribution to this region of the heart. In situ hybridization of transverse serial sections with probes recognizing BMP4 (C, C′) and BMP2 (D, D′) demonstrate that the mesocardial reflections of the dorsal mesocardium express BMP4 (C) but not BMP2 (D). In contrast, little to no BMP4 is expressed in AV junctional myocardium (C′), where BMP2 is abundantly expressed (arrow, D′). FG, foregut; RMR, right mesocardial reflection; LMR, left mesocardial reflection; RV, right ventricle; RA, right atrium; LA, left atrium; AVJ, atrioventricular junction.
BMPs may signal in a paracrine fashion. Due to the close proximity of the mesocardial reflections to the SHF population that will form the DMP, this population was assessed for expression of the receptor Alk3, known to interact with BMP4 and BMP731. Consistent with BMP signaling through Alk3 in SHF cells at the venous pole of the heart, Alk3 expression was observed in Isl1-expressing SHF cells of wild-type mouse embryos at ED10 (Fig. 2A).
Figure 2. Expression of Isl1, Alk3 and pSMAD1,5,8 at the venous pole.
Expression of the BMP receptor Alk3 (red, A) by Isl1-expressing SHF cells (green, A) at the venous pole of a wild-type specimen at ED10. Robust expression of phosphorylated SMAD1/5/8, a marker of active canonical BMP signaling, is present within this Isl1-positive population prior to protrusion of the DMP into the common atrium (10ED, B, B′). Isl1 and phosphorylated SMAD1/5/8 are also expressed within the DMP itself (10.5ED, 2C, C′). FG, foregut; MPES, midpharyngeal endothelial strand; DMP, dorsal mesenchymal protrusion
Alk3-stimulated canonical BMP2,4,7 signal transduction is mediated through phosphorylation of the downstream effectors Smad(s) 1, 5, and/or 831. To determine whether the canonical BMP pathway is active in SHF cells at the venous pole, the expression of pSMAD1/5/8 was immunofluorescently evaluated in mouse embryos at ED9.5 and ED10.5. Expression of pSMAD1/5/8 was detected in both the DMP precursor population at ED9.5 (Fig. 2B, B′) and within the DMP itself at ED10.5 (Fig. 2C, C′).
Deletion of Alk3 from the venous pole results in ostium primum defects
In the Mef2c-AHF-cre mouse, regulatory elements from the mouse mef2c gene drive expression of Cre-recombinase exclusively in the AHF (or SHF)25 and its derivatives at both the arterial25 and venous pole21 of the developing heart (Fig. 3B–F). The contribution of the SHF to the venous pole can be traced by crossing Mef2c-AHF-cre mice with floxed R26mT/mG, reporter mice that express membrane-targeted tandem dimer Tomato (mT) prior to cre-mediated excision and membrane-targeted green fluorescent protein (mG) after cre-mediated excision32, 33 (Fig. 3). At the venous pole, expression of the Mef2c-AHF-cre transgene (Fig. 3D) parallels that of Isl1 (Fig. 3B); both Isl1 and the transgene are expressed, prior to protrusion, by dorsal mesocardial SHF cells situated between the common atrium and foregut and, following protrusion, by cells comprising the DMP. While the Mef2c-AHF-cre transgene is also expressed by myocardium of the right ventricle and outflow tract, it is not significantly expressed by AV cushion mesenchyme (Fig. 3E, F).
Figure 3. Genetic manipulation of Alk3 expression in the SHF through use of the Mef2c-AHF-cre transgene.

Males containing an Alk3 floxed allele and/or the Mef2c-AHF-cre transgene (A, blue ears) were mated with floxed female mice (A, pink ears) to generate Mef2cCRE;R26mG reporter embryos (A, top), SHF-Alk3 cko embryos (A, middle, red box), compound SHF-Alk3 cko/Mef2cCRE;R26mG reporter embryos (A, bottom, red box) and corresponding controls (A, green). Within the splanchnic mesoderm at the venous pole, the Mef2c-AHF-cre transgene (D) is expressed by the same SHF population that expresses Isl1 (B). Expression of the transgene is absent from the nearby myocardium (C), the foregut endoderm (asterisk in B, D), and from the AV cushion mesenchyme (E, F). FG, foregut; RMR, right mesocardial reflection; LMR, left mesocardial reflection; RV, right ventricle; RA, right atrium; LV, left ventricle; LA, left atrium; AVC, atrioventricular cushion; PAS, primary atrial septum
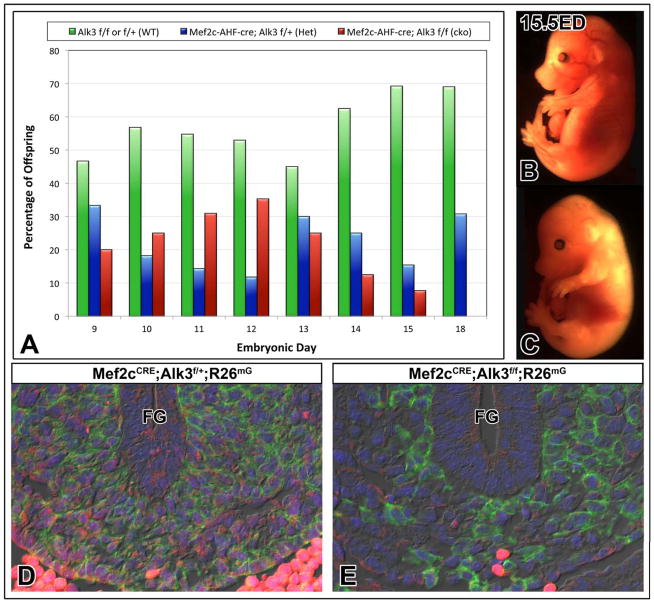
To determine whether BMP signaling is required for development of the DMP precursor population, Alk3-dependent BMP signaling was abrogated from the SHF using an established cre-loxp mating strategy. Mef2c-AHF-cre mice were mated with a) floxed Alk3 mice to generate Mef2c-AHF-cre;Alk3f/f (SHF-Alk3) ckos and b) combination floxed Alk3, floxed R26mT/mG mice to generate Mef2cCRE;Alk3f/f;R26mG ckos (Fig. 3A). Using this approach, thirty-four litters ranging in age from ED9.5 to ED18 were collected. In concordance with Mendelian inheritance, SHF-Alk3 cko specimens comprised approximately 25% of offspring through ED13. At ED15, however, SHF-Alk3 cko specimens comprise less than 10% of progeny. No SHF-Alk3 cko mice were recovered at stages beyond ED15.5 (Fig. 4A). Inspection of external features associated with developmental landmarks, such as closure of the posterior neuropore or lens vesicle, did not indicate developmental delay in SHF-Alk3 cko specimens (cf. Fig. 4B and 4C). Although an exact cause of death cannot be determined, features associated with hepatomegaly, portal congestion, and abdominal distention (Online Figure I) were noted in Mef2cCRE;Alk3f/f;R26mG and SHF-Alk3 ckos. These findings are consistent with hydrops fetalis, a frequently fatal prenatal form of heart failure that can result from cardiac malformations such as AVSD34, 35.
Figure 4. Deletion of Alk3 from the SHF results in reduced recovery of conditional knockouts.
The graph in panel A shows the percentage of control (green), heterozygous SHF-Alk3 mutants (blue), and homozygous SHF-Alk3 mutants (red) recovered in litters at stages ED9–18 (A). No obvious signs of development delay was noted in SHF-Alk3 homozygous mutants (C) when compared to control littermates (B). Immunofluorescent detection of GFP (green) and Alk3 (red) in Mef2cCRE;Alk3f/+;R26mG (control, D) and Mef2cCRE;Alk3f/f;R26mG (cko, E) embryos at ED11 shows decreased expression of Alk3 in GFP-positive cells of Mef2cCRE;Alk3f/f;R26mG ckos. FG, foregut
To determine whether our experimental approach resulted in effective deletion of Alk3 from the SHF, Alk3 expression was assessed in Mef2cCRE;Alk3f/f;R26mG and SHF-Alk3 ckos. Expression of Alk3 was detected in SHF cells of control littermates, but was absent from SHF cells in SHF-Alk3 and Mef2cCRE;Alk3f/f;R26mG ckos. In both groups, however, Alk3 expression was observed in dorsal mesocardial cells not derived from the SHF (Fig. 4D, E).
To determine how conditional deletion of Alk3 from the SHF affects formation of the AV septal complex, SHF-Alk3 ckos and control specimens at stages ED13.5–15.5 were examined (13 specimens from a total of 7 litters). The AV septal complex was properly formed in all control littermates inspected (Alk3f/f, Mef2c-AHF-cre;Alk3f/+, Mef2cCRE;Alk3f/+;R26mG; 8 specimens from 7 litters). In all SHF-Alk3 ckos, however, we observed an ostium primum defect with a common atrioventricular junction (Fig. 5D, D′). The histological analysis also showed that the AV cushions were fused to one another as well as to the muscular ventricular septum.
Figure 5. Deletion of Alk3 from the SHF results in ostium primum defects.
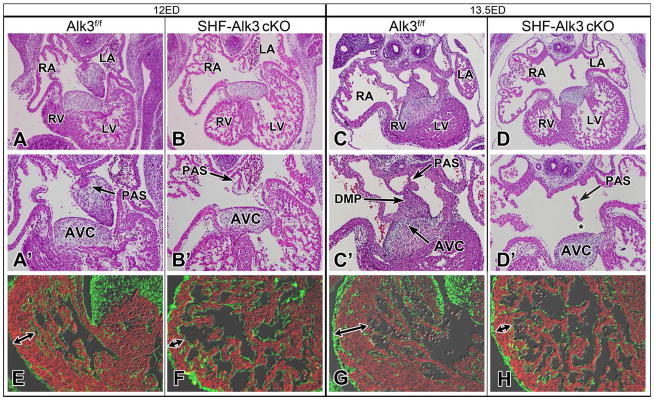
Histological analysis of control (A, C) and SHF-Alk3 cko (B, D) littermates at ED12 and ED13.5 shows that, while the AV valvuloseptal complex is properly formed in controls, SHF-Alk3 ckos are characterized by an ostium primum defect (asterisk, D′). In comparison to control littermates (E, G), the ventricular myocardium of SHF-Alk3 ckos (F, H) is thin (E-F, red MF20, green FLNA). RA, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle; PAS, primary atrial septum; DMP, dorsal mesenchymal protrusion; AVC, atrioventricular cushion
The ventricular myocardium of Mef2cCRE;Alk3f/f;R26mG and SHF-Alk3 ckos (Fig. 5F, H) was thin when compared to control littermates (Fig. 5E, G). Immunofluorescent staining of ventricular myocardium using the proliferation marker pHH3 did not, however, reveal a qualitative difference in proliferation between SHF-Alk3 ckos and control littermates (Online Figure II).
Conditional deletion of Alk3 from the SHF leads to hypoplasia of the DMP-precursor population and failure of the DMP to form
Impaired development of the DMP is implicated in the pathogenesis of AVSDs7–9, 11, 21. To determine whether reduction in BMP signaling alters development of the SHF-derived DMP, SHF-Alk3 cko (n=3) and control Alk3f/f (n=3) specimens at stage ED10.5 were histologically examined and immunohistochemically stained for Isl1. Based on the Isl1 expression profile, a 3D-rendering of the venous pole SHF was generated (Online Figure III). Analysis of these datasets revealed that, when compared to III). Isl1 expression, however, is not an ideal method of assessing SHF contribution in SHF-Alk3 ckos, as the experimental approach could hypothetically alter Isl1 expression within the SHF. Thus, we used Mef2cCRE;Alk3f/+;R26mG and Mef2cCRE;Alk3f/f;R26mG mice in order to trace the SHF lineage at the venous pole in an Isl1-independent fashion. Specimens at ED10.5 were stained for GFP and Isl1 (Fig. 6B, F) to generate 3D-renderings of the venous pole (Fig. 6C, G, D, H). This analysis confirmed that conditional deletion of Alk3 from the SHF results in decreased SHF expansion and failure of the DMP to properly form (Fig. 6G, G′H, H′).
Figure 6. Deletion of Alk3 from the SHF results in abnormal venous pole architecture and malformation of the DMP.
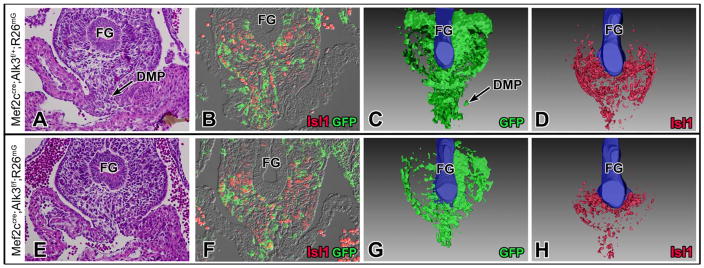
The number of SHF (GFP-positive) cells is reduced in Mef2cCRE;Alk3f/f;R26mG specimens (F, G) when compared to Mef2cCRE;Alk3f/+;R26mG control specimens (B, C) at ED10.5. Based on GFP (green, B, F) and Isl1 expression (red, B, F), a 3D-rendering of SHF contribution to the venous pole was generated using Mef2cCRE;Alk3f/+;R26mG (C, D) and Mef2cCRE;Alk3f/f;R26mG (G, H) specimens at ED10.5. DMP, dorsal mesenchymal protrusion; FG, foregut
pSMAD1/5/8 is reduced in DMP-precursors of SHF-Alk3 cko embryos
To assess whether deletion of Alk3 compromises BMP signaling in the SHF, expression of pSMAD1/5/8 was assessed in SHF-Alk3 cko specimens at ED10.5. Consistent with reduced canonical BMP signaling resulting from decreased availability of the BMP receptor, SHF-Alk3 cko specimens displayed reduced levels of pSMAD1/5/8 in the DMP and nearby dorsal mesocardium (Fig. 7C, D).
Figure 7. Reduced BMP signaling and proliferation in the SHF of SHF-Alk3 cko mutants.

When compared to Alk3 control littermates (A, C), expression of pSMAD1/5/8 (D) is reduced at the venous pole in SHF-Alk3 cko mice (B, D). Further analysis of venous pole SHF cells using Isl1 (green, H, I) as a SHF marker and the proliferation marker Ki67 (red, H, I) demonstrates significantly decreased SHF proliferation in Mef2cCRE;Alk3f/f;R26mG mutants (I) when compared to control littermates (H). Proliferation was also significantly reduced in Mef2cCRE;Alk3f/f;R26mG mutants (G) when GFP was used as the SHF marker (green, G) in combination with Ki67 (red, G). Furthermore, GFP-positive cells comprised a significantly smaller portion of total cell number (E) in Mef2cCRE;Alk3f/f;R26mG mutants. Arrows in F point to a number of GFP-positive;Ki67-positive co-labeled cells, arrows in H point to Isl1-positive;Ki67-positive co-labeled cells. FG, foregut; DMP, dorsal mesenchymal protrusion
Reduction of number of DMP-precursors at the venous pole in SHF-Alk3 cko embryos
Previous studies have shown that proper formation of the DMP is dependent on proliferation and expansion of the SHF DMP-precursor population located behind the dorsal mesocardium11. To determine whether reduction of BMP signaling affects SHF proliferation, sections through the venous pole of ED10–10.5 Mef2cCRE;Alk3f/f;R26mG specimens (n=3) and control Mef2cCRE;Alk3f/+;R26mG specimens (n=4) were labeled using antibodies recognizing GFP, Isl1, and the proliferation marker Ki67. Cell counts revealed that the proliferative index of venous pole SHF cells, irrespective of whether the SHF is defined by Isl1 expression or through use of a cell fate marker, is reduced by approximately two-fold in Mef2cCRE;Alk3f/f;R26mG specimens when compared to control Mef2cCRE;Alk3f/+;R26mG littermates (p=0.02, Fig. 7E–I). Consistently, the proportion of SHF cells comprising the dorsal mesocardium was significantly reduced in Mef2cCRE;Alk3f/f;R26mG mutants (Fig. 7E). Finally, the proportion of dorsal mesocardial GFP-positive cells expressing Isl1 was not significantly different between Mef2cCRE;Alk3f/f;R26mG and Mef2cCRE;Alk3f/+;R26mG groups. This indicates that the venous pole SHF hypoplasia we observed in SHF-Alk3 ckos does indeed reflect decreased SHF cell number, and is not a consequence of decreased Isl1 expression within the SHF cells (Fig. 7E). Although reduced SHF size could have resulted from increased apoptosis, examination of SHF-Alk3 ckos and control littermates at stages ED9.5–13.5 did not reveal any difference in number of apoptotic cells at the venous pole (data not shown).
SHF-Alk3 cko mice do not show premature myocardial differentiation of the SHF
Premature myocardialization of the SHF is implicated in compromised development of the DMP7. To determine whether premature myocardialization contributes to defective DMP development found in SHF-Alk3 knockouts, we assessed the expression of genes typically associated with myocardial differentiation: Nkx2.5, MF20, α-SMA, and α-sarcomeric actin9, 36 (Online Figure IV). This analysis did not reveal premature myocardial differentiation of mesenchymal cells within the SHF DMP-precursor population at any stage examined (ED9.5–13.5).
Early development of the AV cushions is not perturbed in the SHF-Alk3 cko mice
Impaired development of the AV cushions is associated with the pathogenesis of AVSD3–5. Therefore, we examined the developing AV cushions at ED9.5–13.5. Histological analysis of control and SHF-Alk3 cko specimens at ED9.5 did not reveal a difference regarding onset of epithelial-to-mesenchymal transition (Online Figure V). Although ostium primum defects were found in all SHF-Alk3 cko specimens, the overall AV cushion volume was not found to be different in SHF-Alk3 cko specimens when compared to control littermates (Fig. 8E). Consistent with this finding, we did not detect differences in the proliferative index of AV cushion cells in SHF-Alk3 cko and control specimens at ED11, 12, or 13.5 (Fig. 8J). Furthermore, apoptosis was not observed in the AV cushion mesenchyme in either group at these stages investigated (data not shown). Finally, both groups exhibited similar levels of expression of pSMAD1/5/8 and pSMAD3 in the cushions (Online Figure V), indicating that conditional deletion of Alk3 from the SHF does not perturb BMP/TGF signaling within the AV cushions.
Figure 8. AV cushion volume and proliferation of AV cushion mesenchyme is comparable between SHF-Alk3 cko mutants and control specimens.
The proportion of AV cushion cells expressing the proliferation marker Ki67 does not differ between SHF-Alk3 cko mutants and control littermates at ED11 (C, D, J), ED12 (J), or ED13.5 (G, H, J). The volume of the AV cushions also did not differ between the two groups at ED11 (A, B, I), ED12 (I), or ED13.5 (E, F, I). iAVC, inferior atrioventricular cushion; sAVC, superior atrioventricular cushion; PAS, primary atrial septum
DISCUSSION
Proper septation of the four-chambered heart requires the coordinated interaction and maturation of several structures including the primary atrial septum with its mesenchymal cap, and the endocardial cushions. More recently, studies of murine models of congenital heart malformations have revealed that an additional structure is critical for prenatal closure of the ostium primum: the DMP7, 9, 11, 21, 37. While the importance of the SHF-derived DMP to AV septation is now established, the molecular pathways and cellular mechanisms that underlie its formation and maturation are far from fully understood.
BMP signaling and development of the DMP
At least six BMP isoforms (BMP2,4,5,6,7, and 10) are expressed in the developing heart38, 39. Individually, BMP5, 6, and 7 do not appear to have a major role in cardiac development as obvious cardiac malformations are not observed in single knock-outs40. BMP7 is expressed in the developing myocardium41 while BMP6 expression is restricted to the aortic valvar mesenchyme and endothelium of the pulmonary and aortic trunks. BMP2, arguably the most frequently isoform studied, is expressed in the AV myocardium, where it is indispensable for AV cushion formation42. BMP2 deficient mice die before ED12.5 with severe defects in AV cushion morphogenesis30, 42. Loss of BMP10, which is predominantly expressed in the ventricular trabecular myocardium during early heart development, results in embryonic lethality around ED10.0–10.5 due to reduced ventricular mural thickness43. BMP4 is also critical for heart development; BMP4 knockout mice die relatively early in development44 while hypomorphic mice die within one week postnatally. BMP4 hypomorphs are characterized by 100% penetrance of AVSD with common atrioventricular valves45. Myocardial “deletion” of BMP4 through use of compound transgenic mice carrying a cardiac-TroponinT-cre (cTnT-cre) allele, a BMP4tm1 null allele, and a Bmp4loxp-lacZ allele, also resulted in complete penetrance of the AVSD phenotype45. The importance of BMP signaling to atrial and AV septation is also supported by findings in humans as mutations in BMP4 and the BMP receptor Alk2 have been implicated in the etiology of, respectively, atrial septal defects and AVSDs46–48.
Given the emerging insight into the role of the DMP in AV septation, and our longstanding interest in the development of this structure, we sought to determine whether BMP signaling could be involved in DMP development. Our expression studies of the venous pole demonstrated that BMP7 is ubiquitously expressed in the myocardium, that expression of BMP2 is largely absent, and that BMP4 is mainly confined to the mesocardial reflections flanking the developing DMP. Furthermore, we found expression of both activated Smads and Alk3, the BMP receptor known to interact with BMP4 and BMP749, within the SHF cell population that is located in close proximity to the mesocardial reflections and gives rise to the DMP. To determine the significance of BMP signaling in venous pole development, we then conditionally deleted Alk3 from the SHF. This experimental approach resulted in hypoplasia of the DMP and, consequently, a fully penetrant ostium primum defect.
As mentioned earlier, impaired development of the AV endocardial cushions has been associated with the etiology of AVSD3–5, 50, 51. Furthermore, BMPs are known to be involved in the development of the AV cushions3–5. Specifically, myocardially-expressed BMP2 has been shown to be essential for the induction of epithelial-to-mesenchymal transition through its binding to endocardially-expressed Alk342. Therefore, although our cell fate tracing revealed that neither the AV myocardium nor the AV cushion endocardium are SHF-derived, we examined the developing AV cushions to ensure that the development of these structures was not perturbed as a result of conditional deletion of Alk3 from the SHF. Examination of the AV cushions in our SHF-Alk3 knockout mice did not reveal any difference with respect to onset of epithelial-to-mesenchymal transition or expression of markers associated with proliferation, apoptosis, or TGFμ signaling. Furthermore, the volume of the major AV cushions in SHF-Alk3 ckos did not significantly differ from that of control groups at ED11, 12, or 13.5. These findings show that the ostium primum defects observed in our model do not result from impaired formation and/or fusion of the endocardially-derived AV cushions but rather, are a consequence of reduced SHF expansion and malformation of the DMP.
Mechanisms leading to abnormal DMP development
Examination of all SHF-Alk3 cko mice at crucial stages of DMP development and maturation (9.5–13ED) revealed hypoplasia of the SHF at the venous pole and failure of the DMP to develop. Because proper formation and addition of the SHF to the venous pole is a multi-step process, development of this structure may go awry at more than one stage7, 10, 11, 21. The region behind the dorsal mesocardium is largely acellular at ED9.5. However, within less than one day, there is a drastic increase in the number of SHF cells, virtually all of which are actively proliferating. Once these SHF cells protrude into the common atrium and form the DMP, however, they largely become quiescent (not shown). This stark contrast in mitotic activity over such a short developmental window indicates how critical SHF cell-cycle regulation is for proper development of the DMP. In an elegant study, Tian et al. demonstrated that global deletion of Wnt2 results in hypoplasia of the posterior dorsal mesocardium, including the DMP11. Further analysis of the venous pole SHF cells in Wnt2 mutants revealed decreased expression of Lef1, a component of canonical Wnt signaling. While BMP4 has been shown to positively regulate Lef1 expression52, we did not detect a decrease in Lef1 expression in SHF progenitors at the venous pole of SHF-Alk3 cko specimens when compared to control groups (data not shown). From these observations, we infer that the Wnt(2)/β-catenin and BMP signaling pathways might independently regulate development of the DMP by controlling the proliferation and expansion of the population of DMP precursor cells.
A requirement for sonic hedgehog (Shh) signaling in AV development and septation has also been demonstrated7, 8. Through utilization of Mef2c-AHF-cre and floxed Smoothened mice, Goddeeris et al. demonstrated that deletion of the Shh-receptor Smoothened from the SHF results in perturbation of DMP development and, consequently, AVSD7. In the SHF-Smo cko, the compromised development of the DMP was attributed to failure of the DMP precursor population to maintain a mesenchymal phenotype. Premature myocardial differentiation of these cells resulted in their inability to migrate into the common atrium. We examined SHF cells at the venous pole in SHF-Alk3 ckos over the entire known course of normal DMP development (9.5–13ED) but did not observe ectopic/premature expression of genes associated with myocardium, such as Nkx2.5, MF20, α-SMA, and α-sarcomeric actin, when compared to control specimens. Therefore, in our model, failure of venous pole SHF expansion, rather than premature myocardial differentiation, appears to be the major mechanism underlying DMP malformation.
The DMP and the pathogenesis of AVSD
As mentioned above, two morphological subtypes of AVSDs can be distinguished; incomplete and complete AVSDs. Studies on humans with AVSD have shown unequivocally that the AV junction is equally common in both defects2. The main difference between the two subtypes is that the bridging leaflets, which are derived from the major AV cushions, are firmly fused to the crest of the muscular ventricular septum, as well as to each other in most instances of the incomplete (or ostium primum) defect. Here, we demonstrate that inhibition of DMP development by deleting Alk3 from the SHF leads to failure of the base of the primary atrial septum to form and, consequently, the ostium primum to close. In addition, we show that early AV cushion development and fusion is not affected by our experimental approach. The AV septal complex consists of several components20 and correct formation of the valvuloseptal structures depends on proper development of each of them. The fact that perturbation of DMP development in the SHF-Alk3 mouse did not produce defects with shunting at ventricular level suggests that those using models that do present with complete AVSD should pay extra attention to the development of the features of the other components of the AV valvuloseptal complex, in particular the AV cushions.
In summary, our current observations show that the BMP signaling pathway at the venous pole of the heart is required for proper formation of the DMP and, hence, the AV septal complex. These results lend further support to the growing realization that perturbed development of the DMP may play a significant role in the pathogenesis of congenital heart malformations associated with AVSD. Additional research is needed to further unravel the molecular and cellular mechanisms that mediate SHF development at the cardiac venous pole, as these studies may provide invaluable insight into genetic and environmental causes underlying the etiology of AVSD.
Supplementary Material
Novelty And Significance.
What Is Known?
Atrioventricular septal defects (AVSDs) are congenital heart malformations characterized by the presence of an ostium primum defect and a common atrioventricular junction.
AVSDs can result from impaired development of the dorsal mesenchymal protrusion (DMP), a derivative of the second heart field (SHF).
The molecular mechanisms orchestrating the development of the DMP are far from fully understood.
What New Information Does This Article Contribute?
BMP signaling via the BMP receptor Alk3 at the cardiac venous pole is essential for the development of the DMP
Loss of BMP signaling in the SHF at the venous pole leads to DMP malformation and complete penetrance of ostium primum defect
Ostium primum defects can occur regardless of whether AV cushion development is normal or not
Atrioventricular septal defects (AVSDs) are congenital heart malformations in which communication between the left and right atrium is permitted via the so-called ostium primum defect. More recently, studies using murine models have demonstrated an association between ostium primum defects and malformation of the DMP. The findings of this study not only emphasize the importance of the DMP to AV septation, but also provide evidence that BMP signaling is required for DMP development. These results show that loss of BMP signaling from this precursor population results in the malformation of the DMP and a fully penetrant ostium primum defect phenotype. These results provide additional insights into the etiology of cardiac malformations associated with AVSD.
Acknowledgments
SOURCES OF FUNDING
The authors would like to acknowledge the financial support by the following grants: NIH NCRR C06-RR018823, NIH-NCRR C06-RR015455, the “South Carolina COBRE for Developmentally Based Cardiovascular Diseases”, P30 GM103342-01 (A.W.), NIH-NHLBI R01HL084285 (A.W.), NIH 5T32-GM008716-12 (L.E.B.), American Heart Association Grant-in-Aid 09GRNT2060075 (A.W.), American Heart Association Predoctoral grant 11PRE7310036 (L.E.B.), European Community’s Sixth Framework Program Grant LSHM-CT-2005-018630 (MJBvdH), Netherlands Heart Foundation Grant 1996M002 (MJBvdH). This publication was supported by Project 1233 from the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina CTSA, NIH Grant Numbers UL1RR029882 and UL1TR000062 (A.W.). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH and AHA.
Nonstandard Abbreviations
- AHF
anterior heart field
- ASD
atrial septal defect
- AV
atrioventricular
- AVSD
atrioventricular septal defect
- BMP
bone morphogenetic protein
- DMP
dorsal mesenchymal protrusion
- FG
foregut
- iAVC
inferior atrioventricular cushion
- LA
left atrium
- LMR
left mesocardial reflection
- LV
left ventricle
- MPES
midpharyngeal endothelial strand
- PAS
primary atrial septum
- RA
right atrium
- RMR
right mesocardial reflection
- RV
right ventricle
- sAVC
superior atrioventricular cushion
- SHF
second heart field
- VSD
ventricular septal defect
Footnotes
DISCLOSURES
None.
References
- 1.Craig B. Atrioventricular septal defect: From fetus to adult. Heart. 2006;92:1879–1885. doi: 10.1136/hrt.2006.093344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson RH, Wessels A, Vettukattil JJ. Morphology and morphogenesis of atrioventricular septal defect with common atrioventricular junction. World Journal for Pediatric and Congenital Heart Surgery. 2010;1:59–67. doi: 10.1177/2150135109360813. [DOI] [PubMed] [Google Scholar]
- 3.Dor Y, Camenisch TD, Itin A, Fishman GI, McDonald JA, Carmeliet P, Keshet E. A novel role for vegf in endocardial cushion formation and its potential contribution to congenital heart defects. Development. 2001;128:1531–1538. doi: 10.1242/dev.128.9.1531. [DOI] [PubMed] [Google Scholar]
- 4.Gaussin V, Van de Putte T, Mishina Y, Hanks MC, Zwijsen A, Huylebroeck D, Behringer RR, Schneider MD. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor alk3. Proc Natl Acad Sci U S A. 2002;99:2878–2883. doi: 10.1073/pnas.042390499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiltgen GG, Markwald RR, Litke LL. Morphogenetic alterations during endocardial cushion development in the trisomy 16 (down syndrome) mouse. Pediatr Cardiol. 1996;17:21–30. doi: 10.1007/BF02505807. [DOI] [PubMed] [Google Scholar]
- 6.Cole-Jeffrey CT, Terada R, Neth MR, Wessels A, Kasahara H. Progressive anatomical closure of foramen ovale in normal neonatal mouse hearts. Anat Rec (Hoboken) 2012;295:764–768. doi: 10.1002/ar.22432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goddeeris MM, Rho S, Petiet A, Davenport CL, Johnson GA, Meyers EN, Klingensmith J. Intracardiac septation requires hedgehog-dependent cellular contributions from outside the heart. Development. 2008;135:1887–1895. doi: 10.1242/dev.016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann AD, Peterson MA, Friedland-Little JM, Anderson SA, Moskowitz IP. Sonic hedgehog is required in pulmonary endoderm for atrial septation. Development. 2009;136:1761–1770. doi: 10.1242/dev.034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snarr BS, O’Neal JL, Chintalapudi MR, Wirrig EE, Phelps AL, Kubalak SW, Wessels A. Isl1 expression at the venous pole identifies a novel role for the second heart field in cardiac development. Circ Res. 2007;101:971–974. doi: 10.1161/CIRCRESAHA.107.162206. [DOI] [PubMed] [Google Scholar]
- 10.Snarr BS, Wirrig EE, Phelps AL, Trusk TC, Wessels A. A spatiotemporal evaluation of the contribution of the dorsal mesenchymal protrusion to cardiac development. Dev Dyn. 2007;236:1287–1294. doi: 10.1002/dvdy.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian Y, Yuan L, Goss AM, Wang T, Yang J, Lepore JJ, Zhou D, Schwartz RJ, Patel V, Cohen ED, Morrisey EE. Characterization and in vivo pharmacological rescue of a wnt2-gata6 pathway required for cardiac inflow tract development. Dev Cell. 2010;18:275–287. doi: 10.1016/j.devcel.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webb S, Anderson RH, Lamers WH, Brown NA. Mechanisms of deficient cardiac septation in the mouse with trisomy 16. Circ Res. 1999;84:897–905. doi: 10.1161/01.res.84.8.897. [DOI] [PubMed] [Google Scholar]
- 13.Wirrig EE, Snarr BS, Chintalapudi MR, O’Neal JL, Phelps AL, Barth JL, Fresco VM, Kern CB, Mjaatvedt CH, Toole BP, Hoffman S, Trusk TC, Argraves WS, Wessels A. Cartilage link protein 1 (crtl1), an extracellular matrix component playing an important role in heart development. Dev Biol. 2007;310:291–303. doi: 10.1016/j.ydbio.2007.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie L, Hoffmann AD, Burnicka-Turek O, Friedland-Little JM, Zhang K, Moskowitz IP. Tbx5-hedgehog molecular networks are essential in the second heart field for atrial septation. Dev Cell. 2012;23:280–291. doi: 10.1016/j.devcel.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.His W. Die area interposita, die eustachi’sche klappe und die spina vestibuli. Leipzig; Germany: 1880. [Google Scholar]
- 16.Wessels A, Anderson RH, Markwald RR, Webb S, Brown NA, Viragh S, Moorman AF, Lamers WH. Atrial development in the human heart: An immunohistochemical study with emphasis on the role of mesenchymal tissues. Anat Rec. 2000;259:288–300. doi: 10.1002/1097-0185(20000701)259:3<288::AID-AR60>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 17.Soufan AT, van den Hoff MJ, Ruijter JM, de Boer PA, Hagoort J, Webb S, Anderson RH, Moorman AF. Reconstruction of the patterns of gene expression in the developing mouse heart reveals an architectural arrangement that facilitates the understanding of atrial malformations and arrhythmias. Circ Res. 2004;95:1207–1215. doi: 10.1161/01.RES.0000150852.04747.e1. [DOI] [PubMed] [Google Scholar]
- 18.Webb S, Brown NA, Anderson RH. Formation of the atrioventricular septal structures in the normal mouse. Circ Res. 1998;82:645–656. doi: 10.1161/01.res.82.6.645. [DOI] [PubMed] [Google Scholar]
- 19.DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE, VanIperen L, Mentink MM. Development of the pharyngeal arch system related to the pulmonary and bronchial vessels in the avian embryo. With a concept on systemic-pulmonary collateral artery formation. Circulation. 1993;87:1306–1319. doi: 10.1161/01.cir.87.4.1306. [DOI] [PubMed] [Google Scholar]
- 20.Snarr BS, Kern CB, Wessels A. Origin and fate of cardiac mesenchyme. Dev Dyn. 2008;237:2804–2819. doi: 10.1002/dvdy.21725. [DOI] [PubMed] [Google Scholar]
- 21.Briggs LE, Kakarla J, Wessels A. The pathogenesis of atrial and atrioventricular septal defects with special emphasis on the role of the dorsal mesenchymal protrusion. Differentiation. 2012;84:117–130. doi: 10.1016/j.diff.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bleyl SB, Saijoh Y, Bax NA, Gittenberger-de Groot AC, Wisse LJ, Chapman SC, Hunter J, Shiratori H, Hamada H, Yamada S, Shiota K, Klewer SE, Leppert MF, Schoenwolf GC. Dysregulation of the pdgfra gene causes inflow tract anomalies including tapvr: Integrating evidence from human genetics and model organisms. Hum Mol Genet. 2010;19:1286–1301. doi: 10.1093/hmg/ddq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson RH, Webb S, Brown NA. The mouse with trisomy 16 as a model of human hearts with common atrioventricular junction. Cardiovasc Res. 1998;39:155–164. doi: 10.1016/s0008-6363(98)00037-6. [DOI] [PubMed] [Google Scholar]
- 24.Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR. Generation of bmpr/alk3 conditional knockout mice. Genesis. 2002;32:69–72. doi: 10.1002/gene.10038. [DOI] [PubMed] [Google Scholar]
- 25.Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol. 2005;287:134–145. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 26.Wessels A, van den Hoff MJ, Adamo RF, Phelps AL, Lockhart MM, Sauls K, Briggs LE, Norris RA, van Wijk B, Perez-Pomares JM, Dettman RW, Burch JB. Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Dev Biol. 2012;366:111–124. doi: 10.1016/j.ydbio.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dyer LA, Makadia FA, Scott A, Pegram K, Hutson MR, Kirby ML. Bmp signaling modulates hedgehog-induced secondary heart field proliferation. Dev Biol. 2010;348:167–176. doi: 10.1016/j.ydbio.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCulley DJ, Kang JO, Martin JF, Black BL. Bmp4 is required in the anterior heart field and its derivatives for endocardial cushion remodeling, outflow tract septation, and semilunar valve development. Dev Dyn. 2008;237:3200–3209. doi: 10.1002/dvdy.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugi Y, Yamamura H, Okagawa H, Markwald RR. Bone morphogenetic protein-2 can mediate myocardial regulation of atrioventricular cushion mesenchymal cell formation in mice. Dev Biol. 2004;269:505–518. doi: 10.1016/j.ydbio.2004.01.045. [DOI] [PubMed] [Google Scholar]
- 31.Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 32.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 33.Wessels A, van den Hoff M, Adamo R, Phelps A, Lockhart M, Sauls K, Briggs L, Norris RA, van Wijk B, Perez-Pomares J, Dettman R, Burch J. Epicardially-derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Developmental Biology. 2012 doi: 10.1016/j.ydbio.2012.04.020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fauza DO, Wilson JM. Congenital diaphragmatic hernia and associated anomalies: Their incidence, identification, and impact on prognosis. J Pediatr Surg. 1994;29:1113–1117. doi: 10.1016/0022-3468(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 35.Yagel S, Silverman NH, Gembruch J. Fetal cardiology embryology, genetics, physiology, echocardiographic evaluation, diagnosis and perinatal management of cardiac diseases. 2003. [Google Scholar]
- 36.Waller BR, 3rd, Wessels A. Cardiac morphogenesis and dysmorphogenesis. An immunohistochemical approach. Methods Mol Biol. 2000;135:151–161. doi: 10.1385/1-59259-685-1:151. [DOI] [PubMed] [Google Scholar]
- 37.Webb S, Brown NA, Anderson RH. Cardiac morphology at late fetal stages in the mouse with trisomy 16: Consequences for different formation of the atrioventricular junction when compared to humans with trisomy 21. Cardiovasc Res. 1997;34:515–524. doi: 10.1016/s0008-6363(97)00064-3. [DOI] [PubMed] [Google Scholar]
- 38.Somi S, Buffing AA, Moorman AF, Van Den Hoff MJ. Dynamic patterns of expression of bmp isoforms 2, 4, 5, 6, and 7 during chicken heart development. Anat Rec A Discov Mol Cell Evol Biol. 2004;279:636–651. doi: 10.1002/ar.a.20031. [DOI] [PubMed] [Google Scholar]
- 39.van Wijk B, Moorman AF, van den Hoff MJ. Role of bone morphogenetic proteins in cardiac differentiation. Cardiovasc Res. 2007;74:244–255. doi: 10.1016/j.cardiores.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 40.Kim RY, Robertson EJ, Solloway MJ. Bmp6 and bmp7 are required for cushion formation and septation in the developing mouse heart. Dev Biol. 2001;235:449–466. doi: 10.1006/dbio.2001.0284. [DOI] [PubMed] [Google Scholar]
- 41.Kim JS, Viragh S, Moorman AF, Anderson RH, Lamers WH. Development of the myocardium of the atrioventricular canal and the vestibular spine in the human heart. Circ Res. 2001;88:395–402. doi: 10.1161/01.res.88.4.395. [DOI] [PubMed] [Google Scholar]
- 42.Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- 43.Chen H, Shi S, Acosta L, Li W, Lu J, Bao S, Chen Z, Yang Z, Schneider MD, Chien KR, Conway SJ, Yoder MC, Haneline LS, Franco D, Shou W. Bmp10 is essential for maintaining cardiac growth during murine cardiogenesis. Development. 2004;131:2219–2231. doi: 10.1242/dev.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujiwara T, Dehart DB, Sulik KK, Hogan BL. Distinct requirements for extra-embryonic and embryonic bone morphogenetic protein 4 in the formation of the node and primitive streak and coordination of left-right asymmetry in the mouse. Development. 2002;129:4685–4696. doi: 10.1242/dev.129.20.4685. [DOI] [PubMed] [Google Scholar]
- 45.Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL. An essential role of bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joziasse IC, Smith KA, Chocron S, van Dinther M, Guryev V, van de Smagt JJ, Cuppen E, Ten Dijke P, Mulder BJ, Maslen CL, Reshey B, Doevendans PA, Bakkers J. Alk2 mutation in a patient with down’s syndrome and a congenital heart defect. Eur J Hum Genet. 2011;19:389–393. doi: 10.1038/ejhg.2010.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lagendijk AK, Smith KA, Bakkers J. Genetics of congenital heart defects: A candidate gene approach. Trends Cardiovasc Med. 2010;20:124–128. doi: 10.1016/j.tcm.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Smith KA, Joziasse IC, Chocron S, van Dinther M, Guryev V, Verhoeven MC, Rehmann H, van der Smagt JJ, Doevendans PA, Cuppen E, Mulder BJ, Ten Dijke P, Bakkers J. Dominant-negative alk2 allele associates with congenital heart defects. Circulation. 2009;119:3062–3069. doi: 10.1161/CIRCULATIONAHA.108.843714. [DOI] [PubMed] [Google Scholar]
- 49.Heinecke K, Seher A, Schmitz W, Mueller TD, Sebald W, Nickel J. Receptor oligomerization and beyond: A case study in bone morphogenetic proteins. BMC Biol. 2009;7:59. doi: 10.1186/1741-7007-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang B, Weidenfeld J, Lu MM, Maika S, Kuziel WA, Morrisey EE, Tucker PW. Foxp1 regulates cardiac outflow tract, endocardial cushion morphogenesis and myocyte proliferation and maturation. Development. 2004;131:4477–4487. doi: 10.1242/dev.01287. [DOI] [PubMed] [Google Scholar]
- 51.Wu B, Wang Y, Lui W, Langworthy M, Tompkins KL, Hatzopoulos AK, Baldwin HS, Zhou B. Nfatc1 coordinates valve endocardial cell lineage development required for heart valve formation. Circ Res. 2011;109:183–192. doi: 10.1161/CIRCRESAHA.111.245035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kratochwil K, Dull M, Farinas I, Galceran J, Grosschedl R. Lef1 expression is activated by bmp-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev. 1996;10:1382–1394. doi: 10.1101/gad.10.11.1382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.