Abstract
Introduction:
Urinary tract infections (UTI) are a major public health concern in developing countries. Most UTIs are caused by E. coli, accounting for up to 90% of community-acquired UTIs (CAUTI). Recurrent UTI is considered as a major risk factor for urolithiasis. Virulence factors like adhesins and biofilm have been extensively studied by authors on UPEC isolated from recurrent UTI. The studies on isolates from infection stones in kidney are scanty. In a prospective study, we aimed to determine the expression of Haemagglutinins, (Type 1 and P fimbriae), Biofilm production and resistance pattern to common antibiotics of Uropathogenic E.coli (UPEC) isolates from Community acquired Acute Urinary Tract Infection(CAUTI) and Urolithiasis.
Materials and Methods:
A total of 43 UPEC isolates, 23 mid-stream urine (MSU) samples from patients with CAUTI attending Out Patient Departments and 20 from renal calculi of urolithiasis patients at the time of Percutaneous nephrolithostomy (PCNL) were included in the study and the expression of Haemagglutinins,(Type 1 and P fimbriae), Biofilm production and resistance pattern to common antibiotics was assessed.
Results:
A total of 43 UPEC isolates 23 from CAUTI and 20 from renal calculi were tested for production of biofilm and hemagglutinins. In CAUTI, biofilm producers were 56.52% and hemagglutinins were detected in all isolates 100%. In urolithiasis, biofilm producers were 100% but hemagglutinins were detected only in 70% of isolates. All isolates were resistant to multiple antibiotics used. CAUTI isolates were susceptible to 3rd generation cephalosporins, whereas urolithiasis isolates were resistant to 3rd generation cephalosporins and 25% were Extended Spectrum Beta Lactamases ESBL producers.
Conclusions:
HA mediated by type 1 fimbriae plays an important role in CAUTI (P < 0.001 highly significant), whereas, in chronic conditions like urolithiasis, biofilm plays an important role in persistence of infection and the role of hemagglutinins is less.
Keywords: Biofilm, hemagglutination, mannose-resistant hemagglutination, mannose-sensitive hemagglutination, urolithiasis, uropathogenic Escherichia coli
INTRODUCTION
Urinary tract infections (UTIs) are a major public health concern in developing countries. Most UTIs are caused by Escherichia coli, accounting for up to 90% of community-acquired UTIs (CAUTI). The origin of these strains is frequently the patient's own intestinal flora. A subset of fecal E. coli having the virulence factors which enable them to colonise periurethral area, enter urinary tract, and cause symptomatic disease are defined as uropathogenic E. coli (UPEC).[1] Virulence factors of UPEC include the ability to adhere to uroepithelial cells and certain specific serotypes O and K antigens are resistance to phagocytosis and bactericidal action of normal serum. Other factors known to contribute to the virulence are the production of α hemolysins, colicins, aerobactin, cytotoxic necrotizing factor, and cell surface hydrophobicity.[2]
Bacterial adherence not only contributes to colonization but also to invasion, biofilm formation, and host cell damage. The two primary fimbrial adhesins associated with UPEC strains are type 1 and P fimbriae. Type 1 fimbriae mediate adherence largely via the FimH tip adhesin, which recognizes and binds mannosylated moieties on biotic and abiotic surfaces. Within the host, FimH mediates UPEC binding to the bladder epithelium and is also required for proper formation of biofilm-like intracellular bacterial communities within bladder epithelial cells.[3] Expression of P fimbriae is primarily linked to pyelonephritis strains. P fimbriae recognize α-D-galactopyranosyl-(1-4) β-D-galactopyranoside receptor located in the globoseries of glycolipids located on human kidney and erythrocytes. In response to the breach by UPEC into the normally sterile urinary tract, host inflammatory responses are triggered leading to cytokine production, neutrophil influx, the exfoliation of infected bladder epithelial cells, and the generation of reactive nitrogen and oxygen species along with other antimicrobial compounds.[4]
Approximately, 25% of patients with an episode of acute UTI later develop recurrent UTI, an important burden to the health system. The persistence of the same E. coli strain in the urinary tract may be the cause of recurrent UTI and urolithiasis. This may be related to the capacity of bacteria to form biofilms. Biofilm is defined as a structured community of bacterial cells enclosed in a self-produced polymeric matrix and adherent to an inert or living surface. Biofilm can promote persistence in the urinary tract and on biomaterial surfaces by protecting bacteria from the clearing out effect of hydrodynamic forces and the killing activity of host defence mechanisms and antibiotics. Matrix of the biofilm contributes to development of resistance of pathogenic E. coli biofilms and lead to persistent infections.[5]
Recurrent UTI is considered as a major risk factor for urolithiasis. Infection stones which constitute 10%-15% of renal stones are not associated with metabolic abnormalities but are a consequence of mostly luminal events during which microbial proliferation substantially alters urinary chemistry. Association between UTI and renal stone formation is well-known. It is generally assumed that Proteus mirabilis which produces urease are responsible for stone formation. Role of urease nonproducing bacteria like E. coli have remained unclear. Recent studies have shown that P mirabilis inhibited urokinase and E. coli stimulated sialidase. This could favour conversion of uromucoid to mineralizable matrix which lead to renal stone formation.[6] Persistant infection with microbes which modulate these enzymes play role in urolithiasis. Biofilm facilitates persistence leading to persistent UTI which can lead to stone formation. Virulence factors like adhesins and biofilm have been extensively studied by authors on UPEC isolated from recurrent UTI. The studies on isolates from infection stones in kidney are scanty.
We studied biofilm production and hemagglutination as virulence markers of UPEC and their role in CAUTI and urolithiasis.
MATERIALS AND METHODS
The study was conducted in the Department of Microbiology, Gandhi Hospital, Secunderabad for a period of 1 year. Fragmented renal calculi obtained from 72 cases of urolithiasis, which underwent percutaneous nephrolithostomy, were included. Patients with metabolic disorders, immunocompromised status, with stents and patients on antibiotic treatment prior to 1 week were excluded from the study. Mid-stream urine (MSU) specimens were collected from 280 randomly selected patients with acute UTI (CAUTI) attending outpatient department (OPD). All samples were cultured by standard bacteriological technique on blood agar, Mac-Conkey agar. Patients who received antibiotic treatment in the past 1 week were excluded from the study. A total of 43 UPEC isolates, 23 from MSU samples from patients with CAUTI and 20 from renal calculi of urolithiasis patients, were included in the study. All the isolates were cultured identified by standard biochemical tests.[7] They were tested for hemagglutinins, (type 1 and P fimbriae), biofilm production, and resistance pattern to common antibiotics.
Hemagglutination
Presence of type1, P fimbriae was detected by the presence of hemagglutination in the presence and absence of mannose, respectively.[8] The hemagglutination was detected by clumping of erythrocytes by fimbriae of bacteria in the presence of d-mannose. This test was carried out as per the direct bacterial hemagglutination test-slide method and mannose-sensitive and mannose-resistant hemagglutination (MRHA) tests. The strains of E. coli were inoculated into 1% nutrient broth and incubated at 37°C for 48 h for full fimbriation. Blood group “O” red blood cells were then washed thrice in normal saline and made up to a 3% suspension in fresh saline. They were used immediately or within a week when stored at 3-5°C. The slide hemagglutination test was carried out on a multiple-concavity slide. One drop of the red blood cell (RBC) suspension was added to a drop of the broth culture and slide was rocked to and fro at room temperature for 5 min. Presence of clumping was taken as positive for hemagglutination. Mannose-sensitive hemagglutination (MSHA) was detected by the absence of hemagglutination in a parallel set of test in which a drop of 2% w/v d-mannose was added to the red cells and a drop of broth culture. MRHA was detected by the presence of hemagglutination of 3% “O” group human RBC in the presence of 2% mannose.
Biofilm
Ability to form biofilm was tested by Congo red agar (CRA) method.[9] The medium was composed of brain heart infusion broth 37 g/L, sucrose 50 g/L, agar10 g/L, and Congo red 8 g/L. Congo red stain was prepared as a concentrated aqueous solution and autoclaved (12 1°C for 15 min) separately from the other medium constituents and was then added when the agar cooled to 55°C. Plates of the medium were inoculated and incubated aerobically for 24 h at 37°C. Biofilm producers produced black colonies with a dry crystalline consistency. Nonbiofilm producers remained pink. Figure 1-2.
Figure 1.

biofilm positive
Figure 2.

biofilm negative
Antibiotic sensitivity testing
Antibiotic susceptibility of 43 UPEC isolates to common antibiotics like ampicillin (25 μg), gentamicin (10 μg), ciprofloxacin (10 μg), cotrimoxazole (25 μg), norfloxacin (10 μg), cefotaxime (30 μg), ceftazidime (30 μg),) and cephalexin (30 μg) was determined by Kirby Bauer-disk diffusion method (Clinical and Laboratory Standards Institute CLSI 2009).[10] Antibiotics (span diagnostics) were used. E. coli ATCC 25922 was used as control strain.
Those organisms which showed resistance to three or more antibiotics of different structural classes were considered multidrug resistance (MDR). Strains that were resistant to cefotaxime and \or ceftazidime were tested for ESBL production by disc synergy tests.[11] Inoculum was standardized with 0.5 MC Farlands and swabbed on to a 90 mm Muller-Hinton agar plate. A susceptibility disc, containing amoxycillin/clavulanate, was placed in the center of the plate and discs of ceftazidime, cefotaxime were placed 20 mm (center to center) from the amoxycillin/clavulanate disc. The presence of distinctive enhancement of the inhibition zone toward the amoxycillin/clavulanate disc was considered as positive for ESBL production.
Statistical analysis
Statistical analysis was done by chi-square test and level of P < 0.05 was set as significant.
RESULTS
A total of 43 UPEC isolates 23 from CAUTI and 20 from renal calculi were tested for production of biofilm and hemagglutinins. In CAUTI, hemagglutinins were detected in all isolates. In urolithiasis, hemagglutinins were detected only in 70% of isolates. In CAUTI, biofilm producers were 56.52% and in urolithiasis biofilm producers were 100%, These observations were found to be statistically significant (P < 0.05) [Table 1].
Table 1.
Pattern of hemagglutination and biofilm
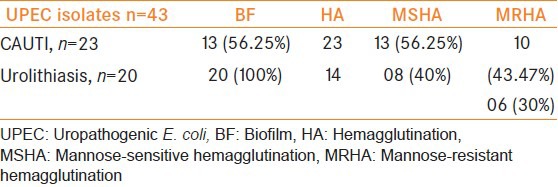
Table 2 shows that out of 21 MSHA isolates, all were biofilm producers and out of 16 MRHA isolates six produced biofilm while 10 did not produce biofilm, P < 0.001 which is statistically significant.
Table 2.
Association of biofilm with HA

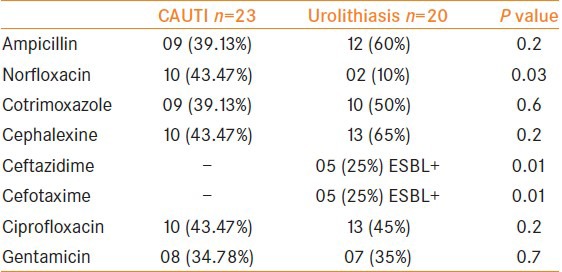
Acute UTI isolates exhibited resistance to ampicillin, cotrimoxazole, cephalexine but the result was not statistically significant (P < 0.03), whereas in urolithiasis five isolates were resistant to 3rd generation cephalosporins and are ESBL+ve (P < 0.01). MDR was found to be 25.56%.
DISCUSSION
The occurrence of virulence factors in UPEC strains strengthens the concept of association of UPEC with urinary pathogenicity. In our study, UPEC isolates from CAUTI and urolithiasis were studied for biofilm, hemagglutinin production.
Hemagglutination is mediated by fimbriae.[1] Virulence determinants like P fimbriae and type 1 fimbriae are more frequent in UPEC. Type 1 fimbriae bind to mannose containing receptors in uroepithelial lining. Type 1 fimbriae, which promote adhesion to host epithelial cells, have been found to be important in the initial steps of biofilm formation.[12] MSHA is mediated by type 1 fimbriae. On the contrary, MRHA activity can be mediated by P fimbriae, X, FIC, and DR fimbriae.[5] In our study all 100% the UPEC strains isolated from cystitis and 70% of isolates from urolithiasis expressed adhesins. Bacterial adhesins not only contribute to colonization but also to invasion and host cell damage.[12] Lower expression of these adhesins in isolates from renal calculi could reflect the loss of adhesins in persistent strains from renal calculi Expression of type 1 fimbriae was higher in cystitis strains in comparison to urolithiasis strains (P < 0.05). Type 1 fimbriae confer binding to uroplakins, which are abundant in the bladder.[12] Expression of P fimbriae was lower in isolates from cystitis as well as renal calculi. Other studies have shown higher expression of P fimbriae in nephritogenic strains as the fimbriae mediate adhesion to receptors in renal tissue.
The capsular polysaccharide of UPEC play a role in evading the immune system by resisting phagocytosis, plays a role in late biofilm development, and also assists in accelerating the urinary stone crystallization process via electrostatic interactions that accumulate urinaryions at the bacterial surface. During the course of UTIs, antibodies that recognize antigenic components of uropathogens are produced. However, proteases targeting immunoglobulins and other host defense components such as complement (C1q and C3) and antimicrobial peptides protect uropathogens from the host response.[13] UPEC typically express an array of fimbrial and afimbrial adhesions, secreted toxins, hemolysin, iron acquisition systems, capsular polysaccharide, and so on, to facilitate extraintestinal survival and enable UPEC to colonize and exert cytopathic effects in uinary tract.[14]
Biofilms are microbial communities of organisms adherent to each other and/or a target surface. Biofilm formation protects bacteria from hydrodynamic flow in the urinary tract, against phagocytosis, host defence mechanisms, as well as antibiotics. More than 50% of all bacterial infections reported involve biofilm formation.[15] A majority of the investigated strains in our study were in vitro positive for biofilm production. The prevalence of biofilm production was, thus, higher than reported in other studies.[12,16] Biofilm formation is more in repeated UTI's. In our study in acute UTI, biofilm formation was 56.52% and in urolithiasis 100%. This shows that biofilm formation was more in urolithiasis which is associated with repeated UTI which correlates with other studies.[16] Previous studies also reported a higher incidence (74%) of biofilm in repeated UTI.[16] However, there are no studies of biofilm formation in isolates from urolithiasis and in our study 100% of the urolithiasis isolates were biofilm producers. In a study from Spain,[15] biofilm-producing UPEC isolates showed a significantly greater type 1 fimbriae expression than nonbiofilm-producing strains, similar observation was made in our study also.
We investigated antibiotic resistance patterns of UPEC isolates [Table 3]. The isolates displayed high resistance to antibiotics tested. Those organisms which showed resistance to three or more antibiotics of different structural classes were considered MDR and it was found to be 25.56%. Acute UTI isolates, exhibited resistance to antibiotics tested. In urolithiasis, five isolates were resistant to 3rd generation cephalosporins and are ESBL +ve (P < 0.01 statistically significant) which reflects their usage as first-line drugs. In our study, BF producing UPEC isolates were more resistant to multiple antibiotics used. Our results are with agreement with other studies.[12] Fluoroquinolones are indeed very effective in stopping the growth of a biofilm.[17] In our hospital for acute UTI in the OPD quinolones more commonly norfloxacin are prescribed as empirical treatment and in chronic cases 3rd generation cephalosporins are prescribed. Antibiotic treatment based on sensitivity pattern of each isolate is to be done instead of empirical treatment.
Table 3.
Antimicrobial resistance pattern of UPEC
CONCLUSION
Our study concludes that, HA mediated by type 1 fimbriae plays an important role in acute UTI, whereas in chronic conditions like urolithiasis, biofilm plays an important role in persistence of infection and the role of hemagglutinins is less. UPEC isolates exhibiting fimbriae and biofilm were resistant to multiple antibiotics. So, periodic review of antibiotic policy needs to be made and reviewed. All patients with repeated UTI should be screened for biofilm formation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Raksha R, Srinivasa H, Macaden RS. Occurrence and characterization of uropathogenic Escherichia coli in urinary tract infections. Indian J Med Microbiol. 2003;21:102–7. [PubMed] [Google Scholar]
- 2.Kausar Y, Chunchanur SK, Nadagir SD, Halesh LH, Chandrasekhar MR. Virulence factors, serotypes and antimicrobial suspectibility pattern of Escherichia coli in urinary tract infections. J Med Sci. 2009;2:47–51. [Google Scholar]
- 3.Hadjifrangiskou M, Hultgren SJ. What does it take to stick around? Molecular insights into biofilm formation by uropathogenic Escherichia coli. Virulence. 2012;3:231–3. doi: 10.4161/viru.19763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiles TJ, Kulesus RR, Mulvey MA. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp Mol Pathol. 2008;85:11–9. doi: 10.1016/j.yexmp.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson JR. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.du Toit, Van Aswegen CH, Steyn PL. Effects of bacteria involved in pathogenesis of infection induced urolithisis on urokinase and sialidase activity. Urol Res. 1992;20:393–7. doi: 10.1007/BF00294494. [DOI] [PubMed] [Google Scholar]
- 7.Betty A. Forbes, Daniel F. Sahm, Alice S. Weissfeld. Bailey and Scott's Diagnostic Microbiology. 12th ed 2007. [Google Scholar]
- 8.Vagarali MA, Karadesai SG, Patil CS, Metgud SC, Mutnal MG. Haemagglutination and siderophore production as the urovirulence markers of uropathogenic Escherichia coli. Indian J Med Microbiol. 2003;21:102–7. doi: 10.4103/0255-0857.38863. [DOI] [PubMed] [Google Scholar]
- 9.Freeman DJ, Falkiner FR, Keane CT. New method for detecting slime production by coagulase negative Staphylococci. J Clin Pathol. 1989;42:872–4. doi: 10.1136/jcp.42.8.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wayne PA Clinical and Laboratory Standards Institute. 8th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2009. CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. [Google Scholar]
- 11.Paterson DL, Bonomo RA. Extended-spectrum β-lactamases: A Clinical update. Clin Microbiol Rev. 2005;18:657–86. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marhova M, Kostadinova S, Stoicova S. Biofilm forming capabilities of UPEC isolates. BMC Biotechnology. 2009;23:616–20. [Google Scholar]
- 13.Jacobsen SM, Stickler DJ, Mobley HL, Shirtliff ME. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microbiol Rev. 2008;21:26–59. doi: 10.1128/CMR.00019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal J, Srivastava S, Singh M. Pathogenomics of uropathogenic E. coli. Indian J Med Microbiol. 2012;30:141–9. doi: 10.4103/0255-0857.96657. [DOI] [PubMed] [Google Scholar]
- 15.Pruss BM, Besemann C, Denton A, Wolfe AJ. Acomplex transcription network controls the early stages of biofilmdevelopment by Escherichia coli. J Bacteriol. 2006;188:3731–9. doi: 10.1128/JB.01780-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soto SM, Smithson A, Martinez JA, Horcajada JP, Mensa J, Vila J. Biofilm formation in uropathogenic Escherichia coli strains: Relationship with prostatitis, urovirulence factors and antimicrobial resistance. J Urol. 2007;177:365–8. doi: 10.1016/j.juro.2006.08.081. [DOI] [PubMed] [Google Scholar]
- 17.Murugan S, Pongiya UD, Peedikayil NJ. Antimicrobial susceptibility pattern of biofilm producing Escherichia coli of urinary tract infections. Curr Res Bacteriol. 2011;4:73–80. [Google Scholar]


