Abstract
Aims:
There are few studies on the pathology of warty carcinoma (WC) of the penis and these have been from South America. Penile cancers are not uncommon in India. We reviewed the frequency of subtypes of penile squamous carcinoma (SC) and the pathological features and outcome of WC when compared to squamous carcinoma-not otherwise specified (SC-NOS). We also compared the clinicopathological features of WC in our series with those published earlier.
Materials and Methods:
We studied 103 cases of penile cancers over 6 years. Cases were classified into different subtypes according to established histologic criteria. Clinicopathologic features were studied in detail and compared among the different subtypes, especially between WC and SC-NOS. The patients were followed-up and disease free survival in months was noted.
Results:
SC-NOS constituted 75.7% of all penile cancer cases in our series. The frequency of other subtypes was WC: 9.7%, verrucous: 3.9%, basaloid type and papillary type: 0.97% each, and mixed types 8.7%. The average tumor size and depth of invasion did not differ significantly between the two subtypes. Frequency of lymphovascular emboli and percentage of lymph node metastasis in WC (30 and 10%) were lesser than in SC-NOS (49.37 and 26.58%), respectively. There were no recurrences after partial penectomy in the WC subtype. In the SC-NOS type, three cases had recurrence after partial/total penectomy.
Conclusion:
Warty carcinoma constitutes nearly 10% of all penile squamous cell cancers. These patients seem to have a less aggressive behavior than SC-NOS.
Keywords: India, cancer, penis, warty carcinoma
INTRODUCTION
Penile cancer frequency varies widely in different parts of the world.[1] There is a relatively high but variable frequency in different parts of India, particularly south India.[2,3,4,5,6] Penile cancers comprised 2% of all cancers in our institution in 1968.[7]
Despite the high frequency of penile squamous carcinomas (SC) in India, there is no published literature on the frequency of its histologic types. Among the various subtypes, warty or condylomatous type of squamous cancer (WC) of the penis is a relatively recently described entity and, barring a few studies from Paraguay and Brazil, very little has been published on this pathology. We reviewed our institutional database of penile cancers to assess the frequency of WC, its clinicopathological characteristics, and identify any differences between WC and squamous carcinoma-not otherwise specified (SC-NOS).
MATERIALS AND METHODS
103 cases of penile carcinoma, who underwent total or partial penectomy between September 2001 and August 2007 at our institution were studies. Patients with only biopsies from the lesion were excluded. After treatment of the primary tumor, all patients were advised inguinal lymphadenectomy (LAD) irrespective of inguinal nodal status and primary tumor histology. Patients who refused to undergo LAD were advised to come for regular follow-up. Patients with pathologically positive inguinal node either on frozen or routine histopathological examination were subjected to pelvic LAD. The specimen were grossed and the location, size, anatomic compartment, and maximum depth of invasion were noted. Sections were taken from area of maximum invasion. The number of blocks of tumor taken per case ranged from two to four. 4 μm sections were stained with hematoxylin and eosin. Microscopic features studied for all 103 cases included grade of tumor, presence of lymphovascular tumor emboli, level of invasion (T stage), and maximum depth of invasion (measured from basement membrane of adjacent normal squamous epithelium to the point of maximum invasion).
Of the 103 cases, 24 tumors with exophytic papillary architecture (verruciform tumors of Cubilla)[8] were studied in detail. In all 24 cases, additional features including the presence of papillae, degree of hyperkeratosis and parakeratosis, keratin cysts, intraepidermal abscesses, presence of koilocytosis, degree of nuclear atypia, and type of tumor-stromal interface (pushing versus irregular, infiltrative) were noted. Adjuvant treatment if any, lymph node (LN) status at the time of presentation and disease free survival in months whenever available were noted. The different subtypes were compared for LN status and depth of invasion and lymphovascular tumor emboli. Although follow-up was not available for all cases, disease free survival in months was noted wherever available.
Verruciform tumors were subtyped according to the criteria defined by Cubilla et al.[8] WC was diagnosed when typical features like papillomatous exophytic growth with rounded or spiky papillae with prominent fibrovascular cores [Figure 1a] and irregular, infiltrative tumor stromal interface [Figure 1b], and conspicuous koilocytosis [Figure 1c], were present throughout the tumor. Koilocytosis was defined as clear perinuclear cytoplasmic haloes, wrinkled, enlarged nuclei, bi or multinucleated cells, and dyskeratosis.[8] When these features were not uniformly present, it was called mixed subtype with components listed in order of predominance.[8]
Figure 1.
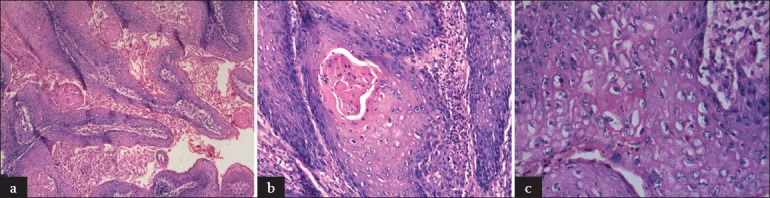
(a) Rounded and spiky papillae of warty carcinoma (H and E, ×100) (b) Irregularly infi ltrating downgrowths (H and E, ×200) (c) Conspicuous koilocytosis (H and E, ×400)
RESULTS
Of the 103 cases, the majority were SC-NOS type. The frequency of subtypes is given in Table 1. There were ten pure WC (9.70% of the total cases), and seven cases with warty and NOS components. Four of these seven had predominant warty component and three of the tumors had predominant NOS component and only focal warty areas. The single case of mixed verrucous and NOS was included under NOS category for purposes of comparison. Among the nonverruciform tumors, there was a single case of basaloid carcinoma. We did not have any rare variants such as sarcomatoid, pseudohyperplastic type, or cuniculatum.
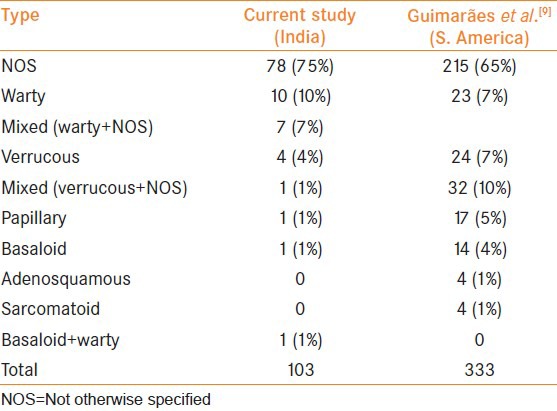
Table 1.
Frequency of histological subtypes of penile squamous carcinoma
Squamous carcinoma-NOS
The clinicopathologic features are summarized in Table 2. Of the 79 cases, 67 had involvement of the glans penis with or without extension into the prepuce and shaft and six cases had only prepucial involvement. In three cases the tumor was mainly in the prepuce with extension into the glans, four cases were in the shaft without involvement of glans. Partial amputation was done in 55 cases, total amputation was done in 21 cases, and two cases underwent circumcision. Eleven cases with LN metastases received chemotherapy. In one case of mixed verrucous and NOS subtypes, a conservative wide local excision of the lesion was done. Inguinal LN dissection was done in 43 cases. Those who did not undergo LN dissection were advised close follow-up. Of the 43, 20 cases showed LN metastasis. One patient had LN metastasis proven by fine needle aspiration cytology (FNAC), but refused surgery. Of the 21 cases with LN metastases, lymphovascular tumor emboli were detected in the penile resection specimen in 71.42%. The average depth of invasion in those cases with LN metastases was 7.33 mm versus 6.04 mm in those cases with negative LN on dissection. Follow-up of 6 months or more was available only in 41 cases and the average follow-up after the diagnosis of disease was 38.6 months with a range of 6-87 months. Of the 41 patients, one developed local recurrence and LN metastases after 33 months of partial amputation, while another developed LN metastases 14 months after total penectomy. One patient who had T4 disease developed local recurrence involving perineal skin and soft tissues 2 months after total penectomy.
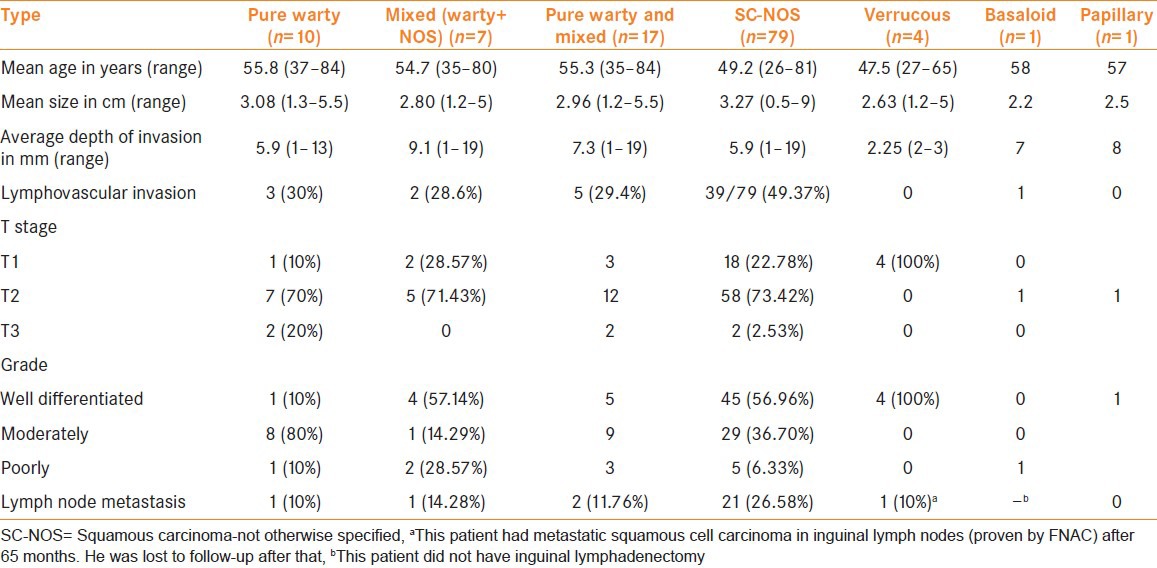
Table 2.
Comparison of clinicopathological features of the different subtypes
24 patients had verruciform tumors [Table 3]; partial amputation was done in 18 patients and total amputation in four. One patient had wide local excision as part of penile conservation and one was lost to follow-up after the diagnosis was made on wide local excision of the lesion.
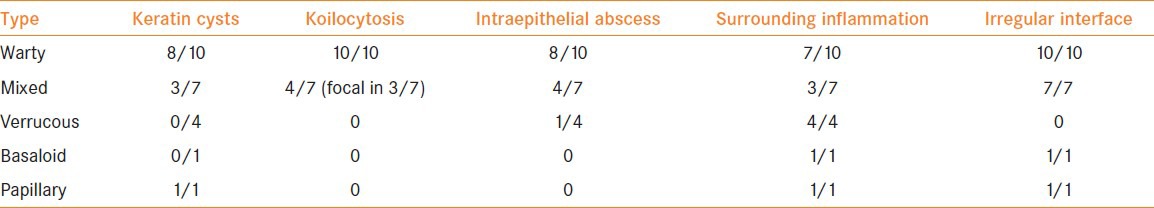
Table 3.
Comparison of microscopic features of the different histologic subtypes under verruciform category
Warty carcinoma
Clinicopathologic features of the 10 patients with Warty carcinoma (WC) are given in Table 2. All ten tumors were located in the glans. There was coronal involvement in four cases and prepucial involvement in two cases. The tumor extended to the shaft in three cases. Multicentricity was seen in one case. Majority of the tumors was moderately differentiated. Partial amputation was done in seven cases and total amputation in three cases. Inguinal LN were enlarged at presentation in six cases (60%). Inguinal LAD was done in two cases and only one had positive LN. This patient was given chemotherapy. The rest were advised close follow-up. Follow up of 6 months or more was available in eight cases. Mean follow-up was 40.63 months with a range of 7-67 months in these 8 patients. One patient who had an initial wide local excision had recurrence after 6 months, at which point, partial amputation was done. No other patient had recurrence during the follow-up period.
Mixed carcinoma with warty and NOS components
The clinicopathologic features of these patients are summarized in Table 2. The tumor was located on the glans in all of the cases and it involved the prepuce in three cases. The tumor extended to the shaft in one case. Multicentricity was not seen in any patient. Enlarged inguinal LN were seen at presentation in four of seven cases. Partial amputation was done in five cases, total amputation in one case and wide local excision in one case as part of penile conservation. Inguinal LAD was done in three cases. One case had positive LN. Follow-up of more than 6 months was available in five cases. Mean follow-up was 41.8 months with a range of 15-89 months. Only one case with wide local excision had recurrence after 89 months.
Verrucous carcinoma
Mean age of presentation, tumor size, and depth of invasion is given in Table 2. The tumors were located in the glans with involvement of corona in all the four cases and prepucial involvement was additionally noted in two cases. One of four tumors extended into the shaft. In one case, the tumor was multicentric with three noncontiguous verruciform tumors. All the four cases were well-differentiated and were of T1 stage. Lymphovascular emboli were not seen in any case.
Partial amputation was done in three cases. One patient who underwent a wide local excision of the tumor for confirmation of diagnosis was lost to follow-up. Only one of the four cases had superficial inguinal LAD and the nodes were negative for tumor. There was no adjuvant treatment in any of the cases. Of the four cases, three had follow-up of 22, 24, and 65 months. One patient, who was seropositive for human immunodeficiency virus (HIV), was found to have metastatic squamous cancer deposits in the inguinal LN by FNAC, 65 months after partial penectomy. He was subsequently lost to follow-up.
There was a single case of papillary carcinoma in our series. It occurred in a 57-year-old male and was located on the glans. The maximum tumor size was 2.5 cm and the depth of invasion was 8 mm. Occasional lymphovascular tumor emboli were seen. Partial amputation and bilateral inguinal LN dissection were done. There were no tumor deposits in the LN.
There was a single case with WC and basaloid components. The patient was a 76-year-old and inguinal LN were not palpable. The tumor involved the glans, prepuce, and corona. He underwent total amputation of the penis. The maximum tumor dimension was 7 cm and the depth of invasion was 12 mm. Lymphovascular tumor emboli were present.
DISCUSSION
The frequency of subtypes of penile cancers in our study is similar to those previously published by Guimarães et al [Table 1].[9] However, the frequency of WC varies from 3.67% to 15.5%.[8,10] Only one case of pure basaloid carcinoma was seen in our series of 103 cases (0.97%). This was much lower when compared to previous data where its incidence varied from 4% to 8%.[9,11] We thus found a higher frequency of warty type and much lower frequency of basaloid type when compared to the studies from Paraguay. This is interesting because both the subtypes are preferentially associated with human papilloma virus (HPV)-16 infection[12] and a possible relation between the two entities has been proposed with the description and characterization of a mixed warty-basaloid variant.[11,13] There is significant variability in the frequency of sub-types of penile cancer geographically.[14,15,16] Rare subtypes like adenosquamous, pseudohyperplastic SC, and carcinoma cuniculatum were not seen in our series. WC was not classified separately in some series. Geographic variations in the prevalence of different HPV subtypes and conditions like lichen sclerosus et atrophicus and phimosis could be some of the reasons for these differences. Further studies on etiopathogenetic factors are required to establish or disprove this hypothesis.
The first description of WC of the penis in English medical literature could be found in the article by Davies et al.,[17] where it has been referred to as malignant condyloma. The first series of penile WC was published in 2000 by Cubilla et al.[8] Similar carcinoma has been described much earlier in the vulva by Rastkar et al.,[18] in 1982. Other groups further studied this entity and found an increased association with HPV infection.[19,20] This is true of penile WC as well.[10] Nearly one in four of our cases (23.3%) were of verruciform category. The type of papillae and degree of hyperkeratosis and parakeratosis did not help in differentiating the different types. Keratin cysts and intraepidermal abscesses were more common in WC. The presence of koilocytosis and type of tumor-stromal interface were the important features differentiating warty subtype from other verruciform tumors.
There are only a few published series on WC of the penis and most of them are from Paraguay and Brazil[8,21]. Our data on these cases is similar to theirs. These patients are generally in their sixth decade and, pathologically, the majority are grade 2 with less than 20% incidence of LN metastasis. In comparison with other subtypes, the mean age of presentation of WC was slightly higher but the average size of the tumor or depth of invasion did not differ significantly. The incidence of lymphovascular tumor emboli and LN metastasis was lower in WC cases (30% and 10%) than in the SC-NOS category (49.4% and 26.6%) respectively. Table 4 show the percentage of cases with lymphovascular tumor emboli in each category with the tumor stage.
Table 4.
Tumor stage for stage comparison of lymphovascular invasion in the different subtypes
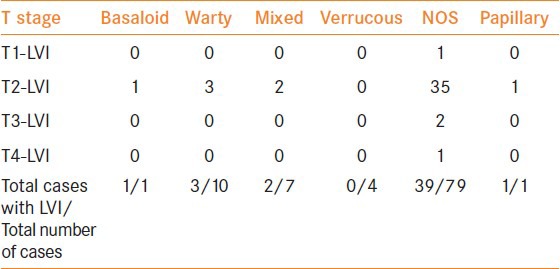
In a study on the relation between histologic subtypes of penile SC and outcome, Cubilla et al.,[22] identified three prognostic groups. The verruciform tumors had a better prognosis than SC of the usual type which in turn had a better prognosis than the basaloid-sarcomatoid group. The authors stated that this could be due to the inherent invasive characteristics of the different subtypes, that is, verruciform tumors had lesser depth of invasion than usual type SC. In our series, the average depth of invasion of the WC cases and the SC-NOS were identical, but WC displayed lesser degree of lymphovascular embolization and inguinal LN metastases.
Guimarães et al.,[9] have documented a 10% recurrence rate for WC when compared to 28% for SC-NOS. The follow-up in our series was not complete. Hence, an exact comparison of disease free survival between the different groups is not possible. However, in the WC cases with follow-up, recurrence was seen in only one case that had only an excision of the tumor. There were no recurrences recorded after partial penectomy. In the SC-NOS group, three had recurrence/metastases, even after total/partial penectomy. Of the six WC cases with clinically palpable inguinal LN, only one had metastasis; while in the mixed (warty and NOS) category, only one of the four cases with enlarged inguinal LN had metastasis. Low recurrence rate and low rate of inguinal LN metastasis necessitates the question whether a conservative surgery without LN dissections would suffice in at least a subset of patients with WC subtype. In a study published earlier by Bhagat et al.,[23] on the same cohort of patients regardless of histological subtype, strongest predictors of inguinal LN metastasis were presence of high grade and lymphovascular invasion in the penile resection specimen and clinically palpable LN. More cases of WC have to be studied to assess whether the predictors are different for this subtype.
Pathologic factors predicting the likelihood of nodal metastases include histological grade of the tumor, level of infiltration, and perineural invasion. Risk stratification systems are based on these three factors.[24] The importance of histological subtype in triggering a prophylactic inguinal LAD is not established. However, in a study by Chaux et al.,[25] warty, papillary, and verrucous histological types of penile cancers were associated with significantly lower rates of recurrence and inguinal LN metastases. Although there seems to be a case for conservative management for these subtypes, larger studies with longer follow-up are necessary before this can be adopted as routine.
CONCLUSIONS
In summary, we have studied the frequency of the different subtypes of penile squamous carcinoma in India and compared it to published literature from Paraguay and Brazil. In India, the frequency of WC appears almost slightly higher. The WC cases in our series, did not differ significantly in mean size or depth of invasion from SC-NOS. Similar to studies from South America, WC cases were found to have lower frequency of lymphovascular invasion and LN metastasis when compared to SC-NOS.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Cubilla AL, Dilner J, Schellhammer PF, Horenbias S, Ayala AG, Reuter VE, et al. Malignant epithelial tumours of penis. In: Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. Pathology and Genetics of Tumors of the Urinary System and Male Genital Organs. Lyon: IARC Press; 2004. pp. 276–290. [Google Scholar]
- 2.Jain SP, Agrawal PK, Jain S, Handa K, Sinha N, Dwivedi JN. Frequency of carcinoma of penis with special reference to North India. Indian J Cancer. 1981;18:250–3. [PubMed] [Google Scholar]
- 3.Kaushal V, Sharma SC. Carcinoma of the penis. A 12-year review. Acta Oncol. 1987;26:413–7. doi: 10.3109/02841868709113709. [DOI] [PubMed] [Google Scholar]
- 4.Prabhakar BR, Gupta S, Prabhakar H. Carcinoma of penis in Punjab. J Indian Med Assoc. 1976;66:55–7. [PubMed] [Google Scholar]
- 5.Reddy CR, Reddy VC, Rao MS. Distribution of malignant tumors in Kurnool. Indian J Cancer. 1967;4:64–71. [PubMed] [Google Scholar]
- 6.Reddy CR, Raghavaiah NV, Mouli KC. Prevalence of carcinoma of penis with special reference to India. Int Surg. 1975;60:474–6. [PubMed] [Google Scholar]
- 7.Thomas JA, Small CS. Carcinoma of penis in Southern India. J Urol. 1968;100:520–6. doi: 10.1016/s0022-5347(17)62563-1. [DOI] [PubMed] [Google Scholar]
- 8.Cubilla AL, Velazques EF, Reuter VE, Oliva E, Mihm MC, Jr, Young RH. Warty (condylomatous) squamous cell carcinoma of the penis: A report of 11 cases and proposed classification of ‘verruciform’ penile tumors. Am J Surg Pathol. 2000;24:505–12. doi: 10.1097/00000478-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Guimarães GC, Cunha IW, Soares FA, Lopes A, Torres J, Chaux A, et al. Penile squamous cell carcinoma clinicopathological features, nodal metastasis and outcome in 333 cases. J Urol. 2009;182:528–34. doi: 10.1016/j.juro.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Bezerra AL, Lopes A, Landman G, Alencar GN, Torloni H, Villa LL. Clinicopathologic features and human papillomavirus DNA prevalence of warty and squamous cell carcinoma of the penis. Am J Surg Pathol. 2001;25:673–8. doi: 10.1097/00000478-200105000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Cubilla AL, Reuter VE, Gregoire L, Ayala G, Ocampos S, Lancaster WD, et al. Basaloid squamous cell carcinoma: A distinctive human papilloma virus-related penile neoplasm: A report of 20 cases. Am J Surg Pathol. 1998;22:755–61. doi: 10.1097/00000478-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Gregoire L, Cubilla AL, Reuter VE, Haas GP, Lancaster WD. Preferential association of human papillomavirus with high-grade histologic variants of penile-invasive squamous cell carcinoma. J Natl Cancer Inst. 1995;87:1705–9. doi: 10.1093/jnci/87.22.1705. [DOI] [PubMed] [Google Scholar]
- 13.Chaux A, Tamboli P, Ayala A, Soares F, Rodríguez I, Barreto J, et al. Warty-basaloid carcinoma: Clinicopathological features of a distinctive penile neoplasm. Report of 45 cases. Mod Pathol. 2010;23:896–904. doi: 10.1038/modpathol.2010.69. [DOI] [PubMed] [Google Scholar]
- 14.Chaux A, Lezcano C, Cubilla AL, Tamboli P, Ro J, Ayala A. Comparison of subtypes of penile squamous cell carcinoma from high and low incidence geographical regions. Int J Surg Pathol. 2010;18:268–77. doi: 10.1177/1066896909339184. [DOI] [PubMed] [Google Scholar]
- 15.Johnson DE, Lo RK, Srigley J, Ayala AG. Verrucous carcinoma of the penis. J Urol. 1985;133:216–8. doi: 10.1016/s0022-5347(17)48887-2. [DOI] [PubMed] [Google Scholar]
- 16.Goodman MT, Hernandez BY, Shvetsov YB. Demographic and pathologic differences in the incidence of invasive penile cancer in the United States, 1995-2003. Cancer Epidemiol Biomarkers Prev. 2007;16:1833–9. doi: 10.1158/1055-9965.EPI-07-0221. [DOI] [PubMed] [Google Scholar]
- 17.Davies SW. Giant condyloma acuminata: Incidence among cases diagnosed as carcinoma of the penis. J Clin Pathol. 1965;18:142–9. doi: 10.1136/jcp.18.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rastkar G, Okagaki T, Twiggs LB, Clark BA. Early invasive and in situ warty carcinoma of the vulva: Clinical, histologic, and electron microscopic study with particular reference to viral association. Am J Obstet Gynecol. 1982;143:814–20. doi: 10.1016/0002-9378(82)90015-1. [DOI] [PubMed] [Google Scholar]
- 19.Kurman RJ, Toki T, Schiffman MH. Basaloid and warty carcinoma of the vulva. Distinctive types of squamous cell carcinomas frequently associated with human papillomaviruses. Am J Surg Pathol. 1993;17:133–45. doi: 10.1097/00000478-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Toki T, Kurman RJ, Park JS, Kessis T, Daniel RW, Shah KV. Probable nonpapillomavirus etiology of squamous cell carcinoma of the vulva in older women: A clinicopathologic study using in situ hybridization and polymerase chain reaction. Int J Gynecol Pathol. 1991;10:107–25. doi: 10.1097/00004347-199104000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Chaux A, Soares F, Rodríguez I, Barreto J, Lezcano C, Torres J, et al. Papillary squamous cell carcinoma, not otherwise specified (NOS) of the penis: Clinicopathologic features, differential diagnosis, and outcome of 35 cases. Am J Surg Pathol. 2010;34:223–30. doi: 10.1097/PAS.0b013e3181c7666e. [DOI] [PubMed] [Google Scholar]
- 22.Cubilla AL, Reuter V, Velazquez E, Piris A, Saito S, Young RH. Histologic classification of penile carcinoma and its relation to outcome in 61 patients with primary resection. Int J Surg Pathol. 2001;9:111–20. doi: 10.1177/106689690100900204. [DOI] [PubMed] [Google Scholar]
- 23.Bhagat SK, Gopalakrishnan G, Kekre NS, Chacko NK, Kumar S, Manipadam MT, et al. Factors predicting inguinal node metastasis in squamous cell cancer of penis. World J Urol. 2010;28:93–8. doi: 10.1007/s00345-009-0421-1. [DOI] [PubMed] [Google Scholar]
- 24.Chaux A, Cubilla AL. Stratification systems as prognostic tools for defining risk of lymph node metastasis in penile squamous cell carcinomas. Semin Diagn Pathol. 2012;29:83–9. doi: 10.1053/j.semdp.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Chaux A, Reuter V, Lezcano C, Velazquez EF, Torres J, Cubilla AL. Comparison of morphologic features and outcomes of resected recurrent and nonrecurrent squamous cell carcinoma of the penis: A study of 81 cases. Am J Surg Pathol. 2009;33:1299–306. doi: 10.1097/PAS.0b013e3181a418ae. [DOI] [PubMed] [Google Scholar]


