Abstract
Pelviureteric junction obstruction (PUJO) of the kidney can lead to a number of different clinical manifestations, which often require surgical intervention. Although the success of pyeloplasty and endopyelotomy are good, there are still a number of patients who fail primary treatment and develop secondary PUJO. These treatment failures can be a challenging cohort to manage. This article aims to provide a comprehensive overview on the surgical options available to the urologist for managing secondary PUJO as well as providing some guidance on assessing factors that will influence management decisions.
Keywords: Endopyelotomy, laparoscopic pyeloplasty, pelviureteric junction obstruction, reconstruction
INTRODUCTION
Pelviureteric junction obstruction (PUJO) of the kidney can cause pain, recurrent urinary tract infections, hydronephrosis and loss of renal unit function. Surgical intervention is often required and numerous treatment strategies have been employed. These modalities have changed with time. PUJO can be considered congenital or acquired and recent algorithms for the management of PUJO have highlighted the importance of establishing intrinsic (e.g. atreteic or stenosed ureteral segment) or extrinsic (e.g. lower pole crossing vessel) compression in deciding optimal primary management.[1]
Following the description in 1949 by Anderson and Hynes, open dismembered pyeloplasty became the gold standard.[2] Endoscopic management in the form of endopyelotomy was introduced in the 1990's and success rates of up to 70-90% quoted.[3] Retrograde and antegrade approaches have been used and laser, cutting balloon and cold knife endopyelotomy are described. Laparoscopic pyeloplasty was first described in 1993[4] and success rates comparable to open pyeloplasty of 84-98% are quoted.[1,5,6,7] Some now believe that laparoscopic pyeloplasty is the new gold standard, having superior outcomes compared to endopyelotomy and less morbidity compared to open pyeloplasty.[1] Other modalities for primary intervention include robotic pyeloplasty, which may have equivalent results to laparoscopy, but is not as widely available and has less follow-up to date.[8]
There appears to be a wide variation in current practice with some centers in the USA and UK utilizing antegrade or retrograde endopyelotomy as primary treatment of PUJO, rather than pyeloplasty.[9,10] Success rates vary depending on the definition of success[11] and the modality of intervention. There is little doubt that there are good outcomes following primary intervention. This means there is minimal data on salvage strategies in those patients who develop a recurrent PUJO following a prior repair a situation termed “secondary PUJO” in this review. The aim of the review is to describe the management options for patients with secondary PUJO.
SECONDARY PUJO ASSESSMENT
Definitions of treatment success and failure vary within the literature. Failure can be considered as the inability to improve symptoms, dynamic renographic parameters, renal unit function or hydronephrosis. Tan et al. defined success in their cohort as drainage on a diuretic renal scan (Whittaker test in equivocal cases) and direct visualization of the PUJ at ureterorenoscopy.[12] Other success criteria have included symptomatic resolution (i.e. more than 80% pain relief) associated with stable or improved renal function and improved washout from the renal pelvis (i.e. T1/2 < 20 min) on renal scan.[1] A combination of reduction of symptoms and an improvement in renogram or intravenous urogram (correct expansion) has also been used.[13] Application of success definitions can be problematic. In one study patients with abnormal renography were considered a success as there was an improvement of renal function, hydronephrosis and symptoms.[12] Equally patients had improved/normalized renograms but were considered failures as they had persistent symptoms.
In long standing obstruction with significant dilatation, renographic or ultrasound scans may not return to “normal” after primary treatment. In this situation, symptomatic improvement and stabilization of renal unit function are the markers of success. Authors have identified lack of consistency in terminology and definition between studies as a particular problem in making clear comparisons between results of different treatments.[7,11] This should be considered when analyzing different treatments and counseling patients.
Failure of primary treatment can be considered as early or late. For the purpose of this review, both scenarios will be termed “secondary PUJO.” The causes of failed treatment include; poor surgical technique, an “irreparable pelvi-caliceal system,” PUJ ischemia with re-stenosis, anastomotic leak with urinoma and fibrosis, a missed crossing vessel which can occur in 18%-50% of cases, ureteric stent malfunction and diabetes.[1,12,14] Most treatment failures present within the first 18 months following the procedure.[1,12] However, secondary PUJO has been identified in patients up to 5 years after primary treatment.[15] This suggests a prolonged follow-up may be necessary in these patients.
It is important to elucidate possible risk factors for failed primary treatment.[12] Dynamic renography is required to assess differential function and the degree of obstruction. If treatment failure is confirmed or suspected, helical computed tomography should be considered to assess for extrinsic causes such as a crossing vessel or fibrosis, the presence of renal calculi and the degree of hydronephrosis. Giving patients realistic expectations is important. Patients should be warned of the risk of kidney loss and further treatment failure. In a cohort of 128 laparoscopic pyeloplastys, 18 were considered failures at a median interval of 2.5 months (84% success of primary treatment). Salvage treatment was only possible for just over half and overall only 44% were salvaged.[12]
Multivariate analysis of patients following laparoscopic pyeloplasty identified diabetes mellitus, longer hospital stay, higher American Society of Anesthesiologists score, an indwelling stent at the time of pyeloplasty and ureteral stent malfunction as risk factors for treatment failure.[12] Following salvage treatment a body mass index >30 and ureteral stent malfunction were identified as risk factors for further failure. The presence of diabetes mellitus has been linked to a poorer outcome in reconstructive procedures of the urinary tract and may be due to the micro vascular changes seen in diabetics.[16] As such, closely regulating blood sugars in the peri-operative period may aid the reconstructive outcome.
MANAGEMENT OPTIONS FOR SECONDARY PUJO
There are a number of options available to the clinician for management of secondary PUJO. The best option for the patient will depend on numerous factors including; individual upper tract anatomy, presence of renal calculi, concurrent medical conditions, informed patient preference, availability and experience of individual techniques, symptoms, renal function and the type of primary treatment modality. Treatment options and predominant management decisions for secondary PUJO are outlined in a flow chart algorithm [Figure 1]. The options to be considered within this review will be:
Figure 1.
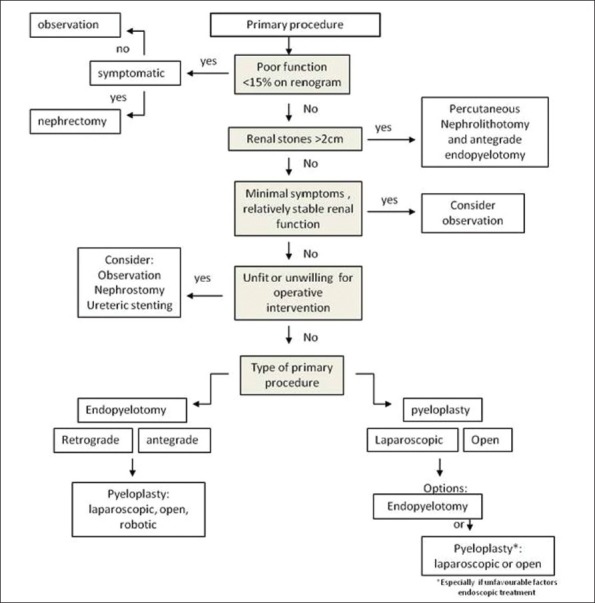
Flow chart algorithm for assessment and treatment of patients with secondary pelviureteric junction obstruction
Antegrade and retrograde endopyelotomy
Pyeloplasty (open, laparoscopic, robotic)
Complex reconstruction; ureterocalycostomy, ileoureteral replacement
Autotransplantation ± boari flap pelvivesicostomy
Long-term ureteric stenting or percutaneous nephrostomy
Nephrectomy (open or laparoscopic)
Observation.
Endoscopic management
Managing failed pyeloplasty can be challenging as previous surgery can result in fibrosis, distorted anatomy and a need for considerable mobilization if open or laparoscopic/robotic surgery is performed. Therefore, an endourological approach can reduce morbidity and provide a shorter hospital stay and quicker post-operative recovery. Many centers appear to utilize endopyelotomy as the mainstay of treatment for the failed pyeloplasty. Endourologic management of PUJO was introduced by Ramsay et al. in 1984[17] and further popularized throughout the 1980s by urologists such as Badlani et al. who developed the term “endopyelotomy.”[18] Retrograde and antegrade approaches are used. Retrograde techniques include the wire cutting balloon (e.g. accusize endopyelotomy) and ureteroscopic laser pyelotomy, usually with the holmium or neodymium-doped yttrium aluminum garnet laser[19] [Figure 2]. Incisions for endopyelotomy should be made in the lateral aspect of the PUJ as anatomical studies have demonstrated there is less chance of encountering a crossing vessel in these region.[20] Usually an eight or 9F ureteric stent is placed afterwards. Percutaneous antegrade endopyelotomy has been well described and, in some experienced centers, results for primary PUJO has approached those of open/laparoscopic pyeloplasty.[9,21] The use of a percutaneous antegrade endopyelotomy in the management of secondary PUJO should certainly be considered if there are concomitant pelvi-calyceal stones, especially >2 cm, which can be managed simultaneously. Both antegrade cold knife and laser endopyelotomy techniques are described.
Figure 2.
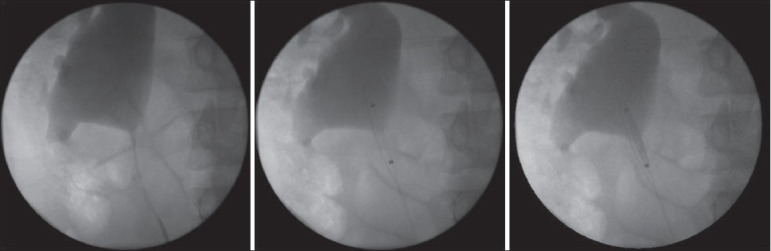
Utilisation of accusize endopyelotomy in the treatment of secondary pelviureteric junction obstruction. The patient had an open pyeloplasty 10 years previously. The markers signifying the proximal and distal extent of the cutting balloon can be clearly seen
Contraindications to endourological management of secondary PUJO are; a long stenosed segment (>2 cm), uncontrolled coagulopathy and active infection. In view of this, accurate pre-operative anatomical assessment of the PUJO and renal vasculature is required with computed tomography and/or retrograde studies. The impact of a crossing vessel on management is controversial. Reports have suggested that this in itself is not a contraindication to primary endoscopic management of the PUJ.[22] However, recent algorithms have suggested that in the presence of extrinsic compression e.g. a crossing vessel, endopyelotomy success rates fall and pyeloplasty should be considered.[1] If a significant crossing vessel is identified in a previous failed endopyelotomy then repeat endopyelotomy is not recommended and as such laparoscopic or open repair is advised.[23]
Endopyelotomy after failed pyeloplasty
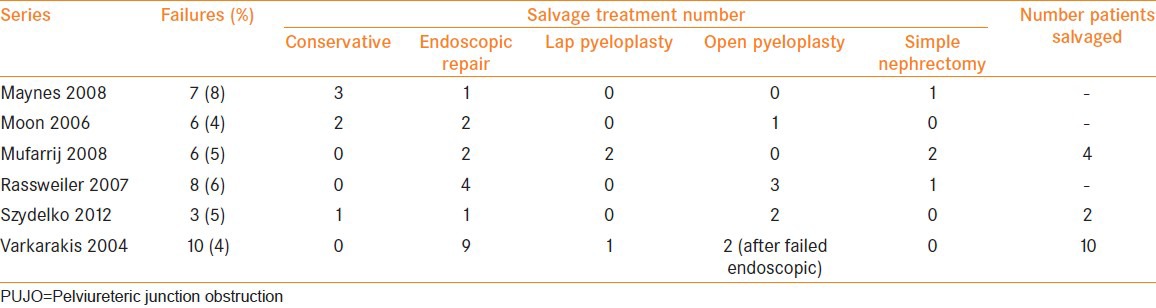
A summary of some contemporary series of failed laparoscopic pyeloplasty are described in Table 1. The success rates of laparoscopic pyeloplasty are 84-98%.[1,6,7,8,24,25] Endopyelotomy used for secondary PUJO in these patients had a success rate of around 70%.[1,12] Not all patients are suitable for endoscopic treatment. In one study, only 10 out of 18 were deemed suitable for endopyelotomy.[12] Similar success rates are seen for patients treated with endopyelotomy (antegrade and retrograde) following previous open pyeloplasty.[25,26] A higher success rate (87.5%) was described in a large series of 72 patients using cold knife antegrade endopyelotomy following failed open pyeloplasty.[27] It would appear that in the context of secondary PUJO, there is little difference in the outcome between an antegrade and retrograde approaches.[28] Some series would suggest that balloon dilatation is not as effective as laser/cutting endopyelotomy, although the number of endoscopic procedures reported in the literature for secondary PUJO are small, making direct comparison difficult.[25,29] Patients tend to choose a minimally invasive technique if given a choice[25] but they should be warned of the need for a third treatment. When endopyelotomy is utilised for patients following a failed pyeloplasty, some authors have suggested little difference in outcomes as compared to endopyelotomy in a primary PUJO.[28,30,31]
Table 1.
Salvage treatment employed for secondary PUJO following laparoscopic pyeloplasty
Endopyelotomy after failed endopyelotomy
Following a failed endopyelotomy, 90% of urologists in the USA would opt for surgical repair (57% open pyeloplasty and 34% laparoscopic pyeloplasty) rather than a repeat endopyelotomy.[12] In a recent large series of 113 laser endopyelotomies, 41 (36%) were for primary treatment failures. Of these, only 10 (25%) had a previous endopyelotomy.[1] This tendency is because repeat endopyelotomy is less effective than open or laparoscopic repair in the salvage setting. In a cohort of 22 patients receiving either antegrade or retrograde endopyelotomy the success rate at 4 years was only 37% following previous endopyelotomy, where as it was 71% following previous open pyeloplasty.[26]
Laparoscopic re-do pyeloplasty
Laparoscopic pyeloplasty has been described by a number of authors following previous open, laparoscopic and endoscopic treatment[1,14,32,33,34,35] [Table 2]. Transperitoneal[33] and retroperitoneal[1] approaches have been successfully used. Different technical approaches to laparoscopic repair of the PUJ have been described [Figure 3]. A re-do Anderson Hynes dismembered pyeloplasty is preferred in cases where there is a missed crossing vessel at the time of primary treatment. A non-dismembered foley V-Y plasty is classically described for a high insertion PUJ. It has been described in secondary PUJO and can be utilized in a retroperitoneal approach. Presence of a crossing vessel, need to reduce the renal pelvis size and a proximal ureteric stricture are relative contraindications to its use in a primary or secondary setting.[36] There does not appear to be a significant difference in the outcome between each technique.[1] Where significant renal pelvis reduction is required, the Culp-DeWeerd pyeloplasty can be used although this can be challenging laparoscopically. Z-plasty and Fenger pyeloplasty have also been described.[1] Placement of a ureteral stent prior to the procedure may help to aid identification of the ureter.
Table 2.
Summarized outcomes of laparoscopic pyeloplasty in patients with secondary PUJO
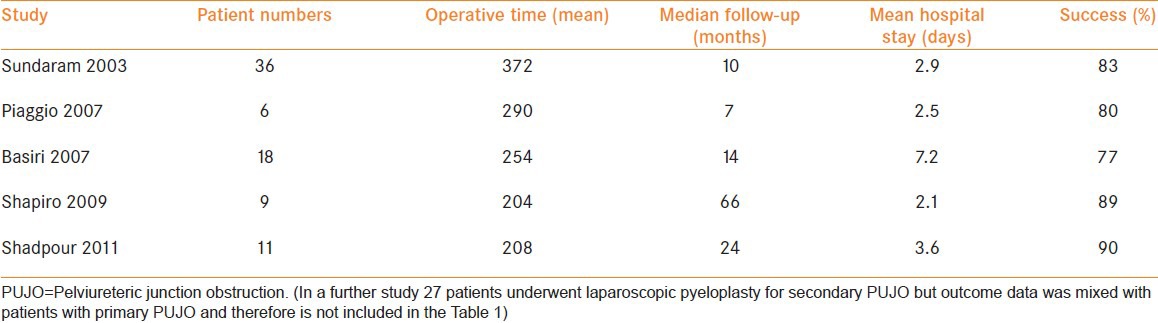
Figure 3.
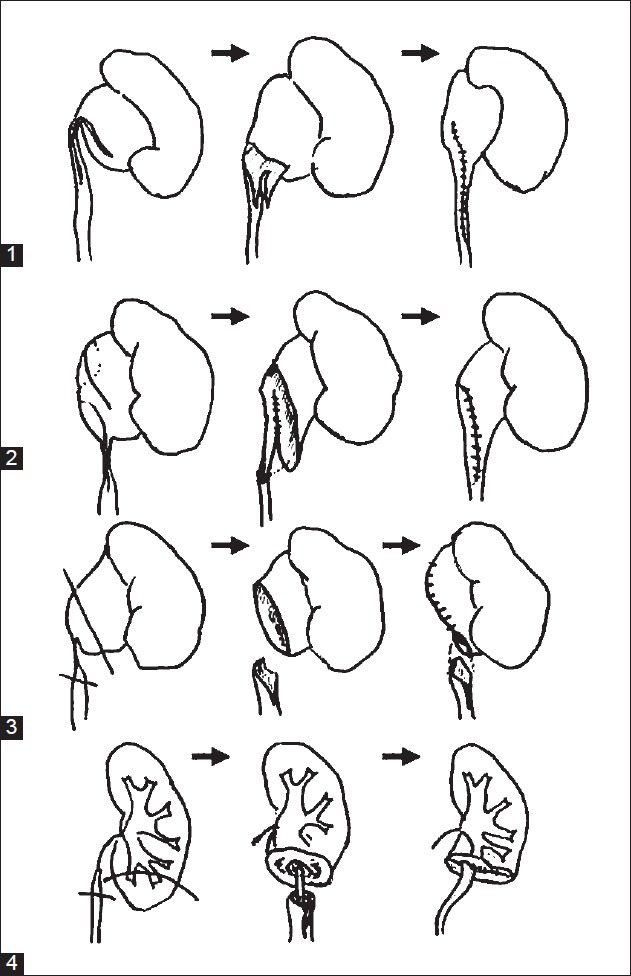
Reconstructive options in the management of primary and secondary pelviureteric junction obstruction. Variations in anatomy may require different surgical techniques. (1) Foley V-Y plasty (2) Culp-deWeerd spiral pyeloplasty (3) Anderson-Hynes dismembered pyeloplasty (4) Uretero-calicostomy
Laparoscopic pyeloplasty following previous endopyelotomy
Due to the poorer outcomes of repeat endopyelotomy following primary endoscopic treatment, laparoscopic pyeloplasty may become the treatment of choice for this cohort of patients. In one series of 143 laparoscopic pyeloplasty's, 21 (15%) had previous endopyelotomy.[1] Sundaram et al.[33] described 33 patients undergoing re-do laparoscopic pyeloplasty after endopyelotomy, with an 83% success rate. The dissection was more difficult and the mean operative time was 6.2 hours. Open conversion occurred in one patient and 8/36 (22%) had some form of post-operative complication. There can still be significant peri-ureteric fibrosis following endopyelotomy making laparoscopy more technically challenging. However in experienced hands it would appear that laparoscopic pyeloplasty can be safely performed in a secondary setting with similar outcomes to that of primary pyeloplasty.
Laparoscopic pyeloplasty following open surgery
Prior open surgery can lead to fibrosis, scarring, poorer access and distorted anatomy. Despite these technically challenging aspects of laparoscopic repair, a recent series described a low complication rate with minimal conversions, major complications or blood transfusions.[14] A success rate of 84% was also reported in 25 patients by Inagaki et al.[37] Shapiro described nine patients who underwent laparoscopic pyeloplasty following a previous open repair.[32] Mean operating time was 204 min and there were no major complications. At 5 years, 8/9 (89%) had a successful outcome.[32] Within the current literature, it is difficult to make clear comparisons in complication rates between primary and secondary pyeloplasty. In experienced hands, similar results to that seen with primary laparoscopic pyeloplasty can be reached and it should remain a procedure performed by technically proficient laparoscopic surgeons with adequate expertise.
Laparoscopic pyeloplasty following previous laparoscopic surgery
Re-do laparoscopic pyeloplasty after a prior laparoscopic pyeloplasty can be a challenging procedure. Although there is little data on the subject, caution has been suggested before considering this technique. In a series of 143 laparoscopic pyeloplastys, eight patients required re-treatment due to failure. No patients had a second laparoscopic procedure. Four patients underwent endopyelotomy, three open pyeloplasty and one open nephrectomy.[1]
Open pyeloplasty for secondary PUJO
Although the proponents of laparoscopic surgery report good outcomes of laparoscopic repair for secondary PUJO, there are those who suggest that following failure with laparoscopic or endoscopic management, open pyeloplasty should be employed.[12] This is based on the likelihood of significant fibrosis and the good results of open pyeloplasty for secondary PUJO. As well as peri-ureteric fibrosis there can also be a relatively long segment of stenosed ureter and wide mobilization of the proximal ureter and kidney is often required. This mobilization then enables bridging of the defect and a tension free anastamosis. For the repair, a dismembered or flap technique is generally utilized. Although these can be complex repairs with difficult dissection,[38] some reports suggest no significant difference in complication or transfusion rates compared to primary repair.[39] Following a prior laparoscopic or open primary repair, excellent results have been reported with up to 100% not requiring further treatment in the medium term.[26] Similar good results are reported for open repair following primary endoscopic treatment with success rates up to 90%.[26,38,39,40] However, the numbers of patients within these reported case series are small. The obvious disadvantage of open pyeloplasty is the increased morbidity associated with a large incision.
Robotic pyeloplasty
Mention should be made of the advancing technique of robotic surgery and management of PUJO. First described by Gettman in 2002 where six pyeloplastys were performed successfully,[41] its use is increasing, in part due to easier intra-corporeal suturing.[42] Some authors feel that the anastamosis achieved is superior to the point where a ureteric stent may not be required.[43] A recently reported large multi-institutional cohort of robotic pyeloplasty with 3 year follow demonstrated equivalent success rates to that of laparoscopic and open pyeloplasty at 97%.[44] However, of the 168 patients reviewed only 21 (12.5%) were performed for secondary PUJO, having had prior surgical treatment. Although the success rate of robotic pyeloplasty in these secondary PUJO procedures were still good, care must be taken in extrapolating these results, especially as there are no studies to date specifically evaluating robotic pyeloplasty in secondary PUJO. Longer term follow-up and further experience is clearly warranted.
Complex reconstruction and auto transplantation
Ureterocalicostomy requires anastomosis of the proximal ureter to a lower pole calyx [Figure 2]. Indications include primary PUJO with intra-renal pelvis, secondary PUJO, complex anatomy and obliterated PUJ/ureter after prior surgery. There is limited data in the literature of its use in primary or secondary PUJO. Success rates of over 80% are described.[45,46,47] Good long-term outcomes in children are also reported.[48] Significant complications can be encountered such as anastomotic leak, re-stenosis and renal artery thrombosis with renal loss.[45] Approximation of ureteric and calyceal urothelium and excision of renal parenchyma in proximity to the anastomosis are considered to be important factors in achieving a durable outcome. Although laparoscopic calicostomy has been described,[49] to date the majority of these procedures are performed open.
In situations where ureteric and renal pelvis repair are not possible ileal interposition or autotransplantation can be considered. Despite ileal interposition being first described in the 1950's,[50] there is limited long-term data concerning patient outcomes, especially in the context of secondary PUJO. Good long-term kidney function can be seen in up to 80% at 5 years.[51] However, up to a quarter of patients may require some form of re-intervention i.e. nephrectomy, PCNL (Percutaneous nephrolithotomy) or re-anastamosis of proximal ileal segment. Urinary tract infections and metabolic acidosis were observed in up to a third.[51] Laparoscopic assisted ileal interposition has been reported with comparable results and reduced morbidity.[52] Alternative to ileal ureter is auto transplantation. Following nephrectomy and bench preparation the kidney is usually re-sited in the right iliac fossa due to better access to the iliac vessels. This can typically be combined with a boari flap and pelvivesicostomy. It has been described in loin-pain hematuria syndrome and renal artery stenosis with good effect. Successful cases of laparoscopically assisted auto-transplantation have also been reported.[53]
Long-term ureteric stenting
Patient preference, clinical, patient or surgical factors may require consideration of long-term ureteric stenting as treatment of secondary PUJO. Negative aspects to this strategy include stent symptoms, stent blockage, urinary tract infection and the need for regular stent changes. Extra-anatomical stents, such as the Detour stent, are used less widely but have the advantage of less stent symptoms and are longer lasting requiring no or fewer changes.[54,55]
Nephrectomy
Open or laparoscopic nephrectomy may be utilized in patients with poor renal function (<15% on renography) and ongoing symptoms. It can also be considered in patients who have good function with severe symptoms and who do not want to consider reconstructive surgery, which carries a risk of failure and the subsequent need for yet more intervention. However patients should be carefully counseled about nephrectomy in the presence of good renal unit function. In chronic pain syndromes, removal of the end organ can still result in ‘phantom’ pain as described in patients post nephrectomy for polycystic kidney disease.[56] Despite the lack of data in the literature on pain in patients having nephrectomy for chronic PUJO, we think patients should still be warned that it is difficult to guarantee that nephrectomy will definitely cure chronic renal pain.
Observation
Observation for patients with failed primary management would appear to be an increasingly viable option.[6,7] It should be considered where there are minimal symptoms and preserved renal function. However, there is little data to suggest how long observation should go on for, how frequently functional assessment should be performed and the likelihood of renal loss over time. Patient factors such as age and co-morbidities need to be carefully assessed. Even if there is a slow reduction in renal unit function it may be that attempted salvage is not warranted in the context of these other factors. Intriguingly spontaneous improvement in renal function and symptoms has been reported in patients undergoing observation.[12]
MANAGEMENT OF PUJO TREATMENT FAILURE IN CHILDREN
In children, the gold standard for primary management remains open dismembered pyeloplasty. However, as with the adult population, there is also increasing use of a laparoscopic approach.[29] Success rates of pyeloplasty are over 90% and although the majority of failures occur within the first 12 months, they can occur up to 3 years post procedure.[57] Ultrasound scan is often used more frequently in follow-up and identifies failures with good accuracy, reducing the need for renography and the associated radiation exposure. Renography can then be reserved for patients with hydronephrosis.[57]
In open pyeloplasty failures, it would appear that endoscopic treatment is inferior to re-do pyeloplasty. In a series of 105 open pyeloplastys, there were seven failures (6%). Five patients had balloon dilatation, with only one success and one patient had failed laser endopyelotomy. Therefore, six patients required open surgery with three re-do pyeloplasty and three ureterocalicostomy.[57] There was dense scarring in all, which may explain the failure of endoscopic intervention, and two undiagnosed crossing vessels. Despite challenging surgical procedures, the success rate was reported as 100% at 18 months. In a subsequent series, 14 patients underwent re-do pyeloplasty (12 open, two lap) and 18 had endoscopic intervention (10 laser endopyelotomy, eight balloon dilatation). At 3 years, the success rates in the endoscopic group was 39% versus 100% in the re-do pyeloplasty group.[58] Factors associated with poor outcome following endoscopic treatment included age >4 years, use of balloon dilatation (only one success) and length of ureteric stricture >5 mm.[58]
FOLLOW-UP AFTER SURGICAL MANAGEMENT OF SECONDARY PUJO
There is debate regarding the optimum length of follow-up after primary pyeloplasty. Some authors have suggested that the follow-up after endopyelotomy should be as long as 3 years.[13] Although the majority of failures were within the 1st year, a significant number of treatment failures occurred after 12 months. Some failures even occurred after 5 years. The resource constraints of patients and many medical institutions mean patients are often not followed up for this length of time. Within the setting of secondary PUJO treatment, it is also unclear how long patients should be followed up after re-do surgery. One approach would suggest close surveillance in the first 12 months and then a bi-annual or annual review for at least 3 years.[15]
CONCLUSIONS
Continued good success rates of primary repair mean that secondary PUJO is a thankfully uncommon scenario for the clinician to manage. However it is important to appreciate the options that can be discussed with the patient if treatment failure occurs. The mode of secondary intervention will be determined by individual upper tract anatomy, concurrent medical conditions, presence of symptoms, renal unit function and the modality of primary treatment. Informed patient preference is a vital part of developing a patient centered management plan. Pragmatically, the local availability of individual techniques and the expertise of urological staff will also have an impact on the final decision.
The lack of standardized terms for treatment success and failure following primary and secondary intervention, makes clear comparisons between studies assessing different treatment modalities difficult.[11] There are no randomized controlled trials assessing different treatments in a secondary treatment context. As experience with secondary treatment grows and further studies are published, we would agree with other authors that standardized terms would aid clinicians in assessing the literature and counseling their patients.[7,11] The small overall numbers of secondary PUJO treatment reported in the literature makes clear conclusions difficult. However, it would appear that following primary pyeloplasty (laparoscopic or open) endopyelotomy carries a good balance of minimal co-morbidities and reasonable success rates. Following primary endopyelotomy there is evidence to support secondary laparoscopic or open pyeloplasty as a good option. As laparoscopic pyeloplasty experience, in particular intra-corporeal suturing, continues to improve, it may be that secondary laparoscopic pyeloplasty develops a wider remit of indications in the management of secondary PUJO. The increasing use of robotic assisted laparoscopic pyeloplasty may also be of use to surgeons attempting surgical repair of difficult secondary PUJO. There will always be particularly challenging cases where more complex reconstruction is required. In these situations patients should be managed within a multi-disciplinary team environment and by surgeons with good experience in upper tract reconstruction.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Rassweiler JJ, Subotic S, Feist-Schwenk M, Sugiono M, Schulze M, Teber D, et al. Minimally invasive treatment of ureteropelvic junction obstruction: Long-term experience with an algorithm for laser endopyelotomy and laparoscopic retroperitoneal pyeloplasty. J Urol. 2007;177:1000–5. doi: 10.1016/j.juro.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JC, Hynes W. Retrocaval ureter: A case diagnosed pre-operatively and treated successfully by a plastic operation. Br J Urol. 1949;21:209–14. doi: 10.1111/j.1464-410x.1949.tb10773.x. [DOI] [PubMed] [Google Scholar]
- 3.Webber RJ, Pandian SS, McClinton S, Hussey J. Retrograde balloon dilatation for pelviureteric junction obstruction: Long-term follow-up. J Endourol. 1997;11:239–42. doi: 10.1089/end.1997.11.239. [DOI] [PubMed] [Google Scholar]
- 4.Schuessler WW, Grune MT, Tecuanhuey LV, Preminger GM. Laparoscopic dismembered pyeloplasty. J Urol. 1993;150:1795–9. doi: 10.1016/s0022-5347(17)35898-6. [DOI] [PubMed] [Google Scholar]
- 5.Jarrett TW, Chan DY, Charambura TC, Fugita O, Kavoussi LR. Laparoscopic pyeloplasty: The first 100 cases. J Urol. 2002;167:1253–6. doi: 10.1016/s0022-5347(05)65276-7. [DOI] [PubMed] [Google Scholar]
- 6.Moon DA, El-Shazly MA, Chang CM, Gianduzzo TR, Eden CG. Laparoscopic pyeloplasty: Evolution of a new gold standard. Urology. 2006;67:932–6. doi: 10.1016/j.urology.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Maynes LJ, Levin BM, Webster TM, Baldwin D, Herrell SD. Measuring the true success of laparoscopic pyeloplasty. J Endourol. 2008;22:1193–8. doi: 10.1089/end.2008.0163. [DOI] [PubMed] [Google Scholar]
- 8.Mufarrij PW, Woods M, Shah OD, Palese MA, Berger AD, Thomas R, et al. Robotic dismembered pyeloplasty: A 6-year, multi-institutional experience. J Urol. 2008;180:1391–6. doi: 10.1016/j.juro.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Rukin NJ, Ashdown DA, Patel P, Liu S. The role of percutaneous endopyelotomy for ureteropelvic junction obstruction. Ann R Coll Surg Engl. 2007;89:153–6. doi: 10.1308/003588407X155824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan BJ, Rastinehad AR, Marcovich R, Smith AD, Lee BR. Trends in ureteropelvic junction obstruction management among urologists in the United States. Urology. 2005;65:260–4. doi: 10.1016/j.urology.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 11.Pouliot F, Lebel MH, Audet JF, Dujardin T. Determination of success by objective scintigraphic criteria after laparoscopic pyeloplasty. J Endourol. 2010;24:299–304. doi: 10.1089/end.2009.0134. [DOI] [PubMed] [Google Scholar]
- 12.Tan HJ, Ye Z, Roberts WW, Wolf JS. Failure after laparoscopic pyeloplasty: Prevention and management. J Endourol. 2011;25:1457–62. doi: 10.1089/end.2010.0647. [DOI] [PubMed] [Google Scholar]
- 13.Albani JM, Yost AJ, Streem SB. Ureteropelvic junction obstruction: Determining durability of endourological intervention. J Urol. 2004;171:579–82. doi: 10.1097/01.ju.0000104801.16269.24. [DOI] [PubMed] [Google Scholar]
- 14.Shadpour P, Haghighi R, Maghsoudi R, Etemedian M. Laparoscopic redo pyeloplasty after failed open surgery. Urol J. 2011;8:31–7. [PubMed] [Google Scholar]
- 15.Dimarco DS, Gettman MT, McGee SM, Chow GK, Leroy AJ, Slezak J, et al. Long-term success of antegrade endopyelotomy compared with pyeloplasty at a single institution. J Endourol. 2006;20:707–12. doi: 10.1089/end.2006.20.707. [DOI] [PubMed] [Google Scholar]
- 16.Breyer BN, McAninch JW, Whitson JM, Eisenberg ML, Mehdizadeh JF, Myers JB, et al. Multivariate analysis of risk factors for long-term urethroplasty outcome. J Urol. 2010;183:613–7. doi: 10.1016/j.juro.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Ramsay JW, Miller RA, Kellett MJ, Blackford HN, Wickham JE, Whitfield HN. Percutaneous pyelolysis: Indications, complications and results. Br J Urol. 1984;56:586–8. doi: 10.1111/j.1464-410x.1984.tb06122.x. [DOI] [PubMed] [Google Scholar]
- 18.Badlani G, Eshghi M, Smith AD. Percutaneous surgery for ureteropelvic junction obstruction (endopyelotomy): Technique and early results. J Urol. 1986;135:26–8. doi: 10.1016/s0022-5347(17)45503-0. [DOI] [PubMed] [Google Scholar]
- 19.Renner C, Frede T, Seemann O, Rassweiler J. Laser endopyelotomy: Minimally invasive therapy of ureteropelvic junction stenosis. J Endourol. 1998;12:537–44. doi: 10.1089/end.1998.12.537. [DOI] [PubMed] [Google Scholar]
- 20.Sampaio FJ, Favorito LA. Ureteropelvic junction stenosis: Vascular anatomical background for endopyelotomy. J Urol. 1993;150:1787–91. doi: 10.1016/s0022-5347(17)35896-2. [DOI] [PubMed] [Google Scholar]
- 21.Gerber GS, Lyon ES. Endopyelotomy: Patient selection, results, and complications. Urology. 1994;43:2–10. doi: 10.1016/s0090-4295(94)80253-x. [DOI] [PubMed] [Google Scholar]
- 22.Lam JS, Cooper KL, Greene TD, Gupta M. Impact of hydronephrosis and renal function on treatment outcome: Antegrade versus retrograde endopyelotomy. Urology. 2003;61:1107–11. doi: 10.1016/s0090-4295(03)00231-0. [DOI] [PubMed] [Google Scholar]
- 23.Nakada SY. Acucise endopyelotomy. Urology. 2000;55:277–82. doi: 10.1016/s0090-4295(99)00393-3. [DOI] [PubMed] [Google Scholar]
- 24.Szydełko T, Kasprzak J, Apoznański W, Zdrojowy R. Laparoscopic anterior y-v pyeloplasty: A valuable treatment option in patients with small or intrarenal pelvis. J Laparoendosc Adv Surg Tech A. 2010;20:627–30. doi: 10.1089/lap.2010.0223. [DOI] [PubMed] [Google Scholar]
- 25.Varkarakis IM, Bhayani SB, Allaf ME, Inagaki T, Ong AM, Kavoussi LR, et al. Management of secondary ureteropelvic junction obstruction after failed primary laparoscopic pyeloplasty. J Urol. 2004;172:180–2. doi: 10.1097/01.ju.0000132142.25717.08. [DOI] [PubMed] [Google Scholar]
- 26.Ng CS, Yost AJ, Streem SB. Management of failed primary intervention for ureteropelvic junction obstruction: 12-year, single-center experience. Urology. 2003;61:291–6. doi: 10.1016/s0090-4295(02)02160-x. [DOI] [PubMed] [Google Scholar]
- 27.Jabbour ME, Goldfischer ER, Klima WJ, Stravodimos KG, Smith AD. Endopyelotomy after failed pyeloplasty: The long-term results. J Urol. 1998;160:690–2. doi: 10.1016/S0022-5347(01)62757-5. [DOI] [PubMed] [Google Scholar]
- 28.Shalhav AL, Giusti G, Elbahnasy AM, Hoenig DM, McDougall EM, Smith DS, et al. Adult endopyelotomy: Impact of etiology and antegrade versus retrograde approach on outcome. J Urol. 1998;160:685–9. doi: 10.1016/S0022-5347(01)62755-1. [DOI] [PubMed] [Google Scholar]
- 29.Piaggio LA, Noh PH, González R. Reoperative laparoscopic pyeloplasty in children: Comparison with open surgery. J Urol. 2007;177:1878–82. doi: 10.1016/j.juro.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 30.Motola JA, Badlani GH, Smith AD. Results of 212 consecutive endopyelotomies: An 8-year followup. J Urol. 1993;149:453–6. doi: 10.1016/s0022-5347(17)36116-5. [DOI] [PubMed] [Google Scholar]
- 31.Kletscher BA, Segura JW. Efficacy of a new endoureterotomy balloon for the treatment of benign ureteral strictures using the porcine model. Urology. 1995;46:168–72. doi: 10.1016/s0090-4295(99)80188-5. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro EY, Cho JS, Srinivasan A, Seideman CA, Huckabay CP, Andonian S, et al. Long-term follow-up for salvage laparoscopic pyeloplasty after failed open pyeloplasty. Urology. 2009;73:115–8. doi: 10.1016/j.urology.2008.08.483. [DOI] [PubMed] [Google Scholar]
- 33.Sundaram CP, Grubb RL, 3rd, Rehman J, Yan Y, Chen C, Landman J, et al. Laparoscopic pyeloplasty for secondary ureteropelvic junction obstruction. J Urol. 2003;169:2037–40. doi: 10.1097/01.ju.0000067180.78134.da. [DOI] [PubMed] [Google Scholar]
- 34.Piaggio LA, Franc-Guimond J, Noh PH, Wehry M, Figueroa TE, Barthold J, et al. Transperitoneal laparoscopic pyeloplasty for primary repair of ureteropelvic junction obstruction in infants and children: Comparison with open surgery. J Urol. 2007;178:1579–83. doi: 10.1016/j.juro.2007.03.159. [DOI] [PubMed] [Google Scholar]
- 35.Basiri A, Behjati S, Zand S, Moghaddam SM. Laparoscopic pyeloplasty in secondary ureteropelvic junction obstruction after failed open surgery. J Endourol. 2007;21:1045–51. doi: 10.1089/end.2006.0414. [DOI] [PubMed] [Google Scholar]
- 36.Kausik S, Segura JW. Surgical management of ureteropelvic junction obstruction in adults. Int Braz J Urol. 2003;29:3–10. doi: 10.1590/s1677-55382003000100002. [DOI] [PubMed] [Google Scholar]
- 37.Inagaki T, Rha KH, Ong AM, Kavoussi LR, Jarrett TW. Laparoscopic pyeloplasty: Current status. BJU Int. 2005;95(Suppl 2):102–5. doi: 10.1111/j.1464-410X.2005.05208.x. [DOI] [PubMed] [Google Scholar]
- 38.Kavoussi LR, Albala DM, Clayman R. Outcome of secondary open surgical procedure in patients who failed primary endopyelotomy. Br J Urol. 1993;72:157–60. doi: 10.1111/j.1464-410x.1993.tb00678.x. [DOI] [PubMed] [Google Scholar]
- 39.Motola JA, Fried R, Badlani GH, Smith AD. Failed endopyelotomy: Implications for future surgery on the ureteropelvic junction. J Urol. 1993;150:821–3. doi: 10.1016/s0022-5347(17)35622-7. [DOI] [PubMed] [Google Scholar]
- 40.Gupta M, Tuncay OL, Smith AD. Open surgical exploration after failed endopyelotomy: A 12-year perspective. J Urol. 1997;157:1613–8. [PubMed] [Google Scholar]
- 41.Gettman MT, Peschel R, Neururer R, Bartsch G. A comparison of laparoscopic pyeloplasty performed with the daVinci robotic system versus standard laparoscopic techniques: Initial clinical results. Eur Urol. 2002;42:453–7. doi: 10.1016/s0302-2838(02)00373-1. [DOI] [PubMed] [Google Scholar]
- 42.Monn MF, Bahler CD, Schneider EB, Sundaram CP. Emerging trends in robotic pyeloplasty for the management of ureteropelvic junction obstruction in adults. J Urol. 2013;189:1352–7. doi: 10.1016/j.juro.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Sethi AS, Regan SM, Sundaram CP. Robot-assisted laparoscopic pyeloplasty with and without a ureteral stent. J Endourol. 2011;25:239–43. doi: 10.1089/end.2010.0192. [DOI] [PubMed] [Google Scholar]
- 44.Sivaraman A, Leveillee RJ, Patel MB, Chauhan S, Bracho JE, 2nd, Moore CR, et al. Robot-assisted laparoscopic dismembered pyeloplasty for ureteropelvic junction obstruction: A multi-institutional experience. Urology. 2012;79:351–5. doi: 10.1016/j.urology.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 45.Ross JH, Streem SB, Novick AC, Kay R, Montie J. Ureterocalicostomy for reconstruction of complicated pelviureteric junction obstruction. Br J Urol. 1990;65:322–5. doi: 10.1111/j.1464-410x.1990.tb14748.x. [DOI] [PubMed] [Google Scholar]
- 46.Osman T, Eltahawy I, Fawaz K, Shoeib M, Elshawaf H, El Halaby R. Ureterocalicostomy for treatment of complex cases of ureteropelvic junction obstruction in adults. Urology. 2011;78:202–7. doi: 10.1016/j.urology.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 47.Matlaga BR, Shah OD, Singh D, Streem SB, Assimos DG. Ureterocalicostomy: A contemporary experience. Urology. 2005;65:42–4. doi: 10.1016/j.urology.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 48.Radford AR, Thomas DF, Subramaniam R. Ureterocalicostomy in children: 12 years experience in a single centre. BJU Int. 2011;108:434–8. doi: 10.1111/j.1464-410X.2010.09925.x. [DOI] [PubMed] [Google Scholar]
- 49.Clayman RV. Laparoscopic ureterocalicostomy: Development of a technique simplified by application of Nitinol clips and a wet monopolar electrosurgery device. J Urol. 2005;174:1849–50. doi: 10.1016/s0022-5347(01)68810-4. [DOI] [PubMed] [Google Scholar]
- 50.Rack FJ. Ureteroileal neocystostomy; use of ileal segment as substitute ureter: Report of a case. J Am Med Assoc. 1953;152:516–7. doi: 10.1001/jama.1953.63690060004010b. [DOI] [PubMed] [Google Scholar]
- 51.Verduyckt FJ, Heesakkers JP, Debruyne FM. Long-term results of ileum interposition for ureteral obstruction. Eur Urol. 2002;42:181–7. doi: 10.1016/s0302-2838(02)00266-x. [DOI] [PubMed] [Google Scholar]
- 52.Stein RJ, Turna B, Patel NS, Weight CJ, Nguyen MM, Shah G, et al. Laparoscopic assisted ileal ureter: Technique, outcomes and comparison to the open procedure. J Urol. 2009;182:1032–9. doi: 10.1016/j.juro.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 53.Bluebond-Langner R, Rha KH, Pinto PA, Varkarakis J, Douyon E, Komotar RJ, et al. Laparoscopic-assisted renal autotransplantation. Urology. 2004;63:853–6. doi: 10.1016/j.urology.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 54.Minhas S, Irving HC, Lloyd SN, Eardley I, Browning AJ, Joyce AD. Extra-anatomic stents in ureteric obstruction: Experience and complications. BJU Int. 1999;84:762–4. doi: 10.1046/j.1464-410x.1999.00315.x. [DOI] [PubMed] [Google Scholar]
- 55.Lloyd SN, Tirukonda P, Biyani CS, Wah TM, Irving HC. The detour extra-anatomic stent – A permanent solution for benign and malignant ureteric obstruction? Eur Urol. 2007;52:193–8. doi: 10.1016/j.eururo.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 56.Steinman TI. Pain management in polycystic kidney disease. Am J Kidney Dis. 2000;35:770–2. doi: 10.1016/s0272-6386(00)70029-1. [DOI] [PubMed] [Google Scholar]
- 57.Thomas JC, DeMarco RT, Donohoe JM, Adams MC, Pope JC, 4th, Brock JW., 3rd Management of the failed pyeloplasty: A contemporary review. J Urol. 2005;174:2363–6. doi: 10.1097/01.ju.0000180420.11915.31. [DOI] [PubMed] [Google Scholar]
- 58.Braga LH, Lorenzo AJ, Skeldon S, Dave S, Bagli DJ, Khoury AE, et al. Failed pyeloplasty in children: Comparative analysis of retrograde endopyelotomy versus redo pyeloplasty. J Urol. 2007;178:2571–5. doi: 10.1016/j.juro.2007.08.050. [DOI] [PubMed] [Google Scholar]


