Abstract
We present a review on the current options for continent urinary diversion and their different indications on the basis of patient selection. In current clinical practice continent urinary diversion is being used world-wide in patients undergoing radical cystectomy and in severe cases of benign bladder pathologies. We also discuss the specific complications of continent urinary diversion and highlight the need to rigorously monitor these patients in the long- term specifically in terms of their renal function and cancer recurrence.
Keywords: Continent urinary diversion, outcomes, surgical techniques
INTRODUCTION
Urologists and patients recognize continent urinary diversion as a successful method of urinary reconstruction following cystectomy for lower urinary tract malignancy or severe functional and anatomical bladder abnormalities. Continent urinary diversion is performed by incorporating segments of the both small and large intestine into the urinary tract to create a urinary reservoir in the suitably selected patient.[1] Such techniques are often described by the intestinal segment used and whether the procedure provides complete continence or acts as a simple conduit conveying urine to the skin. A decision on the method used depends upon the indications for surgery, patient anatomy, renal function and personal choice.
The aim of reconstructive urology is to reproduce a functionally normal lower urinary tract in terms of storage, voiding, continence and preservation of renal function. An ideal method of bladder reconstruction would be non-refluxing, have low pressures, maintain continence and be non-absorptive.[1] In addition, patients actively seek maintenance of normal micturition and an undisturbed body image.
The expectations associated with bladder reconstruction following cystectomy have changed from simple diversion where the upper urinary tract was not protected to current day practice where anatomic and functional reconstruction of the urinary tract is almost equal to the natural, pre-operative state. Studies by Bjerre et al. suggest that continent forms of urinary diversion free from external appliances may be of great psychological and functional benefit in selected patients.[2]
All candidates for bladder reconstruction should undergo pre-operative investigation and counseling. Patients should be informed of potential complications including the impact on sexual function, life-style and body image. A detailed history and examination of the patient are essential. In particular, previous evidence of abdominal/pelvic surgery, irradiation, intestinal resection, renal failure, diverticulitis or inflammatory bowel diseases are important to note when determining method of reconstruction.
In terms of investigations, a complete blood chemistry is important, especially to assess renal function. Upper tract imaging should be performed to assess for evidence of renal scarring, calculus or hydronephrosis. In addition, voiding cystometrograms and cystoscopy should be performed to give an impression of the anatomy and function of the bladder, urethra and sphincter mechanism. Computed tomography scans and colonoscopy may also be performed to formally assess the bowel anatomy. This information is then collated to determine and plan the method of reconstruction.
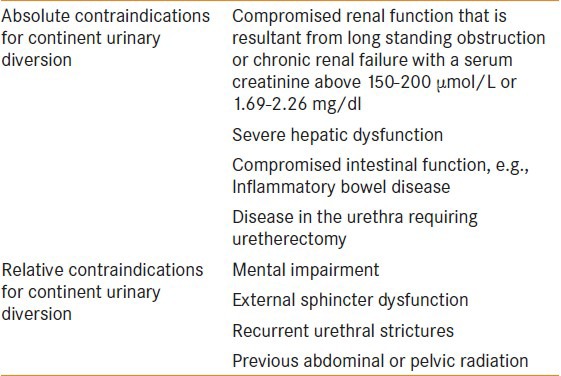
Indications, contraindications and patient selection for continent urinary diversion.
The absolute and relative contraindications[3] for continent urinary diversion are summarized in Table 1.
Table 1.
The absolute and relative contraindications of continent urinary diversion
Continence can be described as patient's ability to retain urine and void voluntarily. The continent urinary diversion technique allows patients an improved quality of life (QOL) by removing the need for a urostomy and the resultant associated social and emotional problems.[1]
Various segments of the intestinal tract can be used during continent urinary diversion. Continent reservoirs can be constructed of small or large bowel or a combination of the two. Principles for successful diversion include the opening and detubularization of the bowel segment.[4] Detubularized bowel segments provide larger capacity at a lower pressure and require a shorter length of intestine than do intact segments. This technique prevents the normal high-pressure contractions of the intestine. Urinary reservoirs should also be created with a large radius providing a larger capacity. The combination of these techniques creates a low pressure, high compliance continent urinary reservoir.[5]
Bissada highlighted the important attributes that all continent urinary diversions should have.[6] These include:
Construction of an adequate volume, low pressure reservoir with high compliance
Reliable continence mechanism
Prevention of intestinal ureteric reflux or stenosis
Simplicity in construction
Avoidance of use of synthetic material
Avoidance of the use of excessive lengths of bowel
Ease of catheterization
Avoidance of revision surgery
Good cosmetic appearance.
Continent urinary diversion can be divided into three major categories. The first group of procedures includes ureterosigmoidostomy and allows the excretion of urine by evacuation. A second group includes orthotopic voiding pouches, in patients with an intact sphincter mechanism. The third group includes continent diversions requiring catheterization for emptying urine from the created reservoir.
URETEROSIGMODIOSTOMY
Ureterosigmoidostomy is a method of urinary diversion away from the lower urinary tract into the sigmoid colon. This continent rectal reservoir technique allows the storage and excretion of urine through the rectum, utilizing the anus for continence.
The ureters should be identified below the common iliac arteries and mobilized carefully. Both ureters are re-implanted separately into the tenia coli using an anti-refluxing technique.
Smith in 1878 performed the first direct anastomosis of the ureters into the colon.[7] The procedure was associated with a high surgical mortality secondary to peritonitis and the development of pyelonephritis. Surgeons recognized that these major complications were the result of ascending infection from the rectum into the kidneys. Resultantly, surgical techniques were developed to implant the ureters into the colon in an anti-refluxing fashion. In 1911, Coffey introduced the concept of tunneling the ureter into the bowel.[8] This technique of continent urinary diversion dominated for several decades however, it is associated with several serious long-term complications including anastomotic colon cancer, ascending urinary infection, hyperchloremic metabolic acidosis, electrolyte imbalance, incontinence and urinary stones.[9]
Despite recommendations to abandon ureterosigmoidostomy as a result of its serious complications, the use of a rectal reservoir has never vanished completely from Urology literature.[10] Rabinovitch et al. considered its use in children during the 1980s.[11] Its resurgence in popularity is secondary to the observation that complications such as bowel frequency and urge incontinence (commonly seen in ureterosigmoidostomy) are abolished if the recto-sigmoid segment is detubularized.
The first attempt to lower rectal reservoir pressures was made by Kock et al. Their method of diversion to the rectum involved an ileal pouch, intussuscepted nipple valve at colorectal junction and antireflux implantation of ureters into the ileal implant.[12]
Fisch et al. developed the Mainz II pouch. They describe a simple detubularized ureterosigmoidostomy procedure. Detubularization of the bowel reduces the frequency and strength of contractions creating a higher capacity reservoir.[13] The authors in this paper[13] reported a 11% incidence of later complications on further experience with 73 patients offered a Mainz II pouch post cystectomy after a mean follow-up period of 127 months as stenosis at the uretral implantation site was most common in 6.8% of patients.
ORTHOTOPIC VOIDING POUCHES
Orthotopic diversion involves the creation of a large capacity, low pressure reservoir from colon or ileum that is connected to the native urethra. Voiding is achieved through relaxation of the external sphincter and an increase in intra-abdominal pressure (Valsalva maneuver). These patients may also need to perform intermittent catheterization (ISC) to relieve urinary retention or for irrigation of excess mucous.[14] the incidence of patients requiring ISC following an orthotopic neo-bladder formation ranges from 4% to 33% in current literature.[14,53]
The complications of neobladder formation may be divided into early and late according to the initial surgical procedure used as the complication may be not be directly related to the neobladder itself. Lee et al.[45] compared 37 Hautmann and 93 Studors ileal neobladdes and found no difference in the complication rates. Several complications are specific to orthotropic urinary diversion compared with ileal conduits. The rates of ventral incisional hernia are higher. This is attributed to chronic abdominal straining to void using the Valsalva maneuver. The risk of fistula formation between the neobladder and other pelvic organs (rectum, vagina and ileum) is still low (<10%) after patients have received radiotherapy.[54]
Patients with hepatic or renal insufficiency are generally not eligible for this procedure. The storage and interaction of urine with the bowel surface can also cause a number of metabolic complications.[1] In addition, patients with prostatic stromal or urethral involvement and many women with bladder neck disease are not suitable for this procedure as the urethra must be removed to ensure cancer control.[1]
All orthotopic neobladder operations share the same principles. Continence is dependent upon preservation of the external sphincteric apparatus and there is a risk of urethral cancer recurrence in those procedures performed for the treatment of bladder cancer.
The decision to proceed with bladder replacement is dependent on the risk of urethral recurrence and the continence of patient. Although initially reserved for male patients, work by Stein et al. has identified that this technique may be appropriate in certain female patients as well.[15]
Various intestinal segments may be used for the creation of a neobladder or reservoir. Bowel segments should be opened and refashioned (detubularized)[Figure 1]. This technique prevents the normal high pressure intestinal contractions. A large radius is also created to ensure a large capacity and lower pressures.[4]
Figure 1.
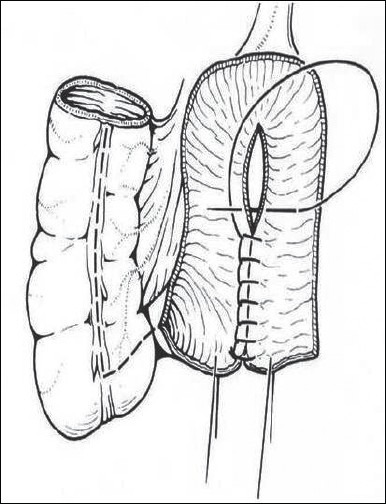
A Mainz ileocecal reservoir
The ileal neobladder is a fully detubularized distal ileal reservoir.[16] A 70 cm segment of ileum is selected ensuring that the terminal ileum is preserved. After the bowel is rejoined, the ileal segment is spatulated at its antimesenteric border. A U-shaped flap is created at the anterior mesenteric border – this is the new bladder neck. The bowel is then arranged in an M or a W shape and the limbs sutured together. A 1 cm button of tissue is removed at the new bladder neck to create the ileourethral anastomosis. A Foley catheter is then placed through the urethra into the ileum. Sutures then join the ileal segment to the urethral stump. LeDuc ureteral implants are created into the posterior ileal segment, which is then closed, creating the pouch.[14]
The Hautmann neobladder (W shaped), uses non-detubularized segments that can be the left intact at either end of the W and the ureters implanted individually into each segment.[17] The larger diameter and lower pressure of these reservoirs compared with non-detubularized bowel has resulted in improved continence rates.[1]
Studer et al. have popularized a low pressure bladder substitute.[18] This technique involves a 60 cm ileal segment. The ileal segment is rotated 120 degrees on its mesentery so that the proximal end reaches the right retroperitoneum. Both ileal ends are oversewn at this point. The distal 40 cm of the ileal segment is opened along the antimesenteric border and folded into a U-shape. The posterior section is joined to the limbs of the U. Standard uretroileal anastomoses are performed at the apex of the ileal segment. The ileal segment is then closed in a cup cystoplasty configuration. The reservoir is then anastomosed to the urethra.[14]
The success of Studer's pouch is the result of using the intact proximal limb to prevent the effects of reflux [Figure 2]. This allows excellent preservation of the upper urinary tracts and urinary continence.
Figure 2.
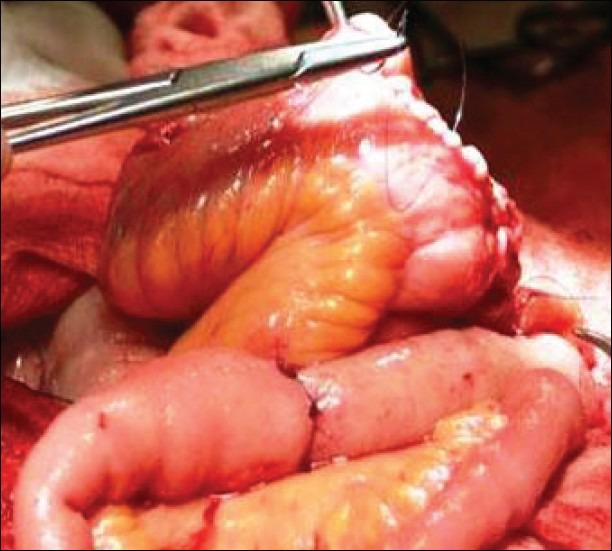
Studer bladder
The hemi-Kock system, constructed from approximately 40 cm of terminal ileum, comprises a detubularized and remodeled ileal plate with a stapled ileoileal intussusception to form a 1-way valve. The Studer method avoids the problems associated with the hemi-kock operation and the complications associated with the nipple valve construction.[14]
A neobladder constructed from the right colon has potential advantages including the use of absorbable staples and the preservation of the terminal ileum for enterohepatic circulation and vitamin B12 absorption.[14] Goldwasser, Mansson and Colleen have both described this technique.[19,20]
The sigmoid colon may also be used to create a voiding reservoir. This bowel segment can be moved easily to the urethral region.[21] In addition, the loss of the sigmoid colon has little impact on the nutritional status/bowel habits of patient. However, unfortunately, this segment of bowel is commonly associated with malignancy or diverticulitis and therefore may not be suitable for long-term diversion.[14] The three key elements that are involved in patients who developed a malignancy at the site of anastomosis between the ureters and sigmoid following a Ureterosigmoidostomy are due to the urinary stream, the fecal stream and the intestinal mucosa at the site of the anastomosis. The pathogenesis of tumor formation in linked to the formation of nitrosamine by fecal flora, but there is conflicting evidence to support this.[55] Current evidence indicates that the interaction of both urine and feces are necessary for carcinogenesis to occur, as perhaps the hydrolytic enzymes in the urine activate the conjugated carcinogens in the stool and the anastomotic site of the two streams are the most active as they have the greatest concentration.[55] Additional mechanisms attributing the development of malignancy at the anastomotic site include the role of the mechanical trauma or suture material causing chronic inflammation and DNA damage leading to the subsequent formation of adenoma or benign juvenile polyp developed may represent a precancerous lesion.
CONTINENT CATHETERISING POUCHES
Continent urinary diversion involves creating a catheterizable urinary reservoir within the abdomen using an ileal segment, the entire right colon or a combination of small and large bowel.[1] A continent reservoir requires the use of a catheter to drain urine from the reservoir several times per day. Failure to catheterize on a regular basis can cause serious problems including acute renal failure, perforation and infection. Suitable patients must therefore have sufficient hand-eye co-ordination and cognitive function to understand and perform ISC.[14]
The location of the catheter portal is commonly located either in the lower abdomen– for cosmetic reasons or at the umbilicus. The umbilicus is preferred for those patients in wheelchairs.
The construction of the continence mechanism for catheterising pouches is complex. Four techniques have been created.[14]
For right colon pouches, appendiceal techniques or ileocaecal valve plications are used
Tapered or imbricated terminal ileum and ileocaecal valve for right colonic pouches
Intussuscepted nipple valve
Hydraulic valve– Benchekroun nipple.
ILEAL RESERVOIRS
The most common continent ileostomy for urinary diversion is the Kock pouch. Kock et al. developed a continent ileal urinary reservoir.[22,23] It is created from 60 to 70 cm of small intestine. The proximal and distal 15 cm are used to create nipple valves for a ureteroileal antireflux anastomoses (inlet) and a catheterisable abdominal stoma (outlet). The nipple valves are created by intusscepting the bowel and stapling and securing it in place. The middle 40 cm of the ileal segment is then opened along the antimesenteric border and folded into a U-shape. The reservoir is then closed by folding the middle segment and suturing it in place. The ureters are then sutured to the proximal nipple valve and the distal nipple valve is brought to the skin as a catheterisable stoma.[14]
Problems with the afferent intusscepted antireflux nipple valve resulted in modifications to the technique. Stein et al. developed the T pouch.[24,25] The T pouch has several advantages over the Kock pouch as it uses a smaller ileal segment to create the antireflux technique, it prevents the need for intussusception, the blood supply is preserved and urine is not in direct contact with the implanted ileal segment.[1]
With fewer complications than the Kock pouch, the technique of creating a T pouch is more commonly used. More recently, Abol-Enein has created a Mansoura pouch, which uses a serosa-lined extramural valve for continence. His technique implants the ureters into a W shaped ileal reservoir through serosa lined extramural tunnels.[26]
Camey described a technique of bladder substitution where an intact segment of ileum was anastomosed directly to the urethra.[27] This involved mobilizing a 40 cm of ileum and anastomosing its midpoint to the urethra. The ureters were then reimplanted to either end of the ileal segment in an antirefluxing fashion. The main problem with this technique was the failure to detubularized the ileal segment resulting in a high incidence of incontinence.[1]
ILEOCECAL RESERVOIRS
Gilchrist et al. were the first to describe the use of the ileocaecal segment when creating continent urinary diversions. The natural anti-reflux mechanism of the ileocaecal valve served as the continence mechanism.[28] The Lundiana pouch was first described in 1977 and again is created from the ileocaecal segment utilizing an intussuscepted ileal nipple valve as the continence mechanism.[29] This work was reproduced by Mansson et al. in 1990.[30] Benchekroun has also produced similar results when developing developed his inkwell valve.[31]
The cathterisable Mainz pouch has been modified over the years again as a result of problems with the nipple valve.[32] The reservoir was created using an antimesenteric opening and spherical reconfiguration of the bowel segment. Ureters were implanted using submucosal tunnels. The intussuscepted terminal ileum was then used as the continence mechanism.
The Mainz group has also developed continence techniques utilizing the appendix. Right colonic pouches are being created using this appendiceal sphincter technology. Riedmiller et al. describe the use of the appendix as stoma.[33] This technique preserves the terminal ileum resulting in fewer metabolic complications. Following the success of this appendiceal continence mechanism they have also developed the construction of a Mitrofanoff (appendiceal) type tube for use in patients with an unsuitable or absent appendix.[34] More recently, Monti et al. have developed the technique using a short segment of ileum to form a pseudoappendix to reimplant into a colonic reservoir.[35]
The Penn pouch was the first continent diversion employing the Mitrofanoff principle, in which the appendix is used as the continence mechanism.[14] Appendiceal continence mechanisms have been reported by Mitrofanoff where the appendix is excised with a button of caecum and reversed on itself before tunneled reimplant.[36] Riedmiller et al. have also reported leaving the appendix attached to the caecum and burying it by rolling it back on itself.[37]
Rowland et al. introduced the Indiana pouch. This technique was a modification of the Mainz pouch where the ureters are implanted along the tenia libera.[38] Continence was ensured using the antireflux mechanism of the ileocaecal valve and the tapered ileal segment. In addition, lembert sutures were taken to reinforce the ileocaecal valve.[14]
Lockhart et al. created the Florida pouch which is formed from the caecum and ascending colon. Utilizing the LeDuc technique, the ureters are re-implanted. The ileocaecal valve along with double plication of the efferent segment creates the continence mechanism.[39] The Miami pouch uses the same bowel segments at the Florida pouch however it differs in that the segments were opened antimesenterically and reformed in a U-shape.[40] The terminal ileum, as the efferent segment, is tapered and reinforced by proximal sutures.
COLONIC RESERVOIRS
In addition to the Florida and the Miami pouches, Leissner et al. have created the transverse pouch (Mainz Pouch III). Consisting of transverse and ascending or descending colon, a U-shaped reservoir is created. Continence is generated from the use of a tailored bowel segment in the anterior pouch wall. This pouch was developed for those patients undergoing previous pelvic radiotherapy.[41]
Excellent continence rates are achieved with all continent catheterizing pouches following urinary diversion. Data on continence rates are summarized in Table 2. In general, continence rates of cutaneous catheterisable pouches are higher than those for orthotopic neobladders.
Table 2.
Continence rates[42]
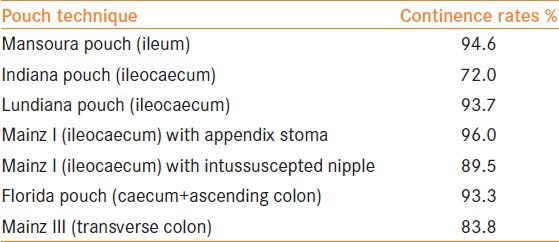
Incontinence rates with catheterizable pouches are low (2-16%).[43,44] Reported continence rates appear to be better for the appendix stoma and the ileocecal valve as compared with the intussuscepted ileal nipple.[44] These findings may be the result of the larger diameter of the intussuscepted nipple, urinary retention due to less catheterization and damage to the efferent limb following catheter insertion.
Night time incontinence is a problem commonly associated with neobladders and can occur in about 20-50% of patients.[45,46] Day time incontinence is lower and ranges from <10% to 33%. Of note, continence rates improve with time in patients with neobladders. This is likely secondary to improved noebladder capacity. Work by Studer et al. identified day time continence rates of 92% and night time rates of 80%, 2 years following neobladder formation.[47] This was further supported by Perimenis et al. who also found that continence rates improved with time and increasing neobladder capacity.[48]
QOL
Adaptation and acceptance of urinary diversion into a patient's life is vital in recovering QOL. There are a number of retrospective studies that have assessed the difference in QOL between continent catheterizable pouches and orthotopic neobladders. No differences were identified between either type of urinary diversion.[49,50,51]
More recently, Large et al. identified no significant differences between either type of urinary diversion in terms of physical, social, emotional, functional and specific health-related QOL.[49] Pazooki et al. identified that the majority of patients were satisfied or very satisfied with their urinary diversion, however, those who received orthotopic neobladder were troubled by urinary leakage.[51]
Somani et al. analyzed the QoL and body image in a prospective cohort study and also performed a systematic review of 40 studies. Comparing CCD to orthotopic bladder substitutions, similar results were obtained between all groups.[52]
CONCLUSION
Continent urinary diversion following radical cystectomy represents an established treatment option. Many different techniques have been developed and improved over the last 50 years.
Different simple and reproducible mechanisms offer highly satisfactory outcomes. Long-term data on different surgical techniques are comparable regarding functional outcomes and QOL. The incidence of complications is acceptable.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Konety B.R, Allareddy V, Herr H. Complications after radical cystectomy: analysis of population-based data. Urology 68. 2006:58–64. doi: 10.1016/j.urology.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 2.Bjerre BD, Johansen C, Steven K. Health-related quality of life after cystectomy: Bladder substitution compared with ileal conduit diversion. A questionnaire survey. Br J Urol. 1995;75:200–5. doi: 10.1111/j.1464-410x.1995.tb07312.x. [DOI] [PubMed] [Google Scholar]
- 3.Park J, Ahn H. Radical cystectomy and orthotopic bladder substitution using ileum. Korean J Urol. 2011 Apr;52:233–240. doi: 10.4111/kju.2011.52.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinman F., Jr Selection of intestinal segments for bladder substitution: Physical and physiological characteristics. J Urol. 1988;139:519–23. doi: 10.1016/s0022-5347(17)42509-2. [DOI] [PubMed] [Google Scholar]
- 5.Nagele U. Urinary diversion following cystectomy. EAU Update Series. 2005;3:129–37. [Google Scholar]
- 6.Bissada NK. Characteristics and use of the in situ appendix as a continent catheterization stoma for continent urinary diversion in adults. J Urol. 1993;150:151–2. doi: 10.1016/s0022-5347(17)35419-8. [DOI] [PubMed] [Google Scholar]
- 7.Smith T. An account of an unsuccessful attempt to treat extroversion of the bladder by a new operation. St Barth Hosp Rep. 1870;15:29. [Google Scholar]
- 8.Pannek J, Senge T. History of urinary diversion. Urol Int. 1998;60:1–10. doi: 10.1159/000030195. [DOI] [PubMed] [Google Scholar]
- 9.Campbells Urology [Google Scholar]
- 10.Gerharz EW, Köhl UN, Weingärtner K, Kleinhans BJ, Melekos MD, Riedmiller H. Experience with the Mainz modification of ureterosigmoidostomy. Br J Surg. 1998;85:1512–6. doi: 10.1046/j.1365-2168.1998.00904.x. [DOI] [PubMed] [Google Scholar]
- 11.Rabinovitch HH. Ureterosigmoidostomy in children – Revival or demise? J Urol. 1980;124:552. doi: 10.1016/s0022-5347(17)55537-8. [DOI] [PubMed] [Google Scholar]
- 12.Kock NG, Ghoneim MA, Lycke KG, Mahran MR. Urinary diversion to the augmented and valved rectum: Preliminary results with a novel surgical procedure. J Urol. 1988;140:1375–9. doi: 10.1016/s0022-5347(17)42049-0. [DOI] [PubMed] [Google Scholar]
- 13.Fisch M, Wammack R, Müller SC, Hohenfellner R. The Mainz pouch II (sigma rectum pouch) J Urol. 1993;149:258–63. doi: 10.1016/s0022-5347(17)36050-0. [DOI] [PubMed] [Google Scholar]
- 14.Benson M, et al. Continent Urinary Diversion. Reconstructive Urology. Urol Clin North Am. 1999;26:125–47. doi: 10.1016/s0094-0143(99)80011-1. [DOI] [PubMed] [Google Scholar]
- 15.Stein J, et al. Orthotopic lower urinary tract reconstruction in women using the Kock ileal neobladder:Updated experiences in 34 patients. J Urol. 1997 Aug;158(2):400–5. [PubMed] [Google Scholar]
- 16.Hautmann RE, Egghart G, Frohneberg D, Miller K. The ileal neobladder. J Urol. 1988;139:39–42. doi: 10.1016/s0022-5347(17)42283-x. [DOI] [PubMed] [Google Scholar]
- 17.Hautmann RE, de Petriconi R, Gottfried HW, Kleinschmidt K, Mattes R, Paiss T. The ileal neobladder: Complications and functional results in 363 patients after 11 years of followup. J Urol. 1999;161:422–7. doi: 10.1016/s0022-5347(01)61909-8. [DOI] [PubMed] [Google Scholar]
- 18.Studer UE, Ackermann D, Casanova GA, Zingg EJ. A newer form of bladder substitute based on historical perspectives. Semin Urol. 1988;6:57–65. [PubMed] [Google Scholar]
- 19.Goldwasser B. The colonic orthotopic bladder. Urology. 1995;45:190–2. doi: 10.1016/0090-4295(95)80003-4. [DOI] [PubMed] [Google Scholar]
- 20.Månsson W, Colleen S. Experience with a detubularised right colonic segment for bladder replacement. Scand J Urol Nephol. 1990;24:53–6. doi: 10.3109/00365599009180360. [DOI] [PubMed] [Google Scholar]
- 21.Reddy PK. Detubularized sigmoid reservoir for bladder replacement after cystoprostatectomy. Preliminary report of new configuration. Urology. 1987;29:625–8. doi: 10.1016/0090-4295(87)90107-5. [DOI] [PubMed] [Google Scholar]
- 22.Kock NG, Nilson AE, Nilsson LO, Norlén LJ, Philipson BM. Urinary diversion via a continent ileal reservoir: Clinical results in 12 patients. J Urol. 1982;128:469–75. doi: 10.1016/s0022-5347(17)53001-3. [DOI] [PubMed] [Google Scholar]
- 23.Jagenburg R, Kock NG, Philipson B. Vitamin B12 absorption in patients with continent ileostomy. Scand J Gastroenterol. 1975;10:141–4. [PubMed] [Google Scholar]
- 24.Stein JP, Lieskovsky G, Ginsberg DA, Bochner BH, Skinner DG. The T pouch: An orthotopic ileal neobladder incorporating a serosal lined ileal antireflux technique. J Urol. 1998;159:1836–42. doi: 10.1016/S0022-5347(01)63170-7. [DOI] [PubMed] [Google Scholar]
- 25.Bochner BH, Stein JP, Ginsberg DA, Kurzrock E, Figueroa A, Skinner DG. A serous lined antireflux valve: in vivo fluorourodynamic evaluation of antireflux continence mechanism. J Urol. 1998;160:112–5. doi: 10.1016/s0022-5347(01)63049-0. [DOI] [PubMed] [Google Scholar]
- 26.Abol-Enein H, Salem M, Mesbah A, Abdel-Latif M, Kamal M, Shabaan A, et al. Continent cutaneous ileal pouch using the serous lined extramural valves. The Mansoura experience in more than 100 patients. J Urol. 2004;172:588–91. doi: 10.1097/01.ju.0000129437.33688.4d. [DOI] [PubMed] [Google Scholar]
- 27.Lilien OM, Camey M. 25-year experience with replacement of the human bladder (Camey procedure) J Urol. 1984;132:886–91. doi: 10.1016/s0022-5347(17)49934-4. [DOI] [PubMed] [Google Scholar]
- 28.Gilchrist RK, Merricks JW, Hamlin HH, Rieger IT. Construction of a substitute bladder and urethra. Surg Gynecol Obstet. 1950;90:752–60. [PubMed] [Google Scholar]
- 29.Mansson W, Sundin T. Experience with a continent caecal reservoir in urinary diversion. Scand J Urol Nephrol. 1978;48(Suppl):4. doi: 10.3109/00365598409180201. [DOI] [PubMed] [Google Scholar]
- 30.Månsson W, Davidsson T, Colleen S. The detubularized right colonic segment as urinary reservoir: Evolution of technique for continent diversion. J Urol. 1990;144:1359–61. doi: 10.1016/s0022-5347(17)39740-9. [DOI] [PubMed] [Google Scholar]
- 31.Benchekroun A, Lakrissa A, Tazi A, Mikou A. [Continent ileo-cecal bladder:33 cases (author's transl)] Chirurgie. 1980;106:668–72. [PubMed] [Google Scholar]
- 32.Thüroff J. W, Riedmiller H, Fisch M, Stein R, Hampel C, Hohenfellner R. “Mainz pouch continent cutaneous diversion,”. British Journal of Urology International. 2010;106(11):1830–1854. doi: 10.1111/j.1464-410X.2010.09773.x. [DOI] [PubMed] [Google Scholar]
- 33.Riedmiller H, Bürger R, Müller S, Thüroff J, Hohenfellner R. Continent appendix stoma: A modification of the Mainz pouch technique. J Urol. 1990;143:1115–7. doi: 10.1016/s0022-5347(17)40200-x. [DOI] [PubMed] [Google Scholar]
- 34.Lampel A, Hohenfellner M, Schultz-Lampel D, Thüroff JW. In situ tunneled bowel flap tubes:2 new techniques of a continent outlet for Mainz pouch cutaneous diversion. J Urol. 1995;153:308–15. doi: 10.1097/00005392-199502000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Monti PR, Lara RC, Dutra MA, de Carvalho JR. New techniques for construction of efferent conduits based on the Mitrofanoff principle. Urology. 1997;49:112–5. doi: 10.1016/S0090-4295(96)00503-1. [DOI] [PubMed] [Google Scholar]
- 36.Duckett JW, Snyder HM., 3rd Use of the Mitrofanoff principle in urinary reconstruction. Urol Clin North Am. 1986;13:271–4. [PubMed] [Google Scholar]
- 37.Riedmiller H, Bürger R, Müller S, et al. Continent appendix stoma: a modification of the Mainz pouch technique. J Urol. 1990 Jun;143:1115–7. doi: 10.1016/s0022-5347(17)40200-x. [DOI] [PubMed] [Google Scholar]
- 38.Rowland RG, Mitchell ME, Bihrle R, Kahnoski RJ, Piser JE. Indiana continent urinary reservoir. J Urol. 1987;137:1136–9. doi: 10.1016/s0022-5347(17)44428-4. [DOI] [PubMed] [Google Scholar]
- 39.Lockhart JL. Remodeled right colon: An alternative urinary reservoir. J Urol. 1987;138:730–4. doi: 10.1016/s0022-5347(17)43355-6. [DOI] [PubMed] [Google Scholar]
- 40.Bejany DE, Politano VA. Stapled and nonstapled tapered distal ileum for construction of a continent colonic urinary reservoir. J Urol. 1988;140:491–4. doi: 10.1016/s0022-5347(17)41699-5. [DOI] [PubMed] [Google Scholar]
- 41.Leissner J, Black P, Fisch M, Höckel M, Hohenfellner R. Colon pouch (Mainz pouch III) for continent urinary diversion after pelvic irradiation. Urology. 2000;56:798–802. doi: 10.1016/s0090-4295(00)00789-5. [DOI] [PubMed] [Google Scholar]
- 42.Michael Rink, Luis Kluth, Eike Eichelberg, Margit Fisch, Roland Dahlem, Rink M, et al. Continent Catheterizable Pouches for Urinary Diversion. Eur Urol Supp. 2010:754–762. [Google Scholar]
- 43.Stolzenburg JU, Schwalenberg T, Liatsikos EN, Sakelaropoulos G, Rödder K, Hohenfellner R, et al. Colon pouch (Mainz III) for continent urinary diversion. BJU Int. 2007;99:1473–7. doi: 10.1111/j.1464-410X.2007.06767.x. [DOI] [PubMed] [Google Scholar]
- 44.Wiesner C, Bonfig R, Stein R, Gerharz EW, Pahernik S, Riedmiller H, et al. Continent cutaneous urinary diversion: Long-term follow-up of more than 800 patients with ileocecal reservoirs. World J Urol. 2006;24:315–8. doi: 10.1007/s00345-006-0078-y. [DOI] [PubMed] [Google Scholar]
- 45.Lee KS, Montie JE, Dunn RL, Lee CT. Hautmann and Studer orthotopic neobladders: A contemporary experience. J Urol. 2003;169:2188–91. doi: 10.1097/01.ju.0000063941.31687.26. [DOI] [PubMed] [Google Scholar]
- 46.Meyer JP, Drake B, Boorer J, Gillatt D, Persad R, Fawcett D. A three-centre experience of orthotopic neobladder reconstruction after radical cystectomy: Initial results. BJU Int. 2004;94:1317–21. doi: 10.1111/j.1464-410X.2004.05164.x. [DOI] [PubMed] [Google Scholar]
- 47.Studer UE, Danuser H, Hochreiter W, Springer JP, Turner WH, Zingg EJ. Summary of 10 years’ experience with an ileal low-pressure bladder substitute combined with an afferent tubular isoperistaltic segment. World J Urol. 1996;14:29–39. doi: 10.1007/BF01836342. [DOI] [PubMed] [Google Scholar]
- 48.Perimenis P, Burkhard FC, Kessler TM, Gramann T, Studer UE. Ileal orthotopic bladder substitute combined with an afferent tubular segment: Long-term upper urinary tract changes and voiding pattern. Eur Urol. 2004;46:604–9. doi: 10.1016/j.eururo.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 49.Large MC, Katz MH, Shikanov S, Eggener SE, Steinberg GD. Orthotopic neobladder versus Indiana pouch in women: A comparison of health related quality of life outcomes. J Urol. 2010;183:201–6. doi: 10.1016/j.juro.2009.08.148. [DOI] [PubMed] [Google Scholar]
- 50.Månsson A, Davidsson T, Hunt S, Månsson W. The quality of life in men after radical cystectomy with a continent cutaneous diversion or orthotopic bladder substitution: Is there a difference? BJU Int. 2002;90:386–90. doi: 10.1046/j.1464-410x.2002.02899.x. [DOI] [PubMed] [Google Scholar]
- 51.Pazooki D, Edlund C, Dahlstrand C, Lindholm E, Törnqvist H, Jonsson O. Continent cutaneous urinary diversion is still a valid alternative after cystectomy for bladder carcinoma. Scand J Urol Nephrol. 2005;39:468–73. doi: 10.1080/00365590500191001. [DOI] [PubMed] [Google Scholar]
- 52.Somani BK, Gimlin D, Fayers P, N’dow J. Quality of life and body image for bladder cancer patients undergoing radical cystectomy and urinary diversion – A prospective cohort study with a systematic review of literature. Urology. 2009;74:1138–43. doi: 10.1016/j.urology.2009.05.087. [DOI] [PubMed] [Google Scholar]
- 53.Yoneda T, Igawa M, Shiina H, Shigeno K, Urakami S. Postoperative morbidity, functional results and quality of life of patients following orthotopic neobladder reconstruction. Int J Urol. 2003;10:119–25. doi: 10.1046/j.1442-2042.2003.00591.x. [DOI] [PubMed] [Google Scholar]
- 54.Mills RD, Studer UE. Female orthotopic bladder substitution: A good operation in the right circumstances. J Urol. 2000;163:1501–4. doi: 10.1016/s0022-5347(05)67651-3. [DOI] [PubMed] [Google Scholar]
- 55.Kälble T, Tricker AR, Berger M, Amelung F, Waldherr R, Hothorn L, et al. Tumor induction in a rat model for ureterosigmoidostomy without evidence of nitrosamine formation. J Urol. 1991;146:862–6. doi: 10.1016/s0022-5347(17)37949-1. [DOI] [PubMed] [Google Scholar]


