Abstract
Patients with a urinary bladder malignancy or severe anatomical/functional bladder abnormalities may be candidates for urinary diversion at the time of cystectomy. Most urinary diversions are constructed from intestinal segments. Urological surgeons who perform urinary diversion surgery should be aware of the physiological and metabolic changes that can occur when intestinal segments are in direct contact with urine. The complications associated with urinary diversion are both acute and chronic. The most important factor associated with the development of metabolic complications following urinary diversion is the length of time that the urine is in contact with the bowel and the type of bowel segment used for urinary diversion. In this review, we describe the metabolic complications associated with urinary diversion, their characteristic clinical presentation, follow-up, and specific treatment.
Keywords: Metabolic complications, physiology, urinary intestinal diversion
INTRODUCTION
Patients with bladder malignancies or severe anatomical/functional bladder abnormalities may be candidates for urinary diversion at the time of cystectomy. The aim of reconstructive urology is to produce a urinary reservoir that is as functionally similar to the native urinary bladder as possible in terms of storage, voiding, continence and preservation of renal function.[1]
The physiological issues with the use of bowel in urinary diversion arise as the intestine itself was never meant to serve either as a conduit for urine or for storage of urine. The patient's compensatory mechanisms initially adapt to the use of bowel and then physiological compensatory mechanisms prevent the onset of the metabolic complications. When a segment of the bowel is removed for this reconstruction, the re-absorption area of the remaining bowel decreases but this segment retains its absorbing and secreting characteristics.[1,2]
The principal issue with the use of bowel in urinary diversion is due to the fact that the bowel continues to produce mucus and continues to perform its main physiological function of secretion and re-absorption. Over time, the mucosa of the colon and the ileum atrophies. The exact mechanism of this process continues to be evaluated, but there is strong evidence to suggest that the lack of stimulation of the bowel mucosa due to the possible lack of feculent matter being in contact with the bowel leads to this.[2,3,4]
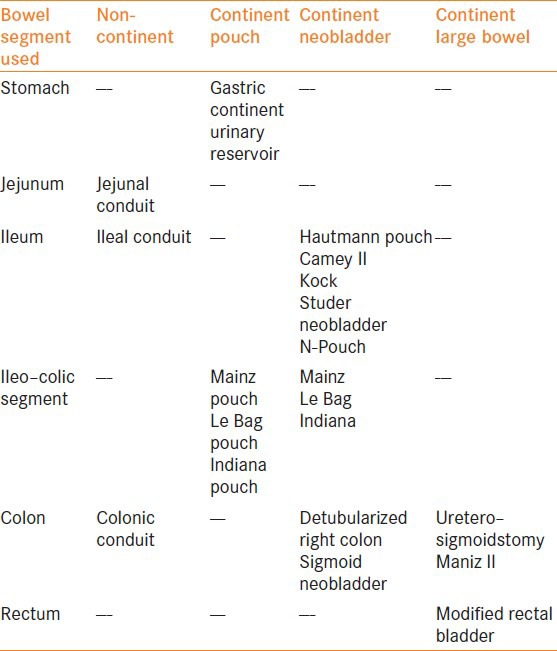
The choice of the type of urinary diversion requires careful clinical and quality of life (QOL) assessments with the patient. The current techniques of urinary diversion range from a uretero–ileo–cutaneostomy[1] to detubularized ileal or ileocolonic segments that can be used to create continent diversions or orthotropic neobladders.[2,3,4,5,6,7,8,9,10,11,12] A summary of the types of urinary diversions is presented in Table 1.
Table 1.
Types of urinary diversion segments
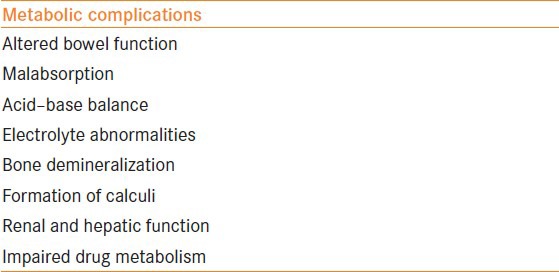
We review the metabolic complications associated with urinary diversion and describe the characteristic clinical presentation, follow-up and treatment of these complications. The extent of the metabolic problem depends on the length and the type of bowel segment used as well as the atrophy of the bowel mucosa subsequent to chronic urinary diversion, baseline renal function, baseline liver function, age, prior chemotherapy/radiotherapy and concomitant comorbidities.[2,5] A summary of the metabolic complications in patients undergoing urinary diversion is presented in Table 2.
Table 2.
Summary of the metabolic complications following urinary diversion
ALTERED BOWEL FUNCTION/MALABSORPTION
A major concern when intestinal segments are used during urinary reconstruction is the resultant effect on bowel function. Patients undergoing a urinary diversion often report diarrhea. This symptom has a significant impact on the patient's QOL.[7] Following the loss of intestinal absorptive surfaces to create a diversion, a number of nutritional complications can also occur. The removal of the terminal ileum or the ileocaecal valve can interfere with the absorption of bile salts that lead to the colonization of bacteria within the terminal ileum leading to the patients developing chronic diarrhea. Hence, the terminal ileum is not routinely used during urinary diversion to prevent the side-effects of Vitamin B12 deficiency. Bile salts are normally reabsorbed and recycled at the terminal ileum. They are essential for fat digestion and for the uptake of fat-soluble vitamins A and D.
The resection of the terminal ileum results in a reduction in bile salt and fat absorption. Larger quantities of bile salts and un-metabolized lipids enter the colon, causing mucosal irritation and steatorrhea. Loss of the ileocaecal valve following resection can also cause diarrhea following bacterial overgrowth of the remaining ileum, reducing its absorptive capacity and thus preventing bile salt and fat absorption.[13] Colonic resections may also cause diarrhea. This occurs when the shortened colonic segment does not absorb the alkaline ileal contents during urinary diversion. This results in diarrhea, dehydration and acidosis. Vitamin B12 is also absorbed at the terminal ileum. Malabsorption has been reported in patients following ileal or gastric resection. Patients with B12 deficiency may present with megaloblastic anemia and peripheral nerve paraesthesia. Because of large B12 stores, deficiencies often do not present for several years.[14] Treatment of persistent diarrhea after urinary diversion consists of cholestyramine (a resin that binds bile salts), increased dietary fiber and the use of Loperamide.
Acid–Base Abnormalities
The principal metabolic abnormality in patients undergoing a urinary diversion is the tendency for patients to develop a respiratory-compensated metabolic acidosis, normally to a very mild degree. This results in further sequelae such as significant demineralization of the skeleton, which has an effect on patient growth, especially in young patients. The patient's baseline renal function is important in the initialization of the compensatory process. The degree, nature and severity of a patient's symptoms largely depend on the type of bowel segment used. The metabolic complications as per the type of bowel segment used are summarized in Table 3.
Table 3.
Metabolic complications and changes using different gut segments
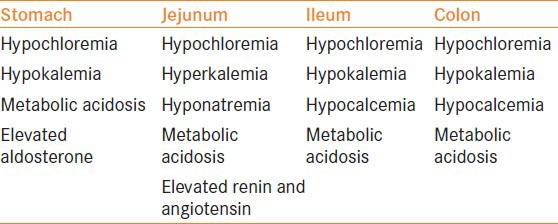
When a patient's jejunum is used, the metabolic complication is hyponatremic, hyperkalemia metabolic acidosis, which can be very severe clinically. The clinical symptoms of the metabolic complications as per the different gut segment used are summarized in Table 4.
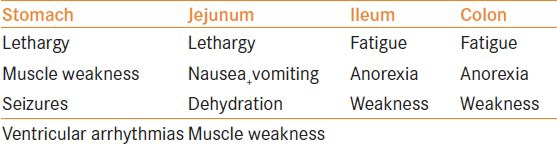
Table 4.
Clinical characteristics of the metabolic complications as per the gut segment used
There is no set percentage on how many patients will develop a metabolic complication following a urinary diversion as the presentation and onset of a metabolic complication differ greatly on the patient's compensatory mechanisms themselves. If a patient's blood plasma chloride level is taken, then approximately 15% of the patients will have a degree of persistent compensatory metabolic acidosis.[15] The exact etiology of metabolic acidosis in patients undergoing a urinary diversion is still unclear, but the work from McDougal and co-workers has largely contributed to our understanding of the pathogenesis of the condition.[16] They[16] showed that water transport across the intestinal epithelium normally follows its osmotic gradient and is dependent on the permeability of the intercellular junction of the luminal cells. The extent of how leaky the intercellular junction is depends on the segment of the gut. With progression of the gut from the stomach to the ileum, the leaky junctions become less leaky. Hence, the stomach is very leaky but the bidirectional fluxes cancel each other. The lack of bidirectional currents makes the jejunum very leaky and hence this is considered to be the reason of the high metabolic complication rates. The ileum is better than the jejunum but is less leaky. The colon is the most efficient segment for a diversion as it has very tight junctions and this reduces the extent of water loss. A hyperchloremic metabolic acidosis is observed with ileal or colonic segments when they are interposed into the urinary tract. In the bowel, sodium and bicarbonate are secreted in exchange for hydrogen and chloride ions.
When urine is in contact with the bowel wall, ammonia, hydrogen and chloride are also reabsorbed. This results in a chronic acid load. A large bowel surface area, prolonged contact time, patient co-morbidities and pre-existing renal failure contribute to the development of metabolic complications. Patients with reservoirs are at increased risk than those with simple conduits due to the prolonged contact time with urine and the larger surface area.[15] Patients may present with weakness, anorexia and vomiting.
A hypochloremia, hypokalemia metabolic acidosis is seen when the stomach is used. This is a significant problem in patients who are dehydrated or who have renal failure as the bicarbonate excretion is impaired.[4] Patients may present with lethargy, seizures, respiratory complications and ventricular arrhythmias.
The use of jejunum in urinary intestinal diversion can result in hyponatremia, hypochloremia, hyperkalemia and acidosis in up to 40% of the patients. The severity of this disorder is dependent on the segment of the jejunum used. The biochemical abnormality seen is the result of increased secretion of sodium and chloride with increased reabsorption of hydrogen and potassium ions. The patient also becomes dehydrated as large volumes of water are secreted with the sodium chloride. This resultant hypovolemia and hyperkalemia stimulates the renin–angiotensin system, causing aldosterone to be released.[4] Patients often present with lethargy, nausea and vomiting, dehydration and muscle weakness.
Electrolyte Abnormalities
Following urinary diversion, there are a number of electrolyte abnormalities that occur when intestinal segments are incorporated into the urinary system. These include hypokalemia, hypocalcemia and hypomagnesemia.[13]
Most electrolyte shifts in the gut are trans-cellular, although a certain degree of para-cellular movement of ions can occur. Sodium is absorbed in much the same way via trans-cellular and para-cellular routes in the ileum and the colon. The ileum absorbs less chloride but more potassium than the colon. In the ileum, the sodium loss is clinically not an issue but in certain cases of severe metabolic acidosis potassium, calcium and magnesium loss can occur that leads to the development of hypokalemia, hypocalcemia and hypomagnesemia. The loss of potassium ions is secondary to intestinal losses and by renal wasting. Patients following urinary diversion can become acidotic as previously mentioned. It is important to be aware of this acid–base balance as correction of the acidosis can worsen the potassium losses.
The mechanism of the development of hyperchloremic acidosis occurs due to ammonia absorption. Ammonium ions dissociate into ammonia and hydrogen. The ammonia then dissociates into cells and the hydrogen ion is actively absorbed in exchange for sodium. The ammonium itself may be absorbed as a substitute for potassium through the potassium channels. This causes the ammonium to enter the ileal or colonic luminal cell, and this is further balanced by the absorption of chloride. Hence, the ammonium and chloride are absorbed and cause a hyperchloremic acidosis and bicarbonate loss.
Hypocalcemia is again the result of renal wasting and the reduction of the body calcium stores. The chronic metabolic acidosis seen following urinary diversion is buffered by bone carbonate. This process releases calcium from the bone. The ongoing acidosis prevents renal calcium re-absorption. Hypocalcemia results in bone demineralization, but this complication is more common in patients with nutritional and renal wasting.
Bone Metabolism
The chronic metabolic hyperchloremic acidosis observed following urinary diversion is buffered by the bone minerals calcium, carbonate and sodium. The mobilization of these minerals results in demineralization of bone. The acidosis observed also impairs the renal activation of vitamin D, which is vital for normal bone mineralization. The intestinal absorption of vitamin D and calcium is also impaired following the loss of absorptive surfaces when intestinal segments are used during diversion.[17] Bone metabolism issues of demineralization are most important in growing children who have undergone a urinary diversion. Why acidosis leads to skeletal demineralization in children and not in adults, including post-menopausal women is still not clear and is a matter of continuing investigation. Acidosis also activates osteoclasts, resulting in bone reabsorption. Because of the use of bowel segments in urinary diversion, intestinal absorption of calcium and vitamin D can also be impaired. Parathormone does not seem to play a role in demineralization after urinary diversion. Again, patients with decreased renal function are particularly at risk for this sequence. Severe bone demineralization leading to osteomalacia in adults or rickets in children is rare. In severe cases, the bone minerals are replaced by osteoid bone, which can result in osteomalacia. Patients may present with pain in their weight-bearing joints.
Calculi
Patients undergoing urinary intestinal diversion are at increased risk of stone formation.[18] Those most susceptible include patients with hyperchloremic metabolic acidosis, chronic urinary tract infections with a urea-splitting organism, e.g. Proteus, Klebsiella and Pseudomonas, and the presence of foreign bodies such as staples or sutures, which can act as a nidus for stone formation.[19]
The development of systemic acidosis increases the risk of stone formation.[19] The prolonged contact of urine with the intestinal surface encourages the exchange of chloride with bicarbonate. The loss of bicarbonate results in acidosis and hypercalciuria, resulting in calcium stones. The use of the ileum in urinary intestinal diversion may result in excess bile salts binding calcium and causing increased absorption of oxalate, increasing the risk of oxalate calculi. In addition, excess conduit length, dehydration and urine stasis may put patients at an increased risk of stone formation.
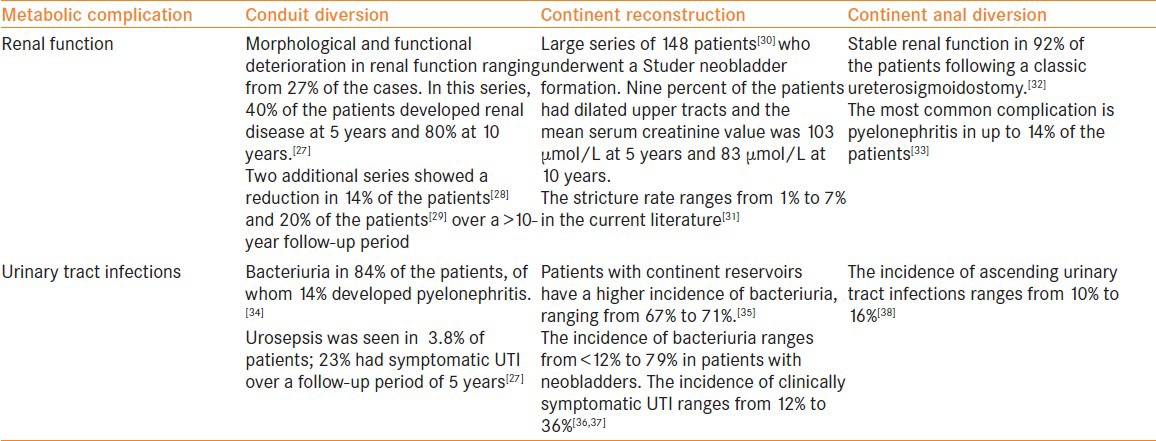
Renal Function
Renal function can be impaired following urinary diversion as a result of ureteric obstruction. This is often the result of stenosis of uretero–intestinal anastomosis. Renal function can also be affected by recurrent urinary infections and urinary lithiasis.[12,13] From the age of 40 years onwards, the glomerular filtration rate (GFR) of adults decreases progressively, with approximately 1 mL/min/1.73 m² from an initial normal value of 100 to 130 mL/min/1.73 m².[20] Factors that impair renal function after urinary diversion are obstruction of the ureters (stenosis of uretero–intestinal anastomosis), recurrent infection and urinary lithiasis. The exact impact of urinary diversion on renal function is not known, and the diagnosis and treatment of upper urinary tract deterioration is essential in preserving renal function. Long-term follow-up with blood tests and imaging is key. In patients with renal failure, there is a trend for using refluxing anastomosis in those patients who provide a low-pressure pouch. In the current literature, there is no single best urinary diversion technique for patients with renal impairment. It has been shown that GFR decreases 15–25% after urinary diversion with a follow-up of 11 years.[20] There is clearly a need for prospective randomized controlled studies on the issue of refluxing versus anti-refluxing anastomosis in continent urinary reconstruction.
In all patients undergoing urinary diversion surgery, life-long monitoring of renal function should be conducted. The use of serum creatinine is not a sensitive parameter to follow the renal function in these patients, and patients should be serially followed-up with renal ultrasound together with serum creatinine.
In patients with renal transplant graft that are scheduled to undergo urinary diversion surgery, caution should be exercised. Studies have shown that the presence of chronic bacteriuria did not affect the renal function in patients with renal transplants undergoing urinary diversion as assessed by the serum creatinine level. Further studies have reported that patients with ileal conduits can safely undergo renal transplantation if they are carefully selected.[21,22] The current estimated risk of renal failure/impairment over 5 years after a urinary diversion approach is 16%,[23] even in patients with previously normal kidneys.
Hepatic Function
Infection with urea-splitting bacteria (Proteus mirabilis, Klebsiella oxytoca, etc.) increases the ammonia load in an acute way. In addition, endotoxins significantly affect hepatic transport and metabolism. There is increased ammonia re-absorption when urine is in contact with the bowel wall. The normal liver can deal with this increased load. However, the presence of urinary tract infections with urea-splitting organisms and urinary tract obstruction can significantly increase the ammonia levels to an extent causing hyperammonemic encephalopathy and even hepatic coma.[24] Because of the limited protein intake, the use of non-absorbable disaccharides (e.g., lactulose enemata) and oral neomycin can diminish the nitrogen load for the patient. Pre-existing hepatic disease obviously will put the patient at a risk for this kind of complication. It is the most frequent cause of altered sensorium in patients with urinary diversion. In patients with non-continent diversion such as an ileal conduit, the occurrence of these complications is extremely rare.
Abnormal Drug Kinetics
Many drugs are normally secreted in urine. Because of the use of the gut during urinary diversion, a large amount of substances is re-absorbed via the intestine. The re-absorption of drugs can result in both diagnostic and therapeutic issues. In patients with diabetes, urine glucose testing is inaccurate due to glucose re-absorption. Drugs that are secreted unchanged in the urine and are absorbed by the intestine can also cause problems. Patients undergoing chemotherapy should have a catheter placed during treatment. Other drugs including Phenytoin, Theofyllin, Lithium, Methotrexate and several antibiotics are known to be absorbed by intestinal segments.[25,26] The clinical significance of this process is difficult to summarize in general terms as an important inter-individual variability in ileal absorption is present.[26] Urologists must be aware of the need for dose adaptations in certain clinical settings.
Prevention of Metabolic Complications following Urinary Diversion
The metabolic complications in patients following urinary diversion can occur from within the peri-operative period to many years following urinary diversion.[23] Although there is an international consensus on the importance of monitoring metabolic acidosis, vitamin B12 levels and upper tract obstruction in patients undergoing urinary diversion, there are no formal guidelines.[23]
When a urological surgeon decides to perform a urinary diversion on the appropriately selected patient, it is important to select the type of diversion and the ideal gut segment that should be used. We summarize the specific metabolic complications depending on the type of the urinary diversion procedure performed in Table 5.
Table 5.
Specific metabolic complications associated with the type of the urinary diversion
Drugs Used in the Management of the Metabolic Complications of Urinary Diversion
Each metabolic complication needs to be treated on an acute basis. However, additional specific complications such as chronic diarrhea after urinary diversion can be treated with cholestyramine, which is a resin that binds bile salts and increases the consumption of dietary fiber intake. In the United Kingdom, the dose of cholestyramine has to be increased gradually from 4 gm twice a day to 8 gm twice a day. With a chronic use of cholestyramine, patients are at risk of developing deficiency of fat-soluble vitamins such as vitamins A, D and K. In patients with persistent diarrhea despite cholestyramine, benefit may be derived from gastrointestinal gut motility inhibitors such as loperamide hydrochloride.[39]
There is variability in the definition of metabolic acidosis. In the current literature, the definition is a venous bicarbonate level less than 21 mmol/L. Alkalinizing therapy is commenced with oral sodium bicarbonate (1–2 g three times a day) as standard routine, but some patients complain of significant gastrointestinal symptoms such as excessive flatulence. In these patients who have significant side-effects with sodium bicarbonate, sodium citrate (1–3 g four times a day) can be tried. Depending on the additional current conditions in which sodium needs to be closely monitored, patients benefit from concomitant treatment with nicotinic acid (500 mg four times a day to 2 g four times a day) or chlorpromazine (25–50 mg four times a day) to diminish the need for alkalinizing agents, through inhibition of cyclic AMP-mediated chloride ion transport.[40,41]
SUMMARY
Urinary diversion is a commonly performed procedure. Surgeons need to be aware of the metabolic changes these patients will undergo. Factors influencing these changes include the bowel segment used and the length of the bowel. Ileal conduits are associated with lower metabolic complications, whereas continent urinary diversion will result in longer contact time between urine and the intestinal segment.
Meticulous pre-operative planning, good surgical technique and excellent post-operative care are vital in minimizing such complications. A good knowledge and understanding of the potential complications is vital. Life-long follow-up of patients with urinary diversion is essential in monitoring of patients for oncological and metabolic complications.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bricker EM. Bladder substitution after pelvic evisceration. Surg Clin North Am. 1950;30:1511–21. doi: 10.1016/s0039-6109(16)33147-4. [DOI] [PubMed] [Google Scholar]
- 2.Hautmann RE, Miller K, Steiner U, Wenderoth U. The ileal neobladder: 6 Years of experience with more than 200 patients. J Urol. 1993;150:40–5. doi: 10.1016/s0022-5347(17)35392-2. [DOI] [PubMed] [Google Scholar]
- 3.Rowland RG. Present experience with the Indiana pouch. World J Urol. 1996;14:92–8. doi: 10.1007/BF00182564. [DOI] [PubMed] [Google Scholar]
- 4.Bissada NK, Herschorn S, Elzawahri A. Urinary conduit formation using retubularized bowel from continent urinary diversion or intestinal augmentations: I. A multi-institutional experience. Urology. 2004;64:485–7. doi: 10.1016/j.urology.2004.04.076. [DOI] [PubMed] [Google Scholar]
- 5.Hautmann RE. Surgery illustrated-surgical atlas ileal neobladders. BJU Int. 2010;105:1024–35. doi: 10.1111/j.1464-410X.2010.09283.x. [DOI] [PubMed] [Google Scholar]
- 6.Studer UE, Varol C, Danuser H. Surgical Atlas Orthotropic ileal neobladders. BJU Int. 2006;98:469–82. doi: 10.1111/j.1464-410X.2006.06383.x. [DOI] [PubMed] [Google Scholar]
- 7.Joniau S, Benijts J, Van Kampen M, De Waele M, Ooms J, Van Cleynenbreugel B, et al. Clinical experience with the N-shaped ileal neobladder: Assessment of complications, voiding patterns, and quality of life in our series of 58 patients. Eur Urol. 2005;47:666–72. doi: 10.1016/j.eururo.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Ghoneim MA, Shaaban AA, Mahran MR, Kock NG. Further experience with the urethral Kock pouch. J Urol. 1992;147:361–5. doi: 10.1016/s0022-5347(17)37238-5. [DOI] [PubMed] [Google Scholar]
- 9.Thüroff JW, Riedmiller H, Fisch M, Stein R, Hampel C, Hohenfellner R. Mainz pouch continent cutaneous diversion. BJU Int. 2010;106:1830–54. doi: 10.1111/j.1464-410X.2010.09773.x. [DOI] [PubMed] [Google Scholar]
- 10.Thuroff JW, Alken P, Engelmann U. The Mainz pouch (mixed augmentation ileum ’n Zecum) for bladder augmentation and continent urinary diversion. Eur Urol. 1985;11:152–60. doi: 10.1159/000472481. [DOI] [PubMed] [Google Scholar]
- 11.Rowland RG, Mitchell ME, Bihrle R. Indiana continent urinary reservoir. J Urol. 1987;137:1136–9. doi: 10.1016/s0022-5347(17)44428-4. [DOI] [PubMed] [Google Scholar]
- 12.Konety BR, Barbour S, Carroll PR. Urinary Diversion and Bladder Substitution. In: Tanagho EA, McAninch JW, editors. Smith's General Urology. 17 ed. New York: McGraw-Hill; 2008. pp. 388–403. [Google Scholar]
- 13.Van der Aa F, Joniau S, Van Den Branden M, Van Poppel H. Metabolic changes after urinary diversion. Adv Urol 2011. 2011 doi: 10.1155/2011/764325. 764325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terai A, Okada Y, Shichiri Y, Kakehi Y, Terachi T, Arai Y, et al. Vitamin B12 deficiency in patients with urinary intestinal diversion. Int J Urol. 1997;4:21–5. doi: 10.1111/j.1442-2042.1997.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 15.Kamidono S, Oda Y, Ogawa T. Clinical study of urinary diversion. II: Review of 41 ileocolic conduit cases, their complications and long term (6-9 years) follow-up. Nishinihon J Urol. 1985;47:415–20. [Google Scholar]
- 16.McDougal WS. Bladder reconstruction following cystectomy by uretero-ileo-colourethrostomy. J Urol. 1986;135:698–701. doi: 10.1016/s0022-5347(17)45822-8. [DOI] [PubMed] [Google Scholar]
- 17.Kawakita M, Arai Y, Shigeno C, Terai A, Okada Y, Takeuchi H, et al. Bone demineralization following urinary intestinal diversion assessed by urinary Pyridium cross-links and dual energy xray absorptiometry. J Urol. 1996;156(2 Pt 1):355–9. doi: 10.1097/00005392-199608000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Terai A, Ueda T, Kakehi Y, Terachi T, Arai Y, Okada Y, et al. Urinary calculi as a late complication of the Indiana continent urinary diversion: Comparison with the kock pouch procedure. J Urol. 1996;155:66–8. [PubMed] [Google Scholar]
- 19.Terai A, Arai Y, Kawakita M, Okada Y, Yoshida O. Effect of urinary intestinal diversion on urinary risk factors for urolithiasis. J Urol. 1995;153:37–41. doi: 10.1097/00005392-199501000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Kristjansson A, Wallin L, Mansson W. Renal function up to 16 years after conduit (refluxing or anti-reflux anastomosis) or continent urinary diversion. 1. Glomerular filtration rate and patency of uretero-intestinal anastomosis. Br J Urol. 1995;76:539–45. doi: 10.1111/j.1464-410x.1995.tb07775.x. [DOI] [PubMed] [Google Scholar]
- 21.Kelly WD, Merkel FK, Markland C. Ileal urinary diversion in conjunction with renal homotransplantation. Lancet. 1966;1:222–6. doi: 10.1016/s0140-6736(66)90049-3. [DOI] [PubMed] [Google Scholar]
- 22.Crowe A, Cairns HS, Wood S, Rudge CJ, Woodhouse CR, Neild GH. Renal transplantation following renal failure due to urological disorders. Nephrol Dial Transplant. 1998;13:2065–9. doi: 10.1093/ndt/13.8.2065. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert SM, Lai J, Saigal CS, Gore JL. Urologic Diseases in America Project. Downstream Complications Following Urinary Diversion. J Urol. 2013;190:916–22. doi: 10.1016/j.juro.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albersen M, Joniau S, Van Poppel H, Cuyle PJ, Knockaert DC, Meersseman W. Urea-splitting urinary tract infection contributing to hyperammonemic encephalopathy. Nat Clin Pract Urol. 2007;4:455–8. doi: 10.1038/ncpuro0877. [DOI] [PubMed] [Google Scholar]
- 25.Davidsson T, Akerlund S, White T, Olaisson G, Månsson W. Mucosal permeability of ileal and colonic reservoirs for urine. Br J Urol. 1996;78:64–8. doi: 10.1046/j.1464-410x.1996.05212.x. [DOI] [PubMed] [Google Scholar]
- 26.Alhasso A, Bryden AA, Neilson D. Lithium toxicity after urinary diversion with ileal conduit. BMJ. 2000;320:1037. [PMC free article] [PubMed] [Google Scholar]
- 27.Iborra I, Casanova JL, Solsona E. Tolerance of external urinary diversion (Bricker) followed for more than 10 years. Eur Urol Supp. 2010;9:736–4. [Google Scholar]
- 28.Fontaine E, Barthelemy Y, Houlgatte A, Chartier E, Beurton D. Twenty-year experience with jejunal conduits. Urology. 1997;50:207–13. doi: 10.1016/S0090-4295(97)00210-0. [DOI] [PubMed] [Google Scholar]
- 29.Jonsson O, Olofsson G, Lindholm E, Törnqvist H. Long-time experience with the Kock ileal reservoir for continent urinary diversion. Eur Urol. 2001;40:632–40. doi: 10.1159/000049849. [DOI] [PubMed] [Google Scholar]
- 30.Thoeny HC, Sonnenschein MJ, Madersbacher S, Vock P, Studer UE. Is ileal orthotopic bladder substitution with an afferent tubular segment detrimental to the upper urinary tract in the long term? J Urol. 2002;168:2030–4. doi: 10.1016/S0022-5347(05)64289-9. [DOI] [PubMed] [Google Scholar]
- 31.Månsson W, Davidsson T, Könyves J, Liedberg F, Månsson A, Wullt B. Continent urinary tract reconstruction - The Lund experience. BJU Int. 2003;92:271–6. doi: 10.1046/j.1464-410x.2003.04330.x. [DOI] [PubMed] [Google Scholar]
- 32.Bissada NK, Morcos RR, Morgan WM, Hanash KA. Ureterosigmoidostomy: Is it a viable procedure in the age of continent urinary diversion and bladder substitution? J Urol. 1995;153:1429–31. doi: 10.1016/s0022-5347(01)67420-2. [DOI] [PubMed] [Google Scholar]
- 33.Bastian PJ, Albers P, Hanitzsch H, Fabrizi G, Casadei R, Haferkamp A, et al. The modified ureterosig- moidostomy (Mainz pouch II) as a continent form of urinary diversion. Urologe A. 2004;43:982–8. doi: 10.1007/s00120-004-0560-3. [DOI] [PubMed] [Google Scholar]
- 34.Bruce AW, Reid G, Chan RC, Costerton JW. Bacterial adherence in the human ileal conduit: A morphological and bacteriological study. J Urol. 1984;132:184–8. doi: 10.1016/s0022-5347(17)49516-4. [DOI] [PubMed] [Google Scholar]
- 35.Mansson W, Colleen S, Mardh PA. The microbial flora of the continent cecal urinary reservoir, its stoma and the peristomal skin. J Urol. 1986;135:247–50. doi: 10.1016/s0022-5347(17)45599-6. [DOI] [PubMed] [Google Scholar]
- 36.Wullt B, Holst E, Steven K, Carstensen J, Pedersen J, Gustafsson E, et al. Microbial flora in ileal and colonic neobladders. Eur Urol. 2004;45:233–9. doi: 10.1016/j.eururo.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Nakano Y, Fujisawa M, Matsui T, Arakawa S, Kamidono S. The significance of the difference in bacterial adherence between bladder and ileum using rat ileal augmented bladder. J Urol. 1999;162:243–7. doi: 10.1097/00005392-199907000-00075. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt JD, Hawtrey CE, Flocks RH, Culp DA. Complications, results and problems of ileal conduit diversions. J Urol. 1973;109:210–6. doi: 10.1016/s0022-5347(17)60390-2. [DOI] [PubMed] [Google Scholar]
- 39.McDougal WS. Bladder reconstruction following cystec-tomy by uretero-ileo-colourethrostomy. J Urol. 1986;135:698–701. doi: 10.1016/s0022-5347(17)45822-8. [DOI] [PubMed] [Google Scholar]
- 40.Koch MO, McDougal SW. Chlorpromazine: Adjuvant therapy for the metabolic derangements created by urinary diversion through intestinal segments. J Urol. 1985;134:165–9. doi: 10.1016/s0022-5347(17)47049-2. [DOI] [PubMed] [Google Scholar]
- 41.Koch MO, McDougal SW. Nicotinic acid: Treatment for the hyperchloremic acidosis following urinary diversion through intestinal segments. J Urol. 1985;134:162–4. doi: 10.1016/s0022-5347(17)47048-0. [DOI] [PubMed] [Google Scholar]


