Abstract
West Nile Virus (WNV) is a mosquito-borne pathogen that impacts the health of its passerine bird hosts as well as incidentally infected humans in the United States. Intensive enzootic activity among the hosts and vectors does not always lead to human outbreaks, as is the situation throughout much of the southeastern United States. In Georgia, substantial yearly evidence of WNV in the mosquito vectors and avian hosts since 2001 has only led to 324 human cases. Although virus has been consistently isolated from mosquitoes trapped in Atlanta, GA, little is known about viral activity among the passerine hosts. A possible reason for the suppression of WNV spillover to humans is that viremic birds are absent from high human-use areas of the city. To test this hypothesis, multiseason, multihabitat, longitudinal WNV surveillance for active WNV viremia was conducted within the avian host community of urban Atlanta by collection of blood samples from wild passerine birds in five urban microhabitats. WNV was isolated from the serum of six blood samples collected from 630 (0.95%) wild passerine birds in Atlanta during 2010–2012, a proportion similar to that found in the Chicago, IL, area in 2005, when over 200 human cases were reported. Most of the viremic birds were Northern Cardinals, suggesting they may be of particular importance to the WNV transmission cycle in Georgia. Results indicated active WNV transmission in all microhabitats of urban Atlanta, except in the old-growth forest patches. The number of viremic birds was highest in Zoo Atlanta, where 3.5% of samples were viremic. Although not significant, these observations may suggest a possible transmission reduction effect of urban old-growth forests and a potential role in WNV amplification for Zoo Atlanta. Overall, spillover to humans remains a rare occurrence in urban Atlanta settings despite active WNV transmission in the avian population.
Key Words: West Nile Virus; Viremia; Spillover; Northern Cardinal; Urban; Zoo; Atlanta, Georgia
Introduction
Emerging infectious zoonotic diseases can quickly devastate naïve wildlife populations and result in public health emergencies. Over the past decades, new diseases have emerged and become concentrated in areas undergoing rapid anthropogenic change, such as urban settings, deforested regions, and areas undergoing intensive farming. When these novel pathogens are successfully introduced into such disturbed settings, they can become established and spread rapidly due to the high density and diversity of both susceptible hosts and disease vectors. Urban settings, in particular, comprise a plethora of disturbed ecosystems and a diversity of wildlife, and the introduction of emerging infectious diseases provides abundant opportunities for pathogen amplification and rapid spread of disease, with major impacts on both human and wildlife health.
Since its introduction to the continental United States in 1999, West Nile virus (WNV) has become enzootic and endemic, spreading from coast to coast in just 4 years (Hayes et al. 2005). Over 36,000 people have been infected (with >1500 fatal cases) (Centers for Disease Control and Prevention 2013), and certain US bird species (crows, blue jays) have been strongly affected (Centers for Disease Control and Prevention 2002). In the eastern United States, WNV transmission between vectors (Culex mosquitoes) and hosts (passerine birds) occurs mostly during late summer in urban settings (Centers for Disease Control and Prevention 2013). Human cases of WNV are the result of spillover from this epizootic cycle, where spillover is defined as occurring when a pathogen is transmitted from an animal to a human, which results in an infection in the human without causing any substantial further human-to-human transmission (Fenton and Pedersen 2005, Lloyd-Smith et al. 2009). Human cases do not necessarily follow intensive enzootic activity, as is the situation in the state of Georgia (and much of the southeastern United States) where WNV is well-documented in the mosquito vectors and avian reservoir hosts (Gibbs et al. 2006a, Vazquez Prokopec et al. 2010), but where a total of only 324 human cases have been reported since 2001 (Centers for Disease Control and Prevention 2013).
In Atlanta, Georgia's major urban center, yearly routine mosquito surveillance has consistently demonstrated active WNV infection in Culex mosquitoes. In addition, both passive dead bird surveillance as well as active live bird surveillance also indicated consistent yearly WNV infection among avian hosts in Atlanta, although budget cuts and other factors have forced suspension of all avian surveillance since 2007 (Allison et al. 2004, Gibbs et al. 2006a, Bradley et al. 2008, Vazquez Prokopec et al. 2010). Consequently, little is known about the prevalence and transmissibility of WNV in avian hosts in Atlanta, particularly in the 6 years since 2007, during which the contributing factors causing yearly recurring WNV outbreaks of widely varying severities have been poorly understood (Centers for Disease Control and Prevention 2013). A possible reason for the suppression of WNV spillover to humans is that viremic birds are absent from high human use areas of the city, resulting in a low probability of exposure to mosquitoes and subsequently to humans (Fenton and Pedersen 2005, Lloyd-Smith et al. 2009). To test this hypothesis, we conducted multiseason, multihabitat, longitudinal WNV surveillance for active WNV viremia within the avian host community of urban Atlanta.
Materials and Methods
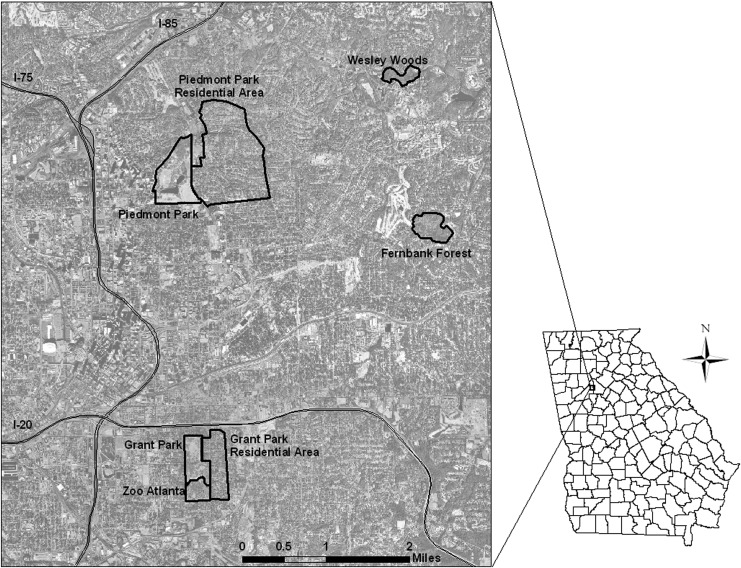
Between early May and early November of 2010–2012, we collected blood samples from wild passerine birds in five urban microhabitats of Atlanta, GA—mixed-use parks, divided into wooded and water sections; residential areas; old-growth forests; and Zoo Atlanta (Fig. 1). The park and residential sites were treated as matched blocks, with residential sampling conducted in the neighborhoods directly east of the parks in areas similar in size to the parks. Parks were divided into two zones. Park–Water contained an artificial water feature (pond or lake) surrounded by public restrooms and other built facilities (public swimming pool, tennis courts, gazebos, or large parking lots); Park–Woods comprised a wooded area with paved walking paths that experienced far less human use.
FIG. 1.
Map of study sites in urban Atlanta, GA, 2010–2012. Grant and Piedmont Parks each included two sampling zones, for a total of nine study sites: (1) a water feature and surrounding built structures; (2) a wooded area and associated walking paths.
During 2010, each habitat type was represented by a single replicate and was sampled in the same order once every 3 weeks between 06:00 and noon, with the residential and park sites represented by the Grant Park (Atlanta's oldest and fourth-largest urban park) area. Samples were collected from 10 properties in the residential zone. This area was selected based on its previous determination as a WNV hotspot and the residents' familiarity with previous WNV surveillance studies (unpublished data). In 2011, we added a replicate for each habitat type, with the additional residential and park sites represented by the Piedmont Park (Atlanta's third-largest urban park) area. Samples were collected from 11 properties and one community garden in the residential zone. Sampling in the Grant Park area was repeated in 2011, with eight properties sampled in the residential zone. With the addition of site replicates in 2011, we reduced the frequency of sampling in each site to once every 4.5 weeks in the same order. In 2012, only a single site (the water zone of Grant Park) was sampled (once every 3 weeks).
Birds were captured using nylon mesh mist nets. After extraction, birds were identified to species, measured, aged, sexed, banded, blood sampled (by jugular venipuncture), and released (Emory University's Institutional Animal Care and Use Committee permit 2001632, Georgia Department of Natural Resources Scientific Collecting Permit 29-WBH-12-1, and Federal Bird Banding Permit 23673). Following collection, blood samples were transported on ice to the laboratory, where they were centrifuged at 10,000 rpm for 10 min for serum collection. After centrifugation, serum was removed and frozen at −80°C until further processing.
Serum samples were screened for circulating virus by inoculating 10 μL of serum into 2-mL cultures of 2-day-old Vero Middle America Research Unit (MARU) cells cultures. Cells were examined daily for 14 days for evidence of cytopathic effects (CPE). If CPE were noted, cells were tested for WNV via VecTest®. WNV was confirmed in VecTest®-positive samples by reverse transcription PCR (RT-PCR), using degenerate WNV-specific primers (WN310F, sense primer, 5′-GTSAACAAAACAAACAGCRATGAA-3′; WN686R, antisense primer, 5′-ACWGMTGAYTTYGTGCACCA-3′) amplifying a 376-bp fragment that spanned both the nucleocapsid and premembrane genes (Allison et al. 2004).
Viral titers from WNV-positive serum samples were measured by plaque assay. Samples were rethawed from −80°C and diluted in MEM to make 10-fold dilutions of 10−1 to 10−6. A 200-μL amount of each dilution was rapidly added to 4-day-old Vero MARU cell cultures on a six-well plate. Plates were rocked every 15 min for 1 h and then overlaid with 4 mL of 1% gum tragacanth/1×minimum essential media (MEM) (supplemented with 2.2 g/L sodium bicarbonate, 3% heat-inactivated fetal bovine serum, 200 units/mL penicillin, 200 μg/mL streptomycin, and 500 ng/mL amphotericin B). Cultures were inactivated on day 6 postadsorption by adding 2 mL of 5% buffered formalin along with 0.25% crystal violet for plaque visualization. After 24 h, plates were rinsed with water and examined for plaques. Dilutions in which 20–100 plaques were distinguishable were used to determine WNV titers (log10 plaque-forming units [pfu]/mL) (Allison et al. 2004).
Statistical analyses comparing differences in proportions for resulting confirmed viremia frequency data were calculated using Pearson chi-squared tests conducted in JMP Pro, Version 10 software (SAS Institute 1989–2013).
Results
During the 3-year study period, 630 unique birds, representing 41 species, were sampled (Table 1). Active WNV infection was detected in 6 of 630 birds (0.95%), from which virus was isolated (Table 2), a proportion within the range found in the Chicago area in 2005 (1.1%) and 2006 (0.3%) when over 200 human cases were reported annually. Four of the six viruses were isolated from 156 samples (2.56%) taken from Northern Cardinals, significantly more than in the 474 samples taken from other bird species (χ2=5.7, p<0.05). One of 131 (0.76%) American Robins and 1 of 47 (2.13%) Carolina Wrens were also viremic. Although only 25.7% (162/630) of samples were taken from hatch-year birds, all but one of the six WNV isolates were obtained from hatch-year birds, which were viremic significantly more often than the 421 older birds (age could not be determined for 47 birds) from which only one isolate was obtained (χ2=9.3, p<0.01).
Table 1.
Avian Species and the Number of Unique Individuals Sampled in Urban Atlanta, GA, 2010–2012
| Species common name | Species name | Number of samples |
|---|---|---|
| Northern Cardinal | Cardinalis cardinalis | 156 |
| American Robin | Turdus migratorius | 131 |
| Carolina Wren | Thryothorus ludovicianus | 47 |
| Northern Mockingbird | Mimus polyglottos | 44 |
| Brown Thrasher | Toxostoma rufum | 41 |
| Gray Catbird | Dumetella carolinensis | 37 |
| European Starling | Sturnus vulgaris | 26 |
| Swainson's Thrush | Catharus ustulatus | 17 |
| Common Grackle | Quiscalus quiscula | 16 |
| Blue Jay | Cyanocitta cristata | 14 |
| Eastern Towhee | Pipilo erythrophthalmus | 14 |
| Tufted Titmouse | Baeolophus bicolor | 11 |
| Wood Thrush | Hylocichla mustelina | 11 |
| Song Sparrow | Melospiza melodia | 9 |
| Eastern Bluebird | Sialia sialis | 6 |
| Gray-Cheeked Thrush | Catharus minimus | 5 |
| Hooded Warbler | Setophaga citrina | 5 |
| White-Breasted Nuthatch | Sitta carolinensis | 5 |
| Brown-Headed Cowbird | Molothrus ater | 3 |
| Eastern Phoebe | Sayornis phoebe | 3 |
| Great-Crested Flycatcher | Myiarchus crinitus | 3 |
| House Finch | Haemorhous mexicanus | 3 |
| Ovenbird | Seiurus aurocapilla | 2 |
| Red-Bellied Woodpecker | Melanerpes carolinus | 2 |
| White-Throated Sparrow | Zonotrichia albicollis | 2 |
| Yellow-Shafted Flicker | Colaptes auratus | 2 |
| Black-and-White Warbler | Mniotilta varia | 1 |
| Chestnut-Sided Warbler | Setophaga pensylvanica | 1 |
| Downy Woodpecker | Picoides pubescens | 1 |
| House Sparrow | Passer domesticus | 1 |
| House Wren | Troglodytes aedon | 1 |
| Indigo Bunting | Passerina cyanea | 1 |
| Kentucky Warbler | Geothlypis formosa | 1 |
| Magnolia Warbler | Setophaga magnolia | 1 |
| Mourning Dove | Zenaida macroura | 1 |
| Northern Waterthrush | Parkesia noveboracensis | 1 |
| Rose-Breasted Grosbeak | Pheucticus ludovicianus | 1 |
| Red-Eyed Vireo | Vireo olivaceus | 1 |
| Red-Winged Blackbird | Agelaius phoeniceus | 1 |
| Veery | Catharus fuscescens | 1 |
| Yellow-Bellied Sapsucker | Sphyrapicus varius | 1 |
| Total | 630 |
Table 2.
West Nile Virus Viremia Titers in Wild Passerines Sampled in Atlanta, GA, 2010–2012
| Species common name | Species name | Age | Location captured | Sample year | Sample month and day | Virus titer (log10 pfu/mL) |
|---|---|---|---|---|---|---|
| Northern Cardinal | Cardinalis cardinalis | Hatch-year | Park–Woods | 2010 | August 13 | 3.74 |
| American Robin | Turdus migratorius | Hatch-year | Park–Woods | 2010 | September 1 | Below detectable levels |
| Northern Cardinal | Cardinalis cardinalis | Hatch-year | Residential | 2011 | July 28 | 3.47 |
| Northern Cardinal | Cardinalis cardinalis | Hatch-year | Zoo Atlanta | 2011 | August 3 | 1.69 |
| Carolina Wren | Thryothorus ludovicianus | After Hatch-Year | Zoo Atlanta | 2011 | August 3 | 4.69 |
| Northern Cardinal | Cardinalis cardinalis | Hatch-year | Park–Water | 2011 | August 9 | 3.87 |
pfu, plaque-forming units.
The old-growth forest sites were the only habitat from which no virus was isolated (out of 97 samples). Two isolates were obtained from 58 Zoo Atlanta samples (3.45%) and two from 122 park-woods samples (1.64%). One isolate was obtained from 126 residential area samples (0.79%) and one from 227 park-water samples (0.44%). No significant differences between microhabitat type and viremia were detected (χ2=6.0, p>0.1). Four of the six isolates were from 2011 (0.95% of 418), two from 2010 (1.42% of 141), and none from 71 samples in 2012, with no significant difference in proportion of viremic birds over the 3 study years (χ2=1.0, p>0.5). Significantly more (4/6) viruses were isolated from the 72 samples taken in August, compared to the 558 samples collected in other months (χ2=18.3, p<0.0001). Detectable viremia levels ranged from 101.69 to 104.69 pfu/mL (mean=104.11 pfu/mL).
Discussion
This is the first report of WNV isolates from live passerines in the state of Georgia, and demonstrates active WNV transmission in Atlanta, with detectable viremia observed in approximately 1% (6) of the 630 birds we captured. These viremia levels from passerines in Atlanta were similar to those from Chicago, but the Chicago area reported more than eight times as many human cases (a difference that cannot be accounted for by human population size differences alone) (Hamer et al. 2008). Thus, despite transmission in the avian population, spillover to humans is a much rarer occurrence in urban Atlanta settings. Our results further confirm that WNV transmission peaks during August, and that hatch-year birds are important amplifying hosts for WNV (Hamer et al. 2008).
Several studies indicate the significance of American Robins as superspreader hosts of WNV (Kilpatrick et al. 2006, Hamer et al. 2009, Simpson and Hurtado 2011), but our results suggest that important regional differences in host importance may exist. Coupled with findings from two studies of WNV antibody prevalence among songbirds in Georgia showing Northern Cardinals having by far the highest seroprevalence (Gibbs et al. 2006, Bradley et al. 2008), our study indicates that Northern Cardinals play an important role in WNV transmission in Georgia. While we isolated virus from only a single American Robin (whose titer was too low for detection by plaque assay), most of the isolates (and a proportion significantly higher than any other avian species) were from Northern Cardinals, which have been shown to be moderately competent as reservoir hosts (Kilpatrick et al. 2007). The four cardinals (2.6% of all of our Northern Cardinal samples) that were viremic had a mean viremia of 103.60 pfu/mL, above the recently proposed 103.4 pfu/mL minimum titer for WNV transmission to feeding mosquitoes (Komar et al. 2003, Wheeler et al. 2012). It is also highly likely that titers obtained as part of this study are lower than at the time of sampling, due to three to four previous freeze–thaw cycles resulting from separate diagnostic testing of samples. Taken together, our results indicate that even moderately competent hosts such as Northern Cardinals may be important for the WNV transmission cycle in Georgia, and we conclude that regional variations in host contribution, with particular attention to Northern Cardinals, should be considered.
Finally, our results indicate active WNV transmission in all microhabitats of urban Atlanta, with the exception of the old-growth forest patches. Although no significant associations between viremia and microhabitat type were detected with the small sample size, the number of viremic birds was highest in Zoo Atlanta, where 3.5% of samples were viremic, a trend that may suggest a potential role in WNV amplification for the Zoo. Zoos represent exclusive settings in which unique combinations of carefully maintained habitats exist together, which include the comingling of exotic and native species, captive and free-roaming wildlife, public and private spaces, anthropogenically changed and natural environments, and insular and connected ecosystems.
Such close proximity of “ecotones” with contrasting resources results in favorable habitats for arthropods while also facilitating their movement between habitats and enhancing their exposure to pathogens; consequently, urban zoos are habitats that may be particularly prone to arthropod-borne diseases. In addition to facilitating transmission through their mixed characteristics, many zoos are built on historical hotspots of human arthropod-borne diseases and are located in or near human population centers and transportation nodes (Adler et al. 2011, Tuten et al. 2012). Given this potential for elevated transmission of arthropod-borne diseases such as WNV in zoos, it is perhaps not surprising that we identified Zoo Atlanta as the habitat with the greatest proportion of viremic birds. The Grant Park area, in which Zoo Atlanta is located, may represent a hotspot of WNV transmission in Fulton County, Atlanta, because there is evidence of relatively high infection rates across hosts and vectors there.
In a study examining the spatial distribution of WNV infection in Atlanta among mosquitoes, humans, and corvid birds (based on dead bird reporting), 6.1–12.0 infected mosquitoes per 1000 were detected in this area, along with significant local clustering of WNV infection. In that study, significant positive spatial clustering of both WNV human incidence and WNV corvid death ratio was also found in the same location, along with a human incidence rate that was 6.5 times higher than the average rate for Atlanta as a whole (Vazquez Prokopec et al. 2010). While the data from that study are too coarse to implicate any of our Grant Park microhabitats, including Zoo Atlanta as a WNV transmission source, they do demonstrate a pattern of consistently high levels of infection in both hosts and reservoirs in the area. Therefore, measuring the role of Zoo Atlanta in the transmission of WNV in Atlanta may be a productive avenue for future research.
No viremic birds were found in the old-growth forest sites. This finding may simply result from a lack of sufficient samples from this microhabitat type that would allow us to detect viremia or it may represent a trend suggesting a possible transmission reduction effect of urban old-growth forests. Other studies provide conflicting results regarding the effect of forests on WNV transmission. One study in Georgia found birds in forested habitats showing WNV seroprevalence at levels nearly as high as birds from urban and suburban sites (Gibbs et al. 2006b), whereas another identified a larger proportion of urban tree cover as significant factor in WNV infection spatial clusters (Vazquez Prokopec et al. 2010). A study from South Dakota even identified forests as a factor contributing to a positive association with WNV risk (Chuang et al. 2012). Increased vegetation levels, especially in urban areas, provide optimal habitats for avian hosts of WNV and facilitate contact between bird species that congregate in these areas, thereby aiding in transmission amplification (Messina et al. 2011). On the other hand, several studies have found significantly reduced WNV incidence in humans (Brown et al. 2008, LaBeaud et al. 2008, Bowden et al. 2011, DeGroote and Sugumaran 2012) or prevalence in birds (Bradley et al. 2008, LaDeau et al. 2011) with increasing forest cover. The negative relationship between WNV transmission and forest habitats may be attributed to the effect of urbanization on increasing the prevalence of preferred larval habitats for the WNV vector species, comprising artificial structures (catchment basins and sewer networks) that fill with eutrophied shallow water, which are rare or absent from forests (LaDeau et al. 2008). These conflicting results with regard to the effect of forest cover on WNV transmission may relate to differing spatial resolutions of the various studies, because they range in scale from considering the presence of forested areas from relatively large-scale county resolutions to much coarser-scale regional resolutions. However, the effect of forest cover at any of these spatial scales may not be reflective of the role of old-growth forest patches within the fine-scale urban habitat mosaic. Therefore, whereas our results show an absence of WNV viremic birds from urban old-growth forest habitats of Atlanta, further study is warranted to determine their overall role within the city and whether they may provide a transmission reduction effect.
Conclusions
This study confirms active WNV transmission in urban Atlanta. We identified detectable viremia in avian hosts at a level comparable to that in cities with much higher rates of WNV spillover to humans, thereby indicating that lack of transmission in the host population does not explain the absence of spillover. We suggest that Northern Cardinals may be particularly important to the WNV transmission cycle in Georgia, and future research is needed to assess the extent (if any) to which their role in transmission can explain the lack of widespread WNV spillover to humans in the southeastern region. Finally, our identification of trends in varying avian viremia levels from different urban microhabitat types within Atlanta, coupled with probable differences in the avian species compositions that reside in these heterogeneous habitats (especially when considering the exotic hosts present in Zoo Atlanta), indicate that future studies on the role of specific habitat types within the fine-scale urban mosaic may shed further light on human risk for WNV and are warranted.
Acknowledgments
We thank J.R. McMillan, Donal Bisanzio, and students in the vector ecology lab at Emory University who assisted in trapping birds; Luis Chaves and Gonzalo Vazquez-Prokopec for suggestions on the study design; and Jennifer Abi Younes for assistance with reverse transcription PCR confirmation of WNV isolates. We also thank James Ballance at Zoo Atlanta, Chris Nelson and Mary Moerlins at the Piedmont Park Conservancy, Chris Showalter at Fernbank Science Center, Jamie Blackburn and Tracy McClendon at Atlanta Botanical Garden, and the residents of the Grant Park and Piedmont Park neighborhoods who graciously allowed us to trap birds on their property.
Funding for this research was provided by National Institutes of Health (NIH) training grant 5T32AI055404-08 (L. Real, PI), Emory University Department of Environmental Studies, and University of Georgia Southeastern Cooperative Wildlife Disease Study (SCWDS).
Author Disclosure Statement
No competing financial interests exist.
References
- Adler PH. Tuten HC. Nelder MP. Arthropods of medicoveterinary importance in zoos. Annu Rev Entomol. 2011;56:123–142. doi: 10.1146/annurev-ento-120709-144741. [DOI] [PubMed] [Google Scholar]
- Allison AB. Mead DG. Gibbs SE. Hoffman DM, et al. West Nile Virus viremia in wild rock pigeons. Emerg Infect Dis. 2004;10:2252–2255. doi: 10.3201/eid1012.040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden SE. Magori K. Drake JM. Regional differences in the association between land cover and West Nile Virus disease incidence in humans in the United States. Am J Trop Med Hygiene. 2011;84:234–238. doi: 10.4269/ajtmh.2011.10-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley CA. Gibbs SE. Altizer S. Urban land use predicts West Nile virus exposure in songbirds. Ecol Applic. 2008;18:1083–1092. doi: 10.1890/07-0822.1. [DOI] [PubMed] [Google Scholar]
- Brown HE. Childs JE. Diuk-Wasser MA. Fish D. Ecological factors associated with West Nile Virus transmission, northeastern United States. Emerg Infect Dis. 2008;14:1539–1545. doi: 10.3201/eid1410.071396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Provisional surveillance summary of the West Nile virus epidemic—United States. Morbid Mortal Weekly Rep. 2002 2002 Jan-Nov;51:1129–1133. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. West Nile Virus Infection. 2013. www.cdc.gov/ncidod/dvbid/westnile/index.htm. [Jul 28;2013 ]. www.cdc.gov/ncidod/dvbid/westnile/index.htm
- Chuang T-W. Hockett CW. Kightlinger L. Wimberly MC. Landscape-level spatial patterns of West Nile Virus risk in the northern Great Plains. Am J Trop Med Hygiene. 2012;86:274–231. doi: 10.4269/ajtmh.2012.11-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGroote JP. Sugumaran R. National and regional associations between human West Nile Virus incidence and demographic, landscape, and land use conditions in the coterminous United States. Vector Borne Zoonotic Dis. 2012;12:657–665. doi: 10.1089/vbz.2011.0786. [DOI] [PubMed] [Google Scholar]
- Fenton A. Pedersen AB. Community epidemiology framework for classifying disease threats. Emerg Infect Dis. 2005;11:1815–1821. doi: 10.3201/eid1112.050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs SEJ. Allison AB. Yabsley MJ. Mead DG, et al. West Nile virus antibodies in avian species of Georgia, USA: 2000–2004. Vector Borne Zoonotic Dis. 2006a;6:57–72. doi: 10.1089/vbz.2006.6.57. [DOI] [PubMed] [Google Scholar]
- Gibbs SEJ. Wimberly MC. Madden M. Masour J, et al. Factors affecting the geographic distribution of West Nile virus in Georgia, USA: 2002–2004. Vector Borne Zoonotic Dis. 2006b;6:73–82. doi: 10.1089/vbz.2006.6.73. [DOI] [PubMed] [Google Scholar]
- Hamer GL. Walker ED. Brawn JD. Loss SR, et al. Rapid amplification of West Nile virus: The role of hatch-year birds. Vector Borne Zoonotic Dis. 2008;8:57–67. doi: 10.1089/vbz.2007.0123. [DOI] [PubMed] [Google Scholar]
- Hamer GL. Kitron UD. Goldberg TL. Brawn JD, et al. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am Soc Trop Med Hygiene. 2009;80:268–278. [PubMed] [Google Scholar]
- Hayes E. Komar N. Nasci RS. Montgomery SP, et al. Epidemiology and transmission dynamics of West Nile Virus disease. Emerg Infect Dis. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick A. Daszak P. Jones MJ. Marra PP, et al. Host heterogeneity dominates West Nile virus transmission. Proc R Soc Lond Series B, Biol Sci. 2006;273:2327–2333. doi: 10.1098/rspb.2006.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM. LaDeau SL. Marra PP. Ecology of West Nile Virus transmission and its impact on birds in the western hemisphere. The Auk. 2007;124:1121–1136. [Google Scholar]
- Komar N. Langevin S. Hinten S. Nemeth N, et al. Experimental infection of North American birds with the New York 1999 strain of West Nile Virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBeaud AD. Gorman AM. Koonce J. Kippes C, et al. Rapid GIS-based profiling of West Nile Virus transmission: Defining environmental factors associated with an urban-suburban outbreak in northeast Ohio, USA. Geospatial Health. 2008;2:215–225. doi: 10.4081/gh.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDeau SL. Marra PP. Kilpatrick AM. Calder CA. West Nile Virus revisited: Consequences for North American ecology. BioScience. 2008;58:937–946. [Google Scholar]
- LaDeau SL. Calder CA. Doran PJ. Marra PP. West Nile Virus impacts in American crow populations are associated with human land use and climate. Ecol Res. 2011;26:909–916. doi: 10.1007/s11284-010-0725-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Smith JO. George D. Pepin KM. Pitzer VE, et al. Epidemic dynamics at the human-animal interface. Science. 2009;326:1362–1367. doi: 10.1126/science.1177345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina JP. Brown W. Amore G. Kitron UD, et al. West Nile Virus in the greater Chicago area: A geographic examination of human illness and risk from 2002 to 2006. URISA J. 2011;23:5–15. [Google Scholar]
- SAS Institute. Cary, NC: 1989–2013. [Google Scholar]
- Simpson J. Hurtado P. Vector host-feeding preferences drive transmission of multi-host pathogens: West Nile virus as a model system. Proc R Soc B. 2011;279:925–933. doi: 10.1098/rspb.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuten HC. Bridges WC. Paul KS. Adler PH. Blood-feeding ecology of mosquitoes in zoos. Medical and Vet Entomol. 2012;26:407–416. doi: 10.1111/j.1365-2915.2012.01012.x. [DOI] [PubMed] [Google Scholar]
- Vazquez Prokopec GM. Eng JV. Kelly R. Mead DG, et al. The risk of West Nile Virus infection is associated with combined sewer overflow streams in urban Atlanta, Georgia, USA. Environ Health Perspect. 2010;118:1382–1388. doi: 10.1289/ehp.1001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler SS. Vineyard MP. Barker CM. Reisen WK. Importance of recrudescent avian infection in West Nile Virus overwintering: Incomplete antibody neutralization of virus allows infrequent vector infection. J Med Entomol. 2012;49:895–902. doi: 10.1603/me11286. [DOI] [PubMed] [Google Scholar]