Abstract
DNA sequences encoding the GroES and GroEL proteins of Orientia tsutsugamushi were amplified by the PCR and sequenced. Pairwise alignment of full-length groES and groEL gene sequences indicated high sequence similarity (90.4–100% and 90.3–100%) in O. tsutsugamushi, suggesting that these genes are good candidates for the molecular diagnosis and phylogenetic analysis of scrub typhus. Comparisons of the 56-kD type-specific antigen (TSA) protein gene and the groES and groEL genes showed that genotypes based on the 56-kD TSA gene were not related to a cluster containing the groES and groEL genes in a dendrogram, suggesting that a gene rearrangement may be associated with homologous recombination in mites.
Key Words: Orientia tsutsugamushi, groES and groEL genes, Phylogeny, Scrub typhus, Japan
Introduction
The bacterium Orientia tsutsugamushi belongs to the family Rickettsiaceae and is transmitted by trombiculid mites such as Leptotrombidium pallidum, Leptotrombidium akamushi, and Leptotrombidium scutellare. It is the causative agent of scrub typhus, an emerging zoonosis, spreading throughout central, southern, and eastern Asia, and Oceania (Kelly et al. 2009). In Korea, 17,451 scrub typhus cases, i.e., 7.2 cases per 100,000 individuals, were reported from 2001 to 2005 (Bang et al. 2008). In Japan, 313–791 cases have been reported annually since 1999, when nationwide patient surveillance began. Less than ten fatal cases of scrub typhus have been reported in Japan each year, but the public health impact of scrub typhus has remained in endemic areas. The number of people that participate in outdoor activities in forests, mountains, and similar areas is increasing in Japan, which increases the risk of transmission. Recently, several research groups conducted field surveys to determine the prevalence of O. tsutsugamushi among wild rodents and mites (Ogawa and Ono 2008). The serological diversity (Tamura et al. 1984) and genotypic diversity (Tamura et al. 2001) of O. tsutsugamushi have also been examined. These studies suggest that many genotypes/serotypes of O. tsutsugamushi are distributed throughout Japan and that the risk of infection with scrub typhus remains high.
Phylogenetic and phylogeographic analyses of O. tsutsugamushi have typically been based on the gene encoding the 56-kD type-specific antigen (TSA) protein thus far. This protein is expressed on the outer membrane surface and is the primary immunogen in human infections that is responsible for eliciting neutralizing antibodies. In general, a 56-kD TSA-based indirect fluorescence antibody technique (IFAT) is used for the diagnosis and classification of serotypes. There is a high diversity of 56-kD TSA genotypes among O. tsutsugamushi, and consequently type-specific PCR based on the 56-kD TSA gene is conducted to identify different genotypes. However, genes encoding major immune antigens that are modulated by the immune response of the host, such as the 56-kD TSA gene, are usually not suitable for phylogenetic and phylogeographic analyses. Housekeeping genes, such as heat shock protein genes or the 16S ribosomal RNA (rRNA) gene, are feasible candidates for such analyses because they are unaffected by the host immune response. The groEL gene, which encodes the 60-kD heat shock protein GroEL, is more diverse than the 16S rRNA gene. The sequences of the groES and groEL genes might be reconstructed more accurately with gene tree reconstructions, similar to 16S rRNA-based phylogeny. Thus, a comparison of the 56-kD TSA gene and the groEL or groES genes would help understand the molecular epidemiology of O. tsutsugamushi.
This study compared the entire groES and groEL gene sequences of six reference samples, i.e., the Kaisei, Sato, Kato, Kuroki, Matsuzawa, and Shimokoshi strains, and 15 field strains of O. tsutsugamushi. Phylogenetic comparisons between the 56-kD TSA gene and the groES or groEL genes suggest that gene rearrangement may be associated with homologous recombination in mites.
Materials and Methods
Genomic DNA was extracted using a PureLink genomic DNA mini kit (Invitrogen, San Diego, CA) from six O. tsutsugamushi reference strains isolated from patients in Japan, i.e., Kaisei, Sato, Kato, Kuroki, Matsuzawa, and Shimokoshi, and 15 field isolates from rodents in Japan, i.e., UAP1, UAP2, UAP4, UAP6, UAP7, FAR1, FAR2, HSB1, HSB2, HSB3, KNP1, KNP2, CMM1, O2, and O3 from infected L929 cells (Fujita et al. 2000, Tamura et al. 2001). DNA samples from two wild rodents in Japan (SH216 and 05-5) and two patients in Shimane, Japan (TD-7 and TD-17) were used for amplification (Tabara et al. 2012). Additionally, three DNA samples, SH205, SH234, and SH245, from rodents that were captured in Japan were also used for amplification. The gene encoding the O. tsutsugamushi 56-kD TSA protein was amplified from DNA samples by PCR using the primer set described by Furuya et al. (1993). To identify long sequences, 1722–1880 bp fragments were obtained using the primers Ot56-M329F (5′-CTAAGGTTAAYTGTARCRAGGTGC-3′) and Ot56-Rev1512R (5′-TCCACATACACACCTTCAGCAGCA-3′). In addition to the 56-kD TSA gene, the groES and groEL genes were also amplified. PCR was performed using oligonucleotide primers, which were newly designed based on the Ikeda strain and other O. tsutsugamushi strains. The primers groEL-14F (5′-TTGTACATRGCGATCAATGTCGT-3′) and groEL-1667R (5′-TAGAAATCCATTCCGCCCATAC-3′) were used for the first-round reaction, and the primers groEL-14F and groEL-772R (5′-GAGCTTCTCCGTCTACATCATCAG-3′) were used for the second-round reactions to detect the groEL gene. In addition, the primers groESL-M594F (5′-GGCTATARGCAATGATATAGCTAAACCAG-3′) and groEL-2258R (5′-GCGAACTATTAGTGCTAGCAACTAC-3′) were newly designed to identify the groESL gene operon of O. tsutsugamushi. First- and second-round PCR were performed using 20-μL reaction mixtures containing 250 μL of deoxyribonucleotide triphosphates (dNTPs), 1 U of Expand Long template DNA polymerase (Roche Applied Science, Penzberg, Upper Bavaria, Germany), and 0.25 μM of each primer.
Initial denaturation was conducted at 94°C for 2 min, followed by 30 cycles of denaturation at 94°C for 40 s, annealing at 55°C for 30 s, and extension at 72°C for 2 min using a GeneAmp PCR 9700 thermal cycler (Perkin-Elmer, Waltham, MA). PCR products were separated with MobiSpin S-400 spin columns (MoBiTec, Goettingen, Germany), and amplicons were sequenced directly using an ABI Prism 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA). Sequences were processed using Genetyx version 9.1 (Genetyx Corporation, Tokyo, Japan) and aligned with CLUSTALW and CLUSTALW2 (Thompson et al. 1994) using publicly available O. tsutsugamushi sequences. The phylogenetic analysis was conducted using Bayesian interference trees, which were generated with Bayesian Metropolis Hastings Markov Chain Monte Carlo (MCMC) tree-sampling methods implemented in MrBayes (Ronquist and Huelsenbeck 2003) using a GTR+I+Γ model of evolution, i.e., the hierarchical likelihood-ratio test (hLRT) in MrModeltest2.3 (http://www.abc.se/∼nylander/mrmodeltest2/mrmodeltest2.html) (Posada and Crandall 1998) with partitioning based on codon positions.
Results
Sequence and phylogenetic analysis of the groESL gene
The 271- to 285-bp and 1651- to 1668-bp groES and groEL genes of O. tsutsugamushi encoded predicted GroES and GroEL proteins with 90–95 and 550–555 amino acids, respectively. The interspecies variation among strains in the groES and groEL genes was negligible (0–5.6% at the nucleotide level and 0–4.0% at the amino acid level), with the exception of the Shimokoshi strain. Analysis of the groESL gene operon, which measures approximately 2.8-kbp and includes the groES and groEL genes of O. tsutsugamushi, revealed four known conserved motifs (two ribosomal binding sites, GGAGGA and AGGAG; one −35 region, TTGAAA; and one −10 region, TATGA) (Stover et al. 1990). A potential E. coli sigma 32 heat shock promoter (TTCACACTTGAA) was also found 5′ upstream of the groES gene. This study confirmed the overall high sequence similarity of the groESL gene operon among strains.
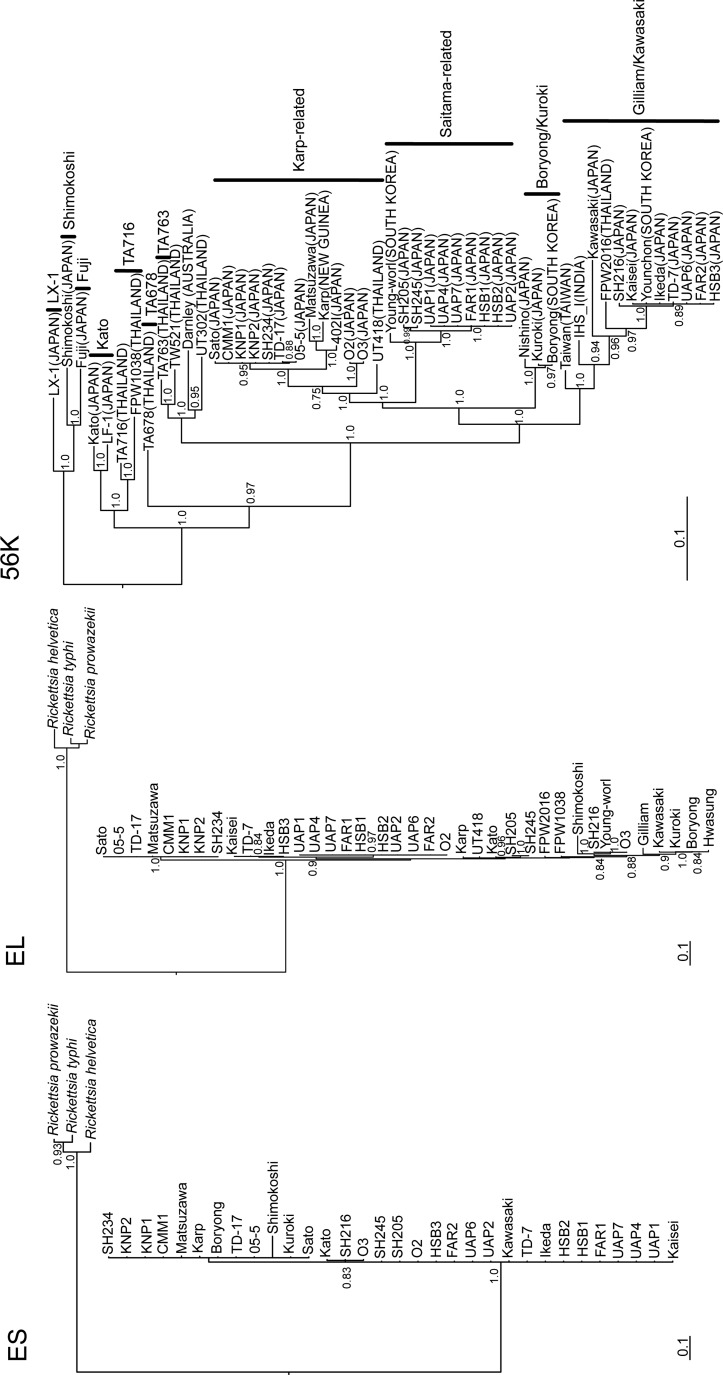
The partially sequenced 56-kD TSA gene, measuring 420–1481 nucleotides in length, was amplified from the genomic DNA of the Sato, CMM1, KNP1, KNP2, SH234, 05-5, and TD-17, and Kaisei, UAP6, FAR2, HSB3, TD-7, and SH216 strains, which showed that the sequences were very similar to those of the Karp-related-type and Gilliam/Kawasaki-type O. tsutsugamushi, respectively (Fig. 1, 56K). Exhaustive phylogenetic analyses based on the nucleotide and deduced amino acid sequences of the groES and groEL genes, which were generated using Bayesian MCMC estimation methods, indicated that both genes were very similar in this species. Interestingly, the two phylogenetic trees were not entirely identical, although they strongly resembled each other. The groES and groEL genes were highly conserved among species, and the differences between strains were less than 9.7%. Thus, highly conserved genes such as groES or groEL genes could be suitable targets for the molecular diagnosis of scrub typhus.
FIG. 1.
Phylogenetic trees generated using the Bayesian Markov chain Monte Carlo estimation methods (MCMC) with the GTR+I+Γ model of evolution, which was estimated from the data based on the alignment of the coding regions of the 271- to 285-nucleotide groES (ES), 1651- to 1668-nucleotide groEL (EL), and 420- to 1481-nucleotide 56-kD type-specific antigen (TSA) (56K) genes of the following Orientia tsutsugamushi strains: Kaisei (56-kD TSA, JX235719; groESL, JX188391) from patient, Sato (56-kD TSA, JX235718; groESL, JX188392) from patient, Kato (56-kD TSA, M63382; groESL, JX188393) from patient, Kuroki (56-kD TSA, M63380; groESL, JX188394) from patient, Matsuzawa (56-kD TSA, AF173043; groESL, KC688324) from patient, Shimokoshi (56-kD TSA, M63381; groESL, JX188395) from patient, UAP1 (56-kD TSA, AF302991; groESL, JX188396) from Apodemus speciosus, UAP2 (56-kD TSA, AF302992; groESL, KC688329) from A. speciosus, UAP4 (56-kD TSA, AF302993; groESL, JX188397) from A. speciosus, USP6 (56-kD TSA, AF302994; groESL, KC688325) from A. speciosus, UAP7 (56-kD TSA, AF302995; groESL, JX188398) from A. speciosus, FAR1 (56-kD TSA, AF302989; groESL, JX188399) from A. speciosus, FAR2 (56-kD TSA, AF302990; groESL, KC688326) from A. speciosus, HSB1 (56-kD TSA, AF302983; groESL, JX188400) from A. speciosus, HSB2 (56-kD TSA, AF302984; groESL, JX188401) from A. speciosus, HSB3 (56-kD TSA, AF302985; groESL, KC688327) from A. speciosus, CMM1 (56-kD TSA, AF302986; groESL, KC688328) from A. speciosus, KNP1 (56-kD TSA, AF302987; groESL, KC688320), KNP2 (56-kD TSA, AF302988; groESL, KC688321), O2 (56-kD TSA, KC688322; groESL, KC688330) from A. speciosus, O3 (56-kD TSA, KC688323; groESL, KC688331) from Apodemus argenteus, SH205 (56-kD TSA, KC693730; groESL, KC688332) from A. speciosus, SH234 (56-kD TSA, KC693731; groESL, KC688333) from A. speciosus, SH245 (56-kD TSA, KC693732; groESL, KC688334) from A. speciosus, TD-7 (56-kD TSA, JX188388; groESL, JX235722) from patient, TD-17 (56-kD TSA, JX188387; groESL, JX235720) from patient, 05-5 (56-kD TSA, JX188390; groESL, JX235721) from rodent, and SH216 (56-kD TSA, JX188389; groESL, JX188402) from Mus musculus. The phylogenetic positions of these strains are also shown relative to the following representative O. tsutsugamushi strains: Gilliam (groESL, AY191585) from patient, Taiwan (56-kD TSA, DQ485289) from patient, Ikeda (56-kD TSA, AF173033; groESL, NC010793) from patient, Younchon (56-kD TSA, U19903) from patient, Karp (56-kD TSA, M33004; groESL, M31887) from patient, Kawasaki (56-kD TSA, M63383; groESL, AY191587) from patient, Boryong (56-kD TSA, AM494475; groESL, NC009488) from patient, Nishino (56-kD TSA, AF173048) from patient, Young-worl (56-kD TSA, AF43141; groESL, AY191588) from patient, 402I (56-kD TSA, AF173047) from patient, Darnley (56-kD TSA, AY860955) from patient, FPW1038 (56-kD TSA, EF213087; groESL, EF551288) from patient, FPW2016 (56-kD TSA, EF213085; groESL, EF551289) from patient, Fuji (56-kD TSA, AF210834) from Leptotrombidium fuji, IHS I (56-kD TSA, DQ530440) from patient, Hwasung (groESL, QY191589) from patient, LF-1 (56-kD TSA, AF173050) from Leptotrombidium fletcheri, LX-1 (56-kD TSA, AF173042) from Leptotrombidium mite, TA678 (56-kD TSA, U19904) from Rattus rattus, TA716 (56-kD TSA, U19905) from Menetes berdmorei, TSA763 (56-kD TSA, RTU80636) from Rattus rajah, TW52-1 (56-kD TSA, AY222630) from Rattus norvegicus, UT302 (56-kD TSA, EF213095) from patient, and UT418 (56-kD TSA, EF213087; groESL, EF551310) from patient, with Rickettsia helvetica (DQ442911), Rickettsia prowazekii (Y15783), and Rickettsia typhi (AF462073) as outgroups. Genotypes based on the 56-kD TSA gene are shown on the right of 56K. The numbers at each node are the posterior node probabilities (>0.70) based on 150,000 trees. Two replicate MCMC runs consisting of six chains of 10 million generations were sampled every 100 generations with a burn-in of 25,000 (25%). The scale bar indicates the nucleotide substitutions per site. The country of origin is shown in parentheses.
Discussion
The present study identified the groESL gene operon of O. tsutsugamushi that includes the groES and groEL genes. The shared identities among strains were 90.4–100% and 90.3–100% for the groES and groEL genes, respectively. Recently, the groEL gene was used to identify Streptococcus, Enterococcus, Staphylococcus, Bartonella, Mycobacterium, and Ehrlichia. The present study demonstrated that the sequences of the heat shock protein genes groES and groEL are highly conserved among O. tsutsugamushi strains. Phylogenetic analyses indicated that the 56-kD TSA gene strongly resembled, but was not identical to, the groEL and groES genes, and these gene sequences are distinctly different from those in Rickettsia.
There are reports that indicate that the 56-kD TSA gene is polymorphic among species (Kollars et al. 2003), but gene-based molecular techniques have not been applied to investigate the relationships of the 56-kD TSA gene. Interestingly, phylogenetic analyses revealed no correlation between the 56-kD TSA gene and geographic location of O. tsutsugamushi strains and vector Leptotrombidium, although the gene is highly polymorphic among species (Fig. 1).
Phylogenetic analysis of the groES, groEL, and TSA genes detected no common clustering in the dendrograms of each phylogenetic tree (Fig. 1). Two and three clusters were detected in the groES and groEL gene-based dendrograms, respectively. The clustered strains did not belong to the same genotype according to analysis of the 56-kD TSA gene. A recent report identified genetic recombination between O. tsutsugamushi and its vector mite (Sonthayanon et al. 2010), which presaged the mismatches between the 56-kD TSA, groES, and groEL genes. Thus, the genomic DNA of O. tsutsugamushi is undoubtedly rearranged in different strains. More importantly, these findings show that the evolutionary history and transmission dynamics of O. tsutsugamushi are far richer and more complex than originally imagined. Given the sympatric and synchronistic coexistence of several O. tsutsugamushi strains and different species of vector mites in the field over evolutionary time, it seems plausible that ongoing exchanges within O. tsutsugamushi continue to drive its evolution. Phylogenetic analyses based on the groESL gene using more isolates will unveil further interesting evolutionary processes that have occurred in O. tsutsugamushi.
Acknowledgments
We thank Masashi Hamada for his technical assistance. This work was supported in part by a grant-in-aid from the Ministry of Health, Labor, and Welfare of Japan (Research on Emerging and Re-emerging Infectious Diseases, Health Science Research Grants), and by a grant-in aid from the Japan Society for the Promotion of Science 24405045 (Scientific Research grant B).
Author Disclosure Statement
No competing financial interests exist.
References
- Bang HA. Lee MJ. Lee WC. Comparative research on epidemiological aspects of tsutsugamushi disease (scrub typhus) between Korea and Japan. Jpn J Infect Dis. 2008;61:148–150. [PubMed] [Google Scholar]
- Fujita H. Watanabe Y. Hagiwara T. Takada N. Trombiculid mites and Orientia tsutsugamushi in field rodents in the northern part of Fukushima prefecture. Ann Rep Ohara Hosp. 2000;43:33–40. [Google Scholar]
- Furuya Y. Yoshida Y. Katayama T. Yamamoto S, et al. Serotype-specific amplification of Rickettsia tsutsugamushi DNA by nested polymerase chain reaction. J Clin Microbiol. 1993;31:1637–1640. doi: 10.1128/jcm.31.6.1637-1640.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ. Fuerst PA. Ching WM. Richards AL. Scrub typhus: The geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis. 2009;48(Suppl 3):S203–S230. doi: 10.1086/596576. [DOI] [PubMed] [Google Scholar]
- Kollars TM., Jr. Bodhidatta D. Phulsuksombati D. Tippayachai B, et al. Short report: Variation in the 56-kD type-specific antigen gene of Orientia tsutsugamushi isolated from patients in Thailand. Am J Trop Med Hyg. 2003;68:299–300. [PubMed] [Google Scholar]
- Ogawa M. Ono T. Epidemiological characteristics of tsutsugamushi disease in Oita Prefecture, Japan: yearly and monthly occurrences of its infections and serotypes of its causative agent, Orientia tsutsugamushi, during 1984–2005. Microbiol Immunol. 2008;52:135–143. doi: 10.1111/j.1348-0421.2008.00024.x. [DOI] [PubMed] [Google Scholar]
- Posada D. Crandall KA. MODELTEST: Testing the model of DNA substitution. Bioinformatics. 1998;14:817–8. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Ronquist F. Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Sonthayanon P. Peacock SJ. Chierakul W. Wuthiekanun V, et al. High rates of homologous recombination in the mite endosymbiont and opportunistic human pathogen Orientia tsutsugamushi. PLoS Negl Trop Dis. 2010;4:e752. doi: 10.1371/journal.pntd.0000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover CK. Marana DP. Carter JM. Roe BA, et al. The 56-kilodalton major protein antigen of Rickettsia tsutsugamushi: Molecular cloning and sequence analysis of the sta56 gene and precise identification of a strain-specific epitope. Infect Immun. 1990;58:2076–2084. doi: 10.1128/iai.58.7.2076-2084.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabara K. Kawabata H. Ando S. Arai S, et al. Epidemiological study of tsutsugamushi disease in Shimane Prefecture, Japan. J Jpn Vet Med Assoc. 2012;65:535–541. [Google Scholar]
- Tamura A. Takahashi K. Tsuruhara T. Urakami H, et al. Isolation of Rickettsia tsutsugamushi antigenically different from Kato, Karp, and Gilliam strains from patients. Microbiol Immunol. 1984;28:873–882. doi: 10.1111/j.1348-0421.1984.tb00743.x. [DOI] [PubMed] [Google Scholar]
- Tamura A. Yamamoto N. Koyama S. Makisaka Y, et al. Epidemiological survey of Orientia tsutsugamushi distribution in field rodents in Saitama Prefecture, Japan, and discovery of a new type. Microbiol Immunol. 2001;45:439–446. doi: 10.1111/j.1348-0421.2001.tb02643.x. [DOI] [PubMed] [Google Scholar]
- Thompson JD. Higgins DG. Gibson TJ. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]