Abstract
Background
The follicular variant of papillary thyroid carcinoma (FVPTC) presents distinct histologic subtypes and molecular genotyping. The preoperative diagnosis of FVPTC through fine-needle aspiration cytology (FNAC) is challenging.
Methods
We reviewed 59 archival thyroid FNAC specimens of surgically confirmed FVPTC according to histologic subtype: encapsulated FVPTC (n=30) and infiltrative FVPTC (n=29). Galectin-3 immunostaining and molecular analyses for BRAF and three RAS genes (NRAS, HRAS, and KRAS) were performed.
Results
FNAC diagnoses of FVPTC included benign (5%), atypia of undetermined significance (19%), follicular neoplasm/suspicious for follicular neoplasm (14%), suspicious for PTC (29%), and PTC (34%). Galectin-3 immunostaining was positive in 50% of FNAC specimens. A BRAF mutation was found only in 14 (24%) tumors with the FNAC diagnosis of PTC or suspicious for PTC: 13 cases with the usual c.1799T>A (p.V600E) mutation and 1 case with a 3 base-pair deletion (c.1799_1801delTGA), resulting in a deletion of lysine at codon 601 and a deletion c.1799_1801delTGA that results in a valine-to-glutamate substitution at codon 600 (p.V600_K601>E) while preserving the reading frame. A BRAF K601E mutation was not found. RAS mutations were observed in 18 (33%) tumors (NRAS, 22%; HRAS, 6%; KRAS, 6%). Mutations of the three RAS genes were detected in codon 61 but not in codons 12 and 13. There was a decreasing trend of RAS mutation rates associated with an increasing risk of malignancy in the FNAC diagnostic categories. The triage efficacy of FNAC to make a recommendation for surgery was 73% for encapsulated tumors and 79% for infiltrative tumors. Addition of galectin-3 or the BRAF test to FNAC showed no significant improvement in the triage efficacy. However, RAS mutations significantly improved the triage efficacy of FNAC. There was no significant difference in the triage efficacy of FNAC, galectin-3 expression, and the prevalence of somatic mutations between encapsulated and infiltrative tumors.
Conclusion
Thyroid FNAC has a low sensitivity for the detection of FVPTC regardless of histologic subtype. Encapsulated FVPTC and infiltrative FVPTC have similar molecular profiles and rates of galectin-3 expression. RAS mutational analysis is more useful than BRAF testing to improve the triage efficacy of FNAC for FVPTC.
Introduction
The follicular variant of papillary thyroid carcinoma (FVPTC) is a common variant comprising 15–20% of all papillary thyroid carcinoma (PTC) (1,2). The diagnosis of FVPTC is based on its histologic features comprising characteristic nuclear features of PTC and an exclusive or predominant (>50%) follicular growth pattern without well-formed papillae (3,4). FVPTC is divided into two subtypes, encapsulated and infiltrative, by the presence or absence of encapsulation (4–6). Encapsulated FVPTC is totally encapsulated by a fibrous tissue and may show tumor capsular or vascular invasion (4–6). The histopathologic diagnosis of infiltrative FVPTC usually poses no problem because the tumor infiltrates the surrounding thyroid parenchyma without a well-defined capsule but with the presence of most of the characteristic nuclear features of PTC. However, the diagnosis of the encapsulated subtype may be particularly difficult because the diagnostic nuclear features of PTC may be moderately developed and intermixed with cells lacking the diagnostic nuclear features in encapsulated FVPTC. Overall, the clinical behavior of infiltrative FVPTC is similar to that of classic PTC with a high rate of lymph node metastasis, whereas encapsulated FVPTC has a behavior much closer to that of follicular adenoma and follicular carcinoma with a low rate of lymph node metastasis (7). In addition to the encapsulated and infiltrative subtypes of FVPTC, a rare aggressive diffuse follicular variant was originally described by Sobrinho-Simões et al. in 1990 (8). The diffuse FVPTC occurs primarily in young females and is characterized by extensive, multifocal involvement of one or both lobes of the thyroid gland (6,9,10).
Fine-needle aspiration cytology (FNAC) is the most reliable and commonly used diagnostic method for thyroid nodules. The Bethesda System for Reporting Thyroid Cytopathology was introduced to reduce ambiguous and implicit thyroid FNAC interpretations through the use of a standardized terminology and general diagnostic categories (11,12). Each diagnostic category has an implied risk of malignancy as well as a recommended clinical management.
The cytopathologic diagnosis of FVPTC is challenging in FNAC because nuclear changes are often subtle and the characteristic nuclear features of PTC can be partially and focally displayed (13–16). The diagnostic sensitivity of FNAC for FVPTC ranges from 9% to 75%, which is very low compared with that of FNAC for classic PTC. FVPTC is a major cause of the cases with false-negative results in FNAC (13–17). Therefore, the diagnosis of FVPTC by cytomorphology alone has limitations and thus requires additional methods such as immunostaining and molecular testing to facilitate the diagnosis of FVPTC.
Galectin-3, a β-galactoside-binding lectin, has been used to aid the differential diagnosis between thyroid cancer and benign thyroid lesions from FNAC samples and surgical tissue specimens (18). Positive galectin-3 immunostaining is observed in almost all classic PTCs, whereas in FVPTC, the positive rate is lower and more focal, and of moderate to weak intensity (18). A difference may exist in galectin-3 expression for FVPTC between postsurgical tissues and clinical FNAC samples.
FVPTCs frequently harbor RAS mutations and have a relatively low rate of BRAF mutations compared to classic PTC (19–21). However, there is controversy about the BRAF and RAS mutational patterns of FVPTC according to its histologic subtypes. Rivera et al. (5) reported that encapsulated FVPTC had a high rate of RAS and an absence of BRAF mutations, while the infiltrative variant showed a molecular profile with a high prevalence of BRAF and a low frequency of RAS mutations. Infiltrative FVPTC had a BRAF and RAS mutational pattern in between follicular neoplasms and classic PTC, whereas the molecular profile of encapsulated FVPTC was similar to follicular neoplasms. However, Gupta et al. (6) found that the two subtypes had similar clinical behaviors and mutation rates for BRAF and RAS.
We therefore analyzed the efficacy of FNAC to make a recommendation for surgery based on the Bethesda System in patients with FVPTC and investigated the molecular profiles of FVPTC according to its histologic subtypes, which may play a role as an adjunctive diagnostic tool along with traditional FNAC.
Materials and Methods
This study was approved by the Institutional Review Board of the Catholic University of Korea Seoul St. Mary's Hospital, and written informed consent was obtained from all subjects or their legal guardians.
Case collection
A total of 59 consecutive patients with FVPTC were enrolled in this study and underwent surgery at Seoul St. Mary's Hospital between January 2011 and April 2012. All histological slides were reviewed independently by three experienced pathologists (S.R.L., C.K.J., and Y.J.C.) with a special interest in thyroid pathology who were familiar with FVPTC histology, and consensus diagnoses were made following the World Health Organization classification (3). All patients enrolled in this study underwent ultrasound-guided FNAC prior to surgery. All FNAC cases were classified into six-tiered diagnostic categories using the Bethesda System for Reporting Thyroid Cytopathology. FVPTC was defined as a papillary carcinoma predominantly composed of follicles (>50% of the tumor) and a complete lack of well-formed papillae. All tumors were classified into encapsulated and infiltrative subtypes based on histologic growth patterns only, irrespective of tumor size. We did not consider whether the tumor was a microcarcinoma, which is defined as a malignant tumor of ≤1 cm in diameter. The rare diffuse follicular variant was not included in this study.
Triage efficacy of FNAC to make a recommendation for surgery was defined as FNAC classification as follicular neoplasm/suspicious for a follicular neoplasm, suspicious for malignancy, or PTC that triggers surgical management. Diagnostic accuracy was defined as FNAC classification as suspicious for malignancy or PTC (17).
Galectin-3 immunohistochemical staining
Immunohistochemical staining was performed on both FNAC-derived cell blocks and corresponding surgical specimens as previously described (22). A mouse monoclonal antibody against galectin-3 (1:800; Novocastra Lab., Newcastle upon Tyne, United Kingdom) was applied for 30 minutes at room temperature. Immunoreaction was detected using the EnVision Plus System (Dako, Carpinteria, CA) and by counterstaining with hematoxylin. Galectin-3 expression was considered positive on both tissue and cell block sections if >5% of tumor cells displayed cytoplasmic and nuclear immunostaining.
Mutational analysis of BRAF and RAS genes
Genomic DNA was isolated from two 10-μm-thick formalin-fixed, paraffin-embedded tissue sections containing a representative portion of each archival tissue block using the QIAamp DNA Mini kit (Qiagen, Hilden, Germany). Tumor areas were manually microdissected from the tissue sections. We performed mutational analysis of exon 15 of the BRAF gene and exons 2 and 3 of the NRAS, HRAS, and KRAS genes using PCR amplification and DNA sequencing, as described earlier (23–25).
Statistical analysis
Continuous parametric data with a normal distribution were compared using the Student's t-test. The nonparametric Mann–Whitney U-test was used to analyze continuous data with a skewed distribution. Categorical data were compared using the chi-squared test. The Fisher's exact test was used when more than 20% of cells had less than 5 expected frequencies for categorical data with a lower number of samples. The linear-by-linear association test was used to analyze the change of molecular genotyping as progressing in severity from benign to malignant FNAC diagnostic categories (ptrend). All analyses were performed with the use of SPSS version 16.0 (SPSS Inc., Chicago, IL). Two-sided p-values of ≤0.05 were considered statistically significant.
Results
Patient characteristics
The study population comprised 59 patients (45 women and 14 men). The age of the patients at the time of surgery ranged from 20 to 79 years (mean 48.4 years). According to the histologic subtypes of FVPTC, 30 of 59 cases were encapsulated variants and 29 were infiltrative variants. There was no significant difference in the mean age between patients (47.4 years) with encapsulated FVPTC and those (49.3 years) with infiltrative FVPTC (p=0.546). The median size of encapsulated and infiltrative tumors was 0.9 and 0.6 cm, respectively (p=0.132). Out of a total of 59 patients, 32 had two or more tumor foci. The largest tumor foci from five patients were classic PTC. Therefore, we excluded the 5 cases and further analyzed the clinicopathologic features of 54 patients according to the histologic subtypes of the FVPTC. There were no significant difference in age, sex, pT stage, extrathyroid extension, multifocality, or lymph node metastasis between patients with encapsulated tumors and those with infiltrative tumors (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/thy).
Fine-needle aspiration cytology
FNAC diagnoses of FVPTC included benign (5%), atypia of undetermined significance (19%), follicular neoplasm/suspicious for a follicular neoplasm (14%), suspicious for PTC (29%), and PTC (34%). There was no significant difference in the distribution of FNAC diagnostic categories between encapsulated and infiltrative FVPTC (Table 1).
Table 1.
Distribution of Fine-Needle Aspiration Cytology Diagnoses According to the Histologic Subtypes of Follicular Variant of Papillary Thyroid Carcinoma
| Diagnostic category | Encapsulated | Infiltrative | Total |
|---|---|---|---|
| Nondiagnostic | 0 | 0 | 0 |
| Benign | 1 (3%) | 2 (7%) | 3 (5%) |
| Atypia of undetermined significance | 7 (23%) | 4 (14%) | 11 (19%) |
| Follicular neoplasm/suspicious for a follicular neoplasm | 3 (10%) | 5 (17%) | 8 (14%) |
| Suspicious for papillary carcinoma | 8 (27%) | 9 (31%) | 17 (29%) |
| Papillary carcinoma | 11 (37%) | 9 (31%) | 20 (34%) |
| Total | 30 (100%) | 29 (100%) | 59 (100%) |
Galectin-3 immunostaining
Out of a total of 59 FNAC specimens, 38 cases had residual follicular cells on cell blocks available for galectin-3 immunostaining. Positive staining for galectin-3 was observed in 19 (50%) FNAC specimens, including 8 (42%) encapsulated and 11 (58%) infiltrative subtypes. Of 58 tissue specimens, 44 cases (76%) containing 19 (66%) encapsulated and 25 (86%) infiltrative subtypes expressed galectin-3 protein. The overall positive rate of galectin-3 expression was higher in tissue specimens (76%) than in cell blocks (50%). There was no significant difference between the positive rates of galectin-3 expression in encapsulated and infiltrative subtypes irrespective of specimen type (Table 2).
Table 2.
Galectin-3 Immunostaining on Fine-Needle Aspiration Cytology and Surgical Specimens According to the Histologic Subtypes of Follicular Variant of Papillary Thyroid Carcinoma
| Specimen type | Galectin-3 | Encapsulated | Infiltrative | p | Total |
|---|---|---|---|---|---|
| Cell block | Negative | 11/19 (58%) | 8/19 (42%) | 0.330 | 19/38 (50%) |
| Positive | 8/19 (42%) | 11/19 (58%) | 19/38 (50%) | ||
| Surgical specimen | Negative | 10/29 (35%) | 4/29 (14%) | 0.066 | 14/58 (24%) |
| Positive | 19/29 (66%) | 25/29 (86%) | 44/58 (76%) |
Molecular analysis
The molecular alterations found in the analyzed FVPTC are summarized in Table 3. A BRAF mutation was detected in 14 (24%) of 58 tumors, including 7 (23%) encapsulated and 7 (25%) infiltrative tumors. The total mutation rate of the three RAS genes was 33% (18/54). Positive cases consisted of NRAS, HRAS, and KRAS mutations in 12 (22%), 3 (6%), and 3 (6%) cases, respectively. RAS mutations were detected in codon 61 of the three RAS genes, but not in codons 12 and 13. There was no statistically significant difference in the prevalence of somatic mutations between encapsulated and infiltrative tumors (Table 4). Among FVPTC, RAS-mutated tumors were significantly larger than non-RAS-mutated tumors (mean 1.3 cm vs. 0.9 cm, p=0.013). However, there were no significant differences in age, sex, pT stage, extrathyroid extension, multifocality, or lymph node metastasis between the RAS mutation status and the clinicopathologic features of FVPTC (Supplementary Table S2).
Table 3.
Frequency of Mutations in Follicular Variant of Papillary Thyroid Carcinoma for the BRAF and RAS Genes
| Nucleotide change | Amino acid change | No. of mutated cases |
|---|---|---|
| BRAF codon 600–601 | ||
| c.1799T>A | p.V600E | 13/58 (22%) |
| c.1799_1801delTGA | p.V600_K601>Ea | 1/58 (2%) |
| NRAS codon 12–13 | ||
| none | ||
| NRAS codon 61 | ||
| c.181C>A | p.Q61K | 3/54 (6%) |
| c.182A>G | p.Q61R | 9/54 (17%) |
| HRAS codon 12–13 | ||
| none | ||
| HRAS codon 61 | ||
| c.182A>G | p.Q61R | 3/54 (6%) |
| KRAS codon 12–13 | ||
| none | ||
| KRAS codon 61 | ||
| c.182A>G | p.G61R | 2/54 (4%) |
| c.180_181TC>AA | p.G61K | 1/54 (2%) |
This rare deletion mutation in the BRAF gene (c.1799_1801delTGA) converts codons 600 GTG (valine) and 601 AAA (lysine) to a new single codon GAA (glutamic acid) (p.V600_K601>E) while preserving the reading frame of the coding region of the gene.
Table 4.
Molecular Genotype According to the Histologic Subtypes of Follicular Variant of Papillary Thyroid Carcinoma
| Genotype | Encapsulated | Infiltrative | p | Total |
|---|---|---|---|---|
| BRAF mutation | 7/30 (23%) | 7/28 (25%) | 0.882 | 14/58 (24%) |
| RAS mutation | 11/28 (39%) | 7/26 (27%) | 0.336 | 18/54 (33%) |
| Somatic mutation | 18/28 (64%) | 14/26 (54%) | 0.435 | 32/54 (59%) |
Comparison of molecular profiles and FNAC results
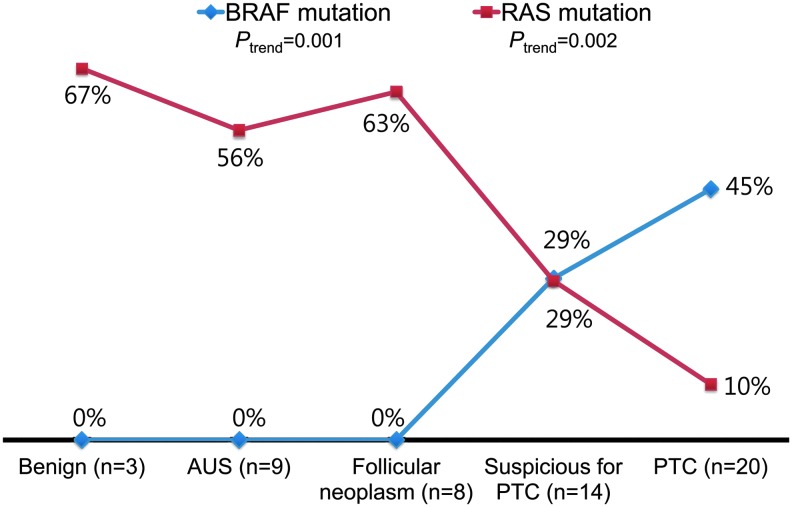
We compared the results of molecular profiles with the diagnostic results of FNAC. A BRAF mutation was found in 45% and 29% of cases that were diagnosed as PTC and suspicious for PTC by FNAC, respectively. However, all cases that were cytopathologically diagnosed as benign, atypia of undetermined significance, or follicular neoplasm/suspicious for a follicular neoplasm showed negative results for BRAF mutations. There was a decreasing trend of RAS mutation rates associated with increasing risk of malignancy in the FNAC diagnostic categories (ptrend=0.002), while there was an increasing trend of BRAF mutation rate among these categories (ptrend=0.001; Fig. 1).
FIG. 1.
Molecular genotype according to the diagnostic categories of fine-needle aspiration cytology. A p-value for trend was analyzed by the linear-by-linear association test (ptrend<0.001). PTC, papillary thyroid carcinoma. Color images available online at www.liebertpub.com/thy
FNAC triage efficacy and diagnostic accuracy
The FNAC triage efficacy for triggering surgery was 76%, and the diagnostic accuracy of FNAC was 63%. There was no significant difference in these diagnostic values of FNAC according to the histologic subtypes of FVPTC. Although molecular testing was done using surgical specimens instead of FNAC specimens, we tried to analyze whether molecular testing can improve the triage efficacy of FNAC (Table 5). Galectin-3 immunostaining and the BRAF mutation test did not improve the triage efficacy or diagnostic accuracy. However, RAS mutation testing significantly improved the triage efficacy of FNAC from 76% to 91% (p<0.001).
Table 5.
Triage Efficacy for Triggering Surgery in Fine-Needle Aspiration Cytology
| Encapsulated | Infiltrative | Total | |
|---|---|---|---|
| FNAC alone | 22/30 (73%) | 23/29 (79%) | 45/59 (76%) |
| FNAC & galectin-3 | 1/11 (9%) | 27/27 (100%) | 28/38 (74%) |
| FNAC & BRAF mutation | 22/30 (73%) | 23/28 (82%) | 45/58 (78%) |
| FNAC & RAS mutation | 26/28 (93%) | 23/26 (89%) | 49/54 (91%) |
| FNAC & somatic mutation | 27/29 (93%) | 25/28 (89%) | 52/57 (91%) |
FNAC, fine-needle aspiration cytology; triage efficacy, defined as classification of an FNAC as papillary thyroid carcinoma, suspicious for malignancy, or follicular neoplasm.
Discussion
Unlike classic PTC, FVPTC shows wide variation in the distribution of characteristic cytologic features of PTC in FNAC specimens and is therefore more challenging to diagnose (17,26,27). The rate of malignant category of FNAC diagnoses in patients with FVPTC ranges from 9% to 38% (13,14,17,26,27). In our study, 20 (34%) of 59 FVPTCs were classified as malignant on the FNAC specimens.
The BRAF V600E mutation constitutes the vast majority of all BRAF alterations detected in thyroid tumors and is found in about 45% of PTC, with a range of 29–83% (28). Another BRAF mutation, K601E, has been reported in up to 9% of FVPTCs (29). The BRAF V600E mutation is present in about 10–20% of FVPTC (27,28,30). We have previously reported that the BRAF V600E mutation was found in 839 (81%) of 1041 Korean patients with PTC (23). In the present study, the prevalence of this mutation was 24% for FVPTC and the BRAF K601E mutation was not found. In a 54-year-old man with a 3.3 cm encapsulated oncocytic FVPTC, we found a deletion of 3 bp, TGA (coding nucleotide 1799–1801), in exon 15 of the BRAF gene. The in-frame deletion of TGA (c.1799_1801delTGA) results in a deletion of lysine at codon 601 and a valine-to-glutamate substitution at codon 600 in the resultant BRAF protein (p.V600_K601>E) while preserving the reading frame of the coding region of the gene.
Our study revealed a distinct stratification in the molecular profile of FVPTC according to the severity of the preceding cytopathologic diagnosis in the Bethesda System. FVPTCs with FNAC diagnoses of “benign,” “atypia of undetermined significance,” or “follicular neoplasm/suspicious for a follicular neoplasm” harbored RAS mutations, but no BRAF mutations. A decreasing trend of RAS mutation rate progressing in severity from benign to malignant FNAC diagnostic categories was detected. One possible reason for the change in the molecular profile according to the cytologic diagnosis is the morphologic heterogeneity of FVPTC. Therefore, the diagnosis of FVPTC may not be so clear by microcopy, especially in the encapsulated subtype. High interobserver variability has been reported for this tumor (31,32). When there was a complete agreement about the diagnosis of FVPTC among the pathologists, the BRAF mutation rate was 25%, whereas no FVPTCs with a low consensus of diagnosis harbored the mutation (33). In the present study, there were no significant differences in the distribution of FNAC diagnoses, galectin-3 positivity, and molecular profiles between encapsulated FVPTCs and infiltrative FVPTCs. FVPTCs with the lesser degrees of cytologic features of PTC were diagnosed as “benign,” “atypia of undetermined significance,” or “follicular neoplasm/suspicious for a follicular neoplasm” and had a high rate of RAS mutations and no BRAF mutations, whereas FVPTCs with readily recognizable cytologic features of PTC were easily diagnosed as malignant/suspicious for malignancy and harbored a high rate of BRAF mutations and a low rate of RAS mutations. Therefore, in clinical practice, the RAS mutation test may be more useful as an ancillary test rather than the BRAF test or galectin-3 immunostaining in the diagnosis of FVPTC using FNAC specimens.
Different specimen types of FNAC and surgical specimens may induce different results of immunostaining and molecular testing in the sample nodule. In our study, cell blocks were available in 38/59 FNAC specimens. We noted galectin-3 positivity of 50% (19/38) in FNAC specimens and 76% (44/58) in surgical tissue. Before this study, we examined the preliminary concordance of molecular tests between FNAC and surgical specimens using 20 cell blocks containing sufficient amounts of follicular cells for molecular testing and corresponding resection specimens. All 20 cases had 100% concordance of molecular analyses for the BRAF and RAS genes between the two types of specimens.
RAS mutations are not specific for malignancy but may occur in benign thyroid lesions. They are found in 40–50% of conventional follicular carcinomas, about 40% of FVPTCs, and about 30% of benign follicular adenomas (34). Despite the lack of specificity for malignancy, it has been reported that RAS mutation testing improves the diagnostic accuracy of FNAC (24). In a large prospective study, a RAS-positive nodule in an FNAC sample had an 87.5% probability of malignancy (24). Furthermore, RAS-positive follicular adenoma is likely to progress to follicular carcinoma and FVPTC (24). For these reasons, surgical resection may be justified in patients with RAS-positive adenomas for the prevention of their disease progression. In the present study, the overall RAS mutation rate of FVPTCs was 33% (18/54). Specifically, the mutation rates were 67%, 56%, and 63% in FNACs diagnosed as “benign,” “atypia of undetermined significance,” or “follicular neoplasm/suspicious for a follicular neoplasm,” respectively. Therefore, our study provides evidence that RAS mutation testing may be diagnostically useful to decrease the false-negative rate. However, routine molecular testing is not recommended for all patients with benign cytologic diagnosis.
Out of the three RAS genes (NRAS, HRAS, and KRAS) found in thyroid cancers, the most frequently affected hot spots are NRAS codon 61 and HRAS codon 61. Much lower mutation rates have been found in KRAS codons 12 and 13 (34,35). In the present study, the NRAS codon 61 mutation was most common. Interestingly, KRAS mutations only involved codon 61, but not codons 12 and 13. These data are similar to those reported in a recent molecular study in which the KRAS codon 61 mutation was found in 1 of 22 encapsulated FVPTCs (6). We therefore suggest that KRAS mutation testing for thyroid cancer should include codon 61 analysis as well as codons 12 and 13.
We observed that FVPTCs with RAS mutations are larger than FVPTCs with wild-type RAS. This finding is in accordance with the data on record (5,19). In follicular thyroid carcinomas, the presence of a RAS mutation is also correlated with a large tumor size (34). The NRAS codon 61 mutation is associated with distant metastasis, and RAS mutations are associated with poor overall patient survival (36). The NRAS codon 61 mutation has also been reported in FVPTCs with distant metastasis (5). Gupta et al. (6) observed that encapsulated FVPTC had a molecular profile and clinical behavior similar to those of infiltrative FVPTC. In the present study, there were no differences in the mutation rates of RAS and BRAF between encapsulated and infiltrative FVPTC. Tumors with RAS mutations showed no significant differences in clinicopathologic features compared to the RAS wild-type group. However, limitations of the present study include a small sample size, retrospective design, and enrollment of patients with tumors of less than 1 cm in size. Further studies are needed to elucidate the role of RAS mutations in FVPTC and to clarify the differences between the clinicopathologic characteristics and prognostic factors in patients with encapsulated and infiltrative FVPTC.
Out of a total of 59 patients, 3 had a benign cytologic diagnosis; however, they underwent surgical excision because of cytologic diagnoses of PTC or follicular neoplasm in the opposite lobe, or another fast growing nodule with suspicious sonographic findings in the same lobe (Supplementary Table S3).
In conclusion, we found that thyroid FNAC had a low sensitivity for detecting FVPTC regardless of histologic subtype. Encapsulated and infiltrative FVPTCs had similar molecular and galectin-3 expression profiles. RAS mutational analysis is a useful ancillary tool to improve the triage efficacy of FNAC for FVPTC, but BRAF testing does not improve the preoperative diagnostic accuracy of FNAC for FVPTC.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the financial support of the Catholic Medical Center Research Foundation made in the program year of 2012. This research was partially supported by a research grant 2011 from Korean Thyroid Association.
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Carcangiu ML. Zampi G. Pupi A. Castagnoli A. Rosai J. Papillary carcinoma of the thyroid. A clinicopathologic study of 241 cases treated at the University of Florence, Italy. Cancer. 1985;55:805–828. doi: 10.1002/1097-0142(19850215)55:4<805::aid-cncr2820550419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Lam AK. Lo CY. Lam KS. Papillary carcinoma of thyroid: a 30-yr clinicopathological review of the histological variants. Endocr Pathol. 2005;16:323–330. doi: 10.1385/ep:16:4:323. [DOI] [PubMed] [Google Scholar]
- 3.LiVolsi VA. Albores-Saavedra J. Asa SL. Baloch ZW. Sobrinho-Simões M. Wenig B. DeLellis RA. Cady B. Mazzaferri EL. Hay I. Fagin JA. Weber AL. Caruso P. Voutilainen PE. Franssila KO. Williams ED. Schneider AB. Nikiforov YE. Rabes HM. Akslen L. Ezzat S. Santoro M. Eng C. Harach HR. Papillary carcinoma. In: DeLellis RA, editor; Lloyd RV, editor; Heitz PU, editor; Eng C, editor. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs. I. ARC Press; Lyon, France: 2004. pp. 57–66. [Google Scholar]
- 4.Nikiforov YE. Ohori NP. Papillary carcinoma. In: Nikiforov YE, editor; Biddinger PW, editor; Thompson LDR, editor. Diagnostic Pathology and Molecular Genetics of the Thyroid. 2nd. Lippincott Williams & Wilkins; Philadelphia, PA: 2012. pp. 183–246. [Google Scholar]
- 5.Rivera M. Ricarte-Filho J. Knauf J. Shaha A. Tuttle M. Fagin JA. Ghossein RA. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010;23:1191–1200. doi: 10.1038/modpathol.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S. Ajise O. Dultz L. Wang B. Nonaka D. Ogilvie J. Heller KS. Patel KN. Follicular variant of papillary thyroid cancer: encapsulated, nonencapsulated, and diffuse: distinct biologic and clinical entities. Arch Otolaryngol Head Neck Surg. 2012;138:227–233. doi: 10.1001/archoto.2011.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghossein R. Encapsulated malignant follicular cell-derived thyroid tumors. Endocr Pathol. 2010;21:212–218. doi: 10.1007/s12022-010-9141-8. [DOI] [PubMed] [Google Scholar]
- 8.Sobrinho-Simões M. Soares J. Carneiro F. Limbert E. Diffuse follicular variant of papillary carcinoma of the thyroid: report of eight cases of a distinct aggressive type of thyroid tumor. Surg Pathol. 1990;3:189–203. [Google Scholar]
- 9.Ivanova R. Soares P. Castro P. Sobrinho-Simoes M. Diffuse (or multinodular) follicular variant of papillary thyroid carcinoma: a clinicopathologic and immunohistochemical analysis of ten cases of an aggressive form of differentiated thyroid carcinoma. Virchows Archiv. 2002;440:418–424. doi: 10.1007/s00428-001-0543-3. [DOI] [PubMed] [Google Scholar]
- 10.Mizukami Y. Nonomura A. Michigishi T. Ohmura K. Noguchi M. Ishizaki T. Diffuse follicular variant of papillary carcinoma of the thyroid. Histopathology. 1995;27:575–577. doi: 10.1111/j.1365-2559.1995.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 11.Baloch ZW. LiVolsi VA. Asa SL. Rosai J. Merino MJ. Randolph G. Vielh P. DeMay RM. Sidawy MK. Frable WJ. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425–437. doi: 10.1002/dc.20830. [DOI] [PubMed] [Google Scholar]
- 12.Lee K. Jung CK. Lee KY. Bae JS. Lim DJ. Jung SL. Application of Bethesda System for reporting thyroid aspiration cytology. Korean J Pathol. 2010;44:521–527. [Google Scholar]
- 13.Goodell WM. Saboorian MH. Ashfaq R. Fine-needle aspiration diagnosis of the follicular variant of papillary carcinoma. Cancer. 1998;84:349–354. doi: 10.1002/(sici)1097-0142(19981225)84:6<349::aid-cncr6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 14.Kesmodel SB. Terhune KP. Canter RJ. Mandel SJ. LiVolsi VA. Baloch ZW. Fraker DL. The diagnostic dilemma of follicular variant of papillary thyroid carcinoma. Surgery. 2003;134:1005–1012. doi: 10.1016/j.surg.2003.07.015. discussion 1012. [DOI] [PubMed] [Google Scholar]
- 15.Lin HS. Komisar A. Opher E. Blaugrund SM. Follicular variant of papillary carcinoma: the diagnostic limitations of preoperative fine-needle aspiration and intraoperative frozen section evaluation. Laryngoscope. 2000;110:1431–1436. doi: 10.1097/00005537-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Tielens ET. Sherman SI. Hruban RH. Ladenson PW. Follicular variant of papillary thyroid carcinoma. A clinicopathologic study. Cancer. 1994;73:424–431. doi: 10.1002/1097-0142(19940115)73:2<424::aid-cncr2820730230>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 17.Kurian EM. Dawlett M. Wang J. Gong Y. Guo M. The triage efficacy of fine needle aspiration biopsy for follicular variant of papillary thyroid carcinoma using the Bethesda reporting guidelines. Diagn Cytopathol. 2012;40(Suppl 1):E69–E73. doi: 10.1002/dc.21718. [DOI] [PubMed] [Google Scholar]
- 18.Chiu CG. Strugnell SS. Griffith OL. Jones SJ. Gown AM. Walker B. Nabi IR. Wiseman SM. Diagnostic utility of galectin-3 in thyroid cancer. Am J Pathol. 2010;176:2067–2081. doi: 10.2353/ajpath.2010.090353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro P. Rebocho AP. Soares RJ. Magalhaes J. Roque L. Trovisco V. Vieira de Castro I. Cardoso-de-Oliveira M. Fonseca E. Soares P. Sobrinho-Simoes M. PAX8-PPARgamma rearrangement is frequently detected in the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2006;91:213–220. doi: 10.1210/jc.2005-1336. [DOI] [PubMed] [Google Scholar]
- 20.Di Cristofaro J. Marcy M. Vasko V. Sebag F. Fakhry N. Wynford-Thomas D. De Micco C. Molecular genetic study comparing follicular variant versus classic papillary thyroid carcinomas: association of N-ras mutation in codon 61 with follicular variant. Hum Pathol. 2006;37:824–830. doi: 10.1016/j.humpath.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 21.Ohori NP. Nikiforova MN. Schoedel KE. LeBeau SO. Hodak SP. Seethala RR. Carty SE. Ogilvie JB. Yip L. Nikiforov YE. Contribution of molecular testing to thyroid fine-needle aspiration cytology of “follicular lesion of undetermined significance/atypia of undetermined significance.”. Cancer Cytopathol. 2010;118:17–23. doi: 10.1002/cncy.20063. [DOI] [PubMed] [Google Scholar]
- 22.Kim ES. Lim DJ. Lee K. Jung CK. Bae JS. Jung SL. Baek KH. Lee JM. Moon SD. Kang MI. Cha BY. Lee KW. Son HY. Absence of galectin-3 immunostaining in fine-needle aspiration cytology specimens from papillary thyroid carcinoma is associated with favorable pathological indices. Thyroid. 2012;22:1244–1250. doi: 10.1089/thy.2011.0166. [DOI] [PubMed] [Google Scholar]
- 23.Jung CK. Im SY. Kang YJ. Lee H. Jung ES. Kang CS. Bae JS. Choi YJ. Mutational patterns and novel mutations of the BRAF gene in a large cohort of Korean patients with papillary thyroid carcinoma. Thyroid. 2012;22:791–797. doi: 10.1089/thy.2011.0123. [DOI] [PubMed] [Google Scholar]
- 24.Nikiforov YE. Steward DL. Robinson-Smith TM. Haugen BR. Klopper JP. Zhu Z. Fagin JA. Falciglia M. Weber K. Nikiforova MN. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009;94:2092–2098. doi: 10.1210/jc.2009-0247. [DOI] [PubMed] [Google Scholar]
- 25.Molinari F. Felicioni L. Buscarino M. De Dosso S. Buttitta F. Malatesta S. Movilia A. Luoni M. Boldorini R. Alabiso O. Girlando S. Soini B. Spitale A. Di Nicolantonio F. Saletti P. Crippa S. Mazzucchelli L. Marchetti A. Bardelli A. Frattini M. Increased detection sensitivity for KRAS mutations enhances the prediction of anti-EGFR monoclonal antibody resistance in metastatic colorectal cancer. Clin Cancer Res. 2011;17:4901–4914. doi: 10.1158/1078-0432.CCR-10-3137. [DOI] [PubMed] [Google Scholar]
- 26.VanderLaan PA. Marqusee E. Krane JF. Features associated with locoregional spread of papillary carcinoma correlate with diagnostic category in the Bethesda System for Reporting Thyroid Cytopathology. Cancer Cytopathol. 2012;120:245–253. doi: 10.1002/cncy.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Proietti A. Giannini R. Ugolini C. Miccoli M. Fontanini G. Di Coscio G. Romani R. Berti P. Miccoli P. Basolo F. BRAF status of follicular variant of papillary thyroid carcinoma and its relationship to its clinical and cytological features. Thyroid. 2010;20:1263–1270. doi: 10.1089/thy.2009.0283. [DOI] [PubMed] [Google Scholar]
- 28.Tufano RP. Teixeira GV. Bishop J. Carson KA. Xing M. BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: a systematic review and meta-analysis. Medicine. 2012;91:274–286. doi: 10.1097/MD.0b013e31826a9c71. [DOI] [PubMed] [Google Scholar]
- 29.Trovisco V. Soares P. Preto A. de Castro IV. Lima J. Castro P. Maximo V. Botelho T. Moreira S. Meireles AM. Magalhaes J. Abrosimov A. Cameselle-Teijeiro J. Sobrinho-Simoes M. Type and prevalence of BRAF mutations are closely associated with papillary thyroid carcinoma histotype and patients' age but not with tumour aggressiveness. Virchows Archiv. 2005;446:589–595. doi: 10.1007/s00428-005-1236-0. [DOI] [PubMed] [Google Scholar]
- 30.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 31.Lloyd RV. Erickson LA. Casey MB. Lam KY. Lohse CM. Asa SL. Chan JK. DeLellis RA. Harach HR. Kakudo K. LiVolsi VA. Rosai J. Sebo TJ. Sobrinho-Simoes M. Wenig BM. Lae ME. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol. 2004;28:1336–1340. doi: 10.1097/01.pas.0000135519.34847.f6. [DOI] [PubMed] [Google Scholar]
- 32.Elsheikh TM. Asa SL. Chan JK. DeLellis RA. Heffess CS. LiVolsi VA. Wenig BM. Interobserver and intraobserver variation among experts in the diagnosis of thyroid follicular lesions with borderline nuclear features of papillary carcinoma. Am J Clin Pathol. 2008;130:736–744. doi: 10.1309/AJCPKP2QUVN4RCCP. [DOI] [PubMed] [Google Scholar]
- 33.Wallander M. Layfield LJ. Jarboe E. Emerson L. Liu T. Thaker H. Holden J. Tripp S. Follicular variant of papillary carcinoma: reproducibility of histologic diagnosis and utility of HBME-1 immunohistochemistry and BRAF mutational analysis as diagnostic adjuncts. Appl Immunohistochem Mol Morphol. 2010;18:231–235. doi: 10.1097/PAI.0b013e3181c61cdd. [DOI] [PubMed] [Google Scholar]
- 34.Nikiforov YE. Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7:569–580. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez-Medarde A. Santos E. Ras in cancer and developmental diseases. Genes Cancer. 2011;2:344–358. doi: 10.1177/1947601911411084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukahori M. Yoshida A. Hayashi H. Yoshihara M. Matsukuma S. Sakuma Y. Koizume S. Okamoto N. Kondo T. Masuda M. Miyagi Y. The associations between RAS mutations and clinical characteristics in follicular thyroid tumors: new insights from a single center and a large patient cohort. Thyroid. 2012;22:683–689. doi: 10.1089/thy.2011.0261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.