Abstract
Background
Patients are commonly referred to cancer genetics services when all affected family members are deceased. This makes genetic testing and risk assessment more difficult, reducing the benefit from screening and prophylactic treatment.
Methods
Observational, retrospective, cohort study of 508 randomly selected patients referred to a regional cancer genetics unit, using review of case notes to explore whether a simple clinical “3, 2, 1” family history rule could have been used to improve timely and appropriate referrals for genetic assessment. The 3, 2, 1 criteria are: three affected relatives with the same/associated cancers, across two generations, with at least one person affected age <50 years.
Results
Most (71% [362]) genetic risk assessment referrals were in unaffected individuals and 22% (80) of these were referred after all affected family members had died, including 24% (19) who lost their last remaining affected relative in the previous year. Most (59% [301]) referrals met all 3, 2, 1 criteria, and 67% of these could have been made earlier in clinical practice. A further 23% (115) met two of the three criteria.
Conclusion
Using a simple “3, 2, 1” family rule in cancer care and particularly in palliative care could enable earlier cancer genetic risk assessment for unaffected relatives, improving the potential to benefit from targeted screening and intervention.
Introduction
Cancer is one of the leading causes of death worldwide, with an estimated 157,275 deaths from cancer in the United Kingdom (UK) in 2010 alone.1 The etiology of most tumors involves a complex interplay of genetic and environmental factors. However, some tumors are due to a single germline mutation in an individual's genetic makeup, inherited in a Mendelian pattern. One percent of the UK population is thought to carry single gene alterations predisposing them to certain types of cancer, which is collectively known as familial inherited tumor susceptibility syndrome (FITSS).2 To detect families with a FITSS germline, genetic testing looking for mutations is required. This testing should ideally be on living, affected family members to remove the ambiguity that arises from indirect testing (i.e., testing at-risk relatives). The latter can be problematic, as a negative (uninformative) result does not exclude FITSS in the family; it only indicates that the tested individual does not carry the mutation. In this situation, every at-risk relative may still require full sequencing as opposed to complete sequencing of the affected individual and targeted cascading to other relatives if appropriate. Additionally, the indirect testing of potentially at-risk relatives creates problems with analyzing the significance of missense variants,3 ultimately making the entire process less cost-effective and creating greater anxiety and uncertainty within families.4
Therefore, making the most appropriate referrals to cancer genetics requires vigilance on the part of health care professionals along a patient's cancer pathway, with those involved in diagnosis having the first and palliative care often the last opportunity. During this treatment journey, answers to questions positively correlating to Knudson's hypothesis5 should be recognized regarding family history, multiple cancer cases in multiple generations, young age of onset, multiple tumors in the same individual, and patterns of related tumors, for example, multiple incidences of breast and ovarian cancer. These should trigger a referral to cancer genetics for further counseling and analysis.
Positive identification of a germline mutation allows for significant potential for families to benefit from more targeted screening and increased surveillance. It also empowers patients with knowledge of their health that may affect their lifestyle choices. There may be particular opportunities for preventative interventions such as prophylactic mastectomy, which in BRCA1 carriers reduces the risk of breast cancer by more than 90%,6 or preventative aspirin treatment in patients at high risk of bowel cancer, which reduces the risk of developing malignancy by 60%.7 Equally, negative germline testing provides significant reassurance for individuals and their families who have experienced cancer. Timely and accurate calculation of familial risk can reduce suffering and facilitate targeting of interventions efficiently.
Although the above scenario is the ideal, currently, referrals to cancer genetics are often delayed, resulting in missed opportunities for life-saving interventions. There are a significant number of mutation carriers that remain unidentified.8–10 Lower referral rates and poor access for the socioeducationally disadvantaged and minority ethnic populations are a further concern.11 Clinicians may struggle to make referrals for many reasons including time constraints, insufficient knowledge of genetics and referral pathways, or simply having never considered making a referral to cancer genetics.12,13 The death of a relative is a common trigger for referral to the genetics service, but, as highlighted above, this raises difficulties, as direct testing can no longer be achieved. Palliative care, commonly at the end of the cancer pathway, may present the last opportunity to achieve direct testing; however, genetics is often not incorporated into the palliative care program14 and therefore represents a key education area. We have devised a “3, 2, 1” criteria, based on the Amsterdam criteria for hereditary nonpolyposis colorectal cancer, which may aid detection of FITSS in clinical practice.15 The 3, 2, 1 rule is based on three criteria: three affected relatives with the same/associated cancers, occurring across two generations, with at least one affected individual age <50 years. This simple 3, 2, 1 rule for triaging family histories from all clinical specialties may identify suitable patients for a timely cancer genetics referral, enabling germline DNA analysis if appropriate, and reducing the need for problematic indirect testing in families.
This study suggests a potential approach to triage for cancer genetics referrals and highlights the need for such a model in reducing the interval between the last affected individual dying and referral times, thus reducing referrals where DNA has not been able to be stored. Appropriate, timely referrals allowing for germline analysis will increase opportunities for diagnostic and predictive genetic testing, to enable more targeted screening and preventative strategies for very high-risk groups. This can enable definitive reassurance for relatives shown not to be at risk, thus allowing more targeted counseling support for other families.
Aim
This study aimed to identify the quality of, and reason for, referral to cancer genetics services, and to explore whether a clinical 3, 2, 1 family history rule could be used to facilitate earlier and more appropriate referrals for genetic assessment, in comparison with conventional single-case histopathological criteria.
Ultimately, education of primary care clinicians and targeted education of specialties such as gynecology, colorectal surgery, and palliative care to improve 1- and 5-year cancer mortality figures is needed. Increasing detection of FITSS would reduce premature cancer deaths under the age of 75 by allowing clinicians to target screening and offer life-saving interventions if necessary.
Materials and Methods
We undertook a retrospective case review of randomly allocated active files of 508 patients who were the first referred family members to a regional cancer genetics unit assessed for familial cancer risk in the preceding 3 years (2010–2012). We determined the cause of the referral, exploring its instigation and identifying documented patient concerns and medical questions asked by the referring clinician. Referring medical specialty, reasons for referral, family history at point of referral, and ethnicity were extracted from family history questionnaires completed by patients on initial clinic appointments. In cases where all affected patients were deceased the year/month of death of the last living affected relative was identified. Referral reasons were categorized and recorded qualitatively, with methods informed by prior piloting and development of data collection and entry procedures.
Results
Referral of affected individuals
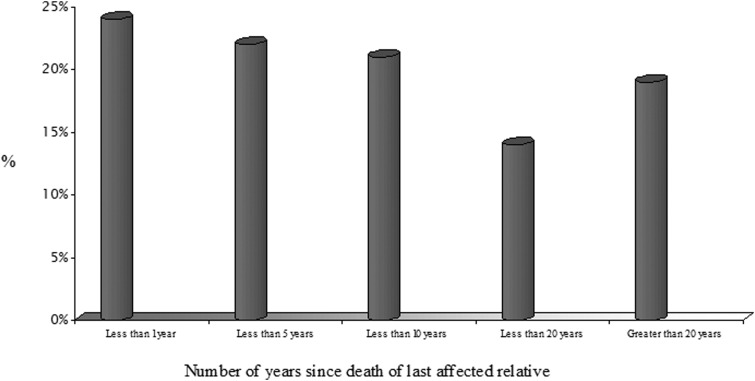
One-half (51%) of the patients referred to the department had a family history of breast cancer, 28% colorectal cancer, 10% ovarian cancer, and the remaining 11% other various tumors. Less than one-third (146, 29%) of referrals were individuals with an existing cancer diagnosis, allowing the offer of direct genetic testing and then effective cascading to appropriate unaffected at-risk individuals in their family. However, 71% (362) of referrals made for assessment of familial cancer risk, genetic counseling, and potential intervention were in unaffected individuals, making analysis immediately more difficult. Of the 362 referrals, 111 (22% of total) cases were in families where all affected family members were already deceased before the referral was made (average of 11 years) and 24% of these 111 families had lost their last remaining affected relative in the 12 months preceding the referral (Fig. 1).
FIG. 1.
Time elapsed between death of the last affected family member and referral to cancer genetics assessment for cases where all affected relatives were deceased (n=111 referrals).
Cases referred meeting the 3, 2, 1 criteria
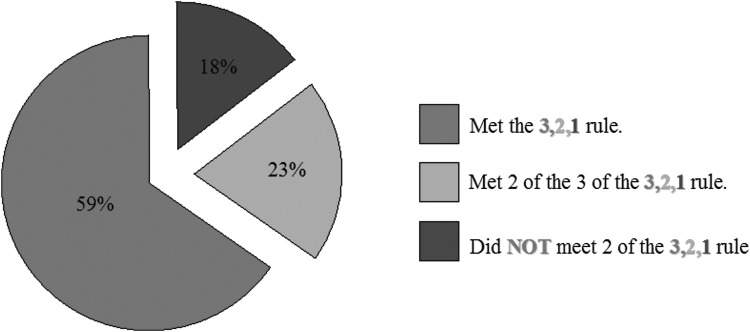
Most cases (416, 82%) met at least two of the 3, 2, 1 criteria. All three criteria points of the 3, 2, 1 rule were met by 301 (59%) cases. Of the 508 cases reviewed, only 18% (92) failed to meet a minimum of two of the three criteria (Fig. 2)
FIG. 2.
Number of referrals fulfilling the “3, 2, 1” rule criteria.
Sources and reasons for referral
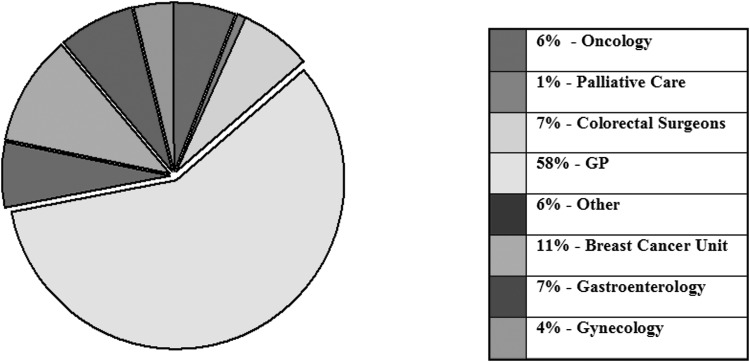
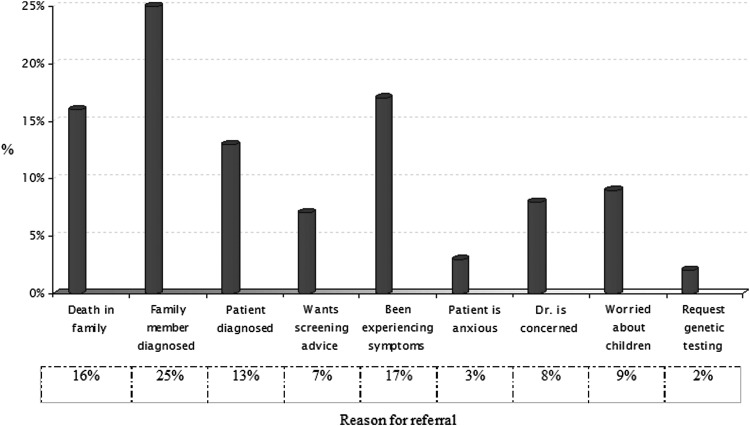
Most (295, 58%) referrals for genetic cancer risk assessment and counseling originated from primary care rather than from secondary care sources, with breast-related specialties being the most likely to refer, and palliative care the least likely (Fig. 3). Wide ranges of factors were identified as primary reasons for referral (Fig. 4). These differed in emphasis for cases from black and minority ethnic groups (BME) (Fig. 5).
FIG. 3.
Source for cancer genetics referrals.
FIG. 4.
Reasons for referral across all specialties.
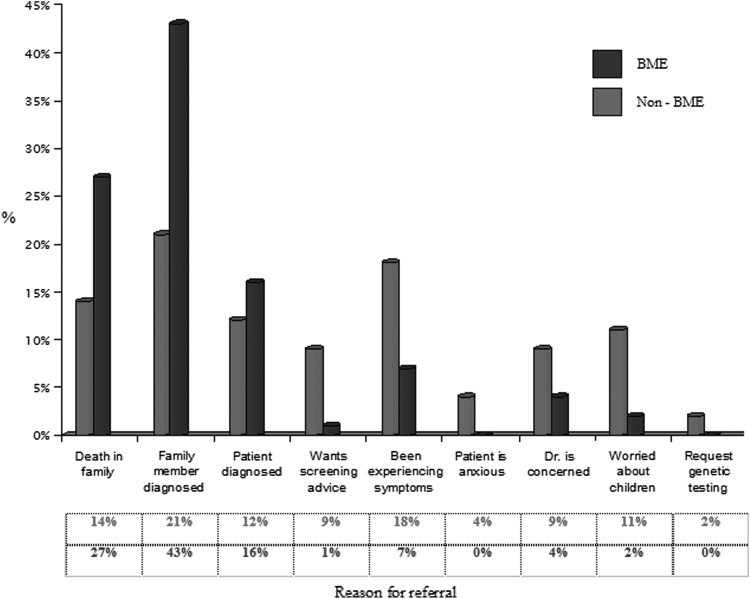
FIG. 5.
Reasons for referral across all specialties, comparing BME with non-BME groups.
Summary of results
Our results demonstrated that in 508 cancer genetics referrals, 22% (111) of patients with a family history of cancer were referred when all of the affected members of the family were already deceased. Of these 111 patients, the referral was made on average 11 years too late, with 24% of the affected family members dying within the year prior to referral (Fig. 1). This suggests that timelier referrals and thus appropriate testing is yet to be realized.
Discussion
The use of our proposed 3, 2, 1 rule might facilitate significantly greater and earlier referrals of patients at risk of FITSS. Approximately two-thirds of referrals in this study could have been made earlier. This would reassure “not at risk” family member and increase the opportunities for more targeted screening and cancer prevention. Our data suggest that this simple 3, 2, 1 model could detect a large proportion of eligible at-risk families in various cancer care settings. With educational support and training, this model could be developed to identify those who would most benefit from cancer genetics assessment.
One in a hundred people have FITSS caused by the inheritance of an alteration in a single gene; however, most of these individuals are unknown to cancer genetics specialists. Missed opportunities to prevent and detect familial cancer often result in young adults with children being diagnosed with incurable tumors.16 This genetic testing determines whether the condition is inherited and due to a single mutation, and helps guide the need for targeted screening and preventative surgery or chemotherapy that may reduce mortality and improve survival for relatives at risk.17 If tumors are detected at an early stage, then expensive treatments with high associated morbidity can often be avoided.18 Examples include breast screening with magnetic resonance imaging (MRI) for women at very high risk for breast cancer,19 prophylactic aspirin for patients at high risk for bowel cancer,7 and aromatase inhibitors for certain women at risk for breast cancer.20
This triage system could be used by practitioners throughout the cancer care pathway, from general practice to palliative medicine. Although there are a number of opportunities where pedigree determination and causal analysis could occur throughout the cancer pathway, this study highlights that this is not taking place in a consistent manner. Palliative care is ultimately the last opportunity to identify the affected individuals where storing DNA would help to clarify risk for family members, but referrals from this discipline were remarkably low (Fig. 3). In some families, genetic counseling about familial cancer risk is beneficial and reassuring. However, for others who may not have been aware of the risk involved, it becomes a difficult subject to discuss, particularly when dealing with cancer diagnosis, treatment, or terminal illness within the family. The question of when and how this should best be done during cancer care is key for future care development and recommendations, and in particular requires qualitative work with patients, families, and health professionals, in addition to contributions from health psychology, palliative care, and the wider cancer sector.
The results also demonstrate a significant difference in reason for referral among BME patient populations compared with those from other ethnic backgrounds. BME patient groups were more than twice as likely to be referred following a recent family member being diagnosed with a malignancy (43% for BME versus 21% for non-BME patients). BME patient groups were also more likely to have been referred because of the recent death of a family member (27% for BME versus 14% for non-BME). Also of note was that non-BME patient groups were nine times more likely than BME patient groups to request referral in order to obtain screening advice. These findings could help explain the worrying disparity in referral rates observed nationally to cancer genetics. Current literature highlights major concerns that individuals from BME groups are not being referred at a rate in proportion to their population size nationally.21 This concern might be considered in the development of services and educational programs in primary and secondary care to address variation in referral rates in our increasingly ethnically diverse populations.
Strengths and limitations
This study of a large, randomly selected patient cohort offers new insights into current practice in cancer genetics referral and the potential for its improvement. Substantive data have been gathered retrospectively, but it is recognized that this process relied on the data being initially recorded in routine practice from family history questionnaires, referral letters, and consultation records. Further research using prospective methods, including defined case report instruments, and across multiple regional sites with additional detailed exploration of individual contexts and influences on referral using qualitative research with referrers and patients is now needed.
Acknowledgments
The study was funded by Macmillan Cancer Support and the National Institute of Health Research (NIHR), as part of the programme of the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) for Leicestershire, Northamptonshire, and Rutland (LNR). The views expressed in this study are not necessarily those of the NIHR. University of Leicester Audit number: 5821.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Cancer Research UK: Cancer mortality statistics – UK. http://info.cancerresearchuk.org/cancerstats/mortality/ 2010. [Feb 7;2010 ]. http://info.cancerresearchuk.org/cancerstats/mortality/
- 2.Ponder BAJ. Inherited predisposition to cancer. Trends Genetics. 1990;6:213–218. doi: 10.1016/0168-9525(90)90181-5. [DOI] [PubMed] [Google Scholar]
- 3.Tavtigian SV. Greenblatt MS. Lesueur F. Byrnes GB. In silico analysis of missense substitutions using sequence-alignment based methods. Hum Mutat. 2008;11:1327–1336. doi: 10.1002/humu.20892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michie S. Bobrow M. Marteau TM. Predictive genetic testing in children and adults: a study of emotional impact. J Med Genetics. 2001;38(8):519–526. doi: 10.1136/jmg.38.8.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine AJ. Tumour suppressor genes. Sci Med. 1995;2:28. [Google Scholar]
- 6.Meijers-Heijboer H. Geel BV. Van Putten WLJ. Henzen-Logmans SC. Seynaeve C. Menke-Pluymers MBE. Baitels CCM. Verhoog LC. Van den Ouweland AMW. Niermeijer MF. Brekelmans CTM. Klijn JGM. Breast Cancer after Prophylactic Bilateral Mastectomy in Women with a BRCA1 or BRCA2 Mutation. N Engl J Med. 2001;345:159–164. doi: 10.1056/NEJM200107193450301. [DOI] [PubMed] [Google Scholar]
- 7.Bastiaannet E. Sampieri K. Dekkers OM. de Craen AJ. van Herk-Sukel MP. Lemmens V. van den Broek CB. Coebergh JW. Herings RM. van de Velde CJ. Fodde R. Liefers GJ. Use of aspirin post diagnosis improves survival for colon cancer patients. Br J Cancer. 2012;106:1564–1570. doi: 10.1038/bjc.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson D. Easton DF. Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94:1358–1365. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 9.Risch HA. McLaughlin JR. Cole DE. Rosen B. Bradley L. Fan I. Tang J. Li S. Zhang S. Shaw PA. Narod SA. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: A kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98:1694–1706. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 10.Malone KE. Daling JR. Doody DR. Hsu L. Bernstein L. Coates RJ. Marchbanks PA. Simon MS. McDonald JA. Norman SA. Strom BL. Burkman RT. Ursin G. Deapen D. Weiss LK. Folger S. Madeoy JJ. Friedrichsen DM. Suter NM. Humphrey MC. Spirtas R. Ostrander EA. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res. 2006;66:8297–8308. doi: 10.1158/0008-5472.CAN-06-0503. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs C. Rawson R. Campion C. Caulfield C. Heath J. Burton C. Kavalier F. Providing a community-based cancer risk assessment service for a socially and ethnically diverse population. Fam Cancer. 2007;6:189–195. doi: 10.1007/s10689-007-9134-z. [DOI] [PubMed] [Google Scholar]
- 12.Baars MJH. Henneman L. Kate LPT. Deficiency of knowledge of genetics and genetic tests among general practitioners, gynecologists, and pediatricians: A global problem. Nat Genetics Med. 2005;7:605–610. doi: 10.1097/01.gim.0000182895.28432.c7. [DOI] [PubMed] [Google Scholar]
- 13.Suther S. Goodson P. Barriers to the provision of genetic services by primary care physicians: A systematic review of the literature. Genetics Med. 2003;5:70–76. doi: 10.1097/01.GIM.0000055201.16487.61. [DOI] [PubMed] [Google Scholar]
- 14.Lillie AK. Clifford C. Metcalfe A. Caring for families with a family history of cancer: Why concerns about genetic predisposition are missing from the palliative agenda. Palliat Med. 2011;25:117–124. doi: 10.1177/0269216310383738. [DOI] [PubMed] [Google Scholar]
- 15.Vasen HF. Mecklin JP. Khan PM. Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC) Dis Colon Rectum. 1991;34:424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 16.Foulkes WD. Inherited susceptibility to common cancers. N Engl J Med. 2008;359:2143–2153. doi: 10.1056/NEJMra0802968. [DOI] [PubMed] [Google Scholar]
- 17.Grann VR. Jacobson JS. Thomason D. Hershman D. Heitjan DF. Neugut AI. Effect of prevention strategies on survival and quality-adjusted survival of women with BRCA1/2 mutations: An updated decision analysis. J Clin Oncol. 2005;20:2520–2529. doi: 10.1200/JCO.2002.10.101. [DOI] [PubMed] [Google Scholar]
- 18.Vasen HFA. Ballegooijen M. Buskens E. Kleibeuker JK. Taal BG. Griffioen G. Nagengast FM. Menko FH. Khan PM. A cost-effectiveness analysis of colorectal screening for hereditary non-polyposis colorectal carcinoma gene carriers. Cancer. 2000;82:1632–1637. [PubMed] [Google Scholar]
- 19.Morrow M. Waters J. Morris E. MRI for breast cancer screening, diagnosis, and treatment. Lancet. 2011;378:1804–1811. doi: 10.1016/S0140-6736(11)61350-0. [DOI] [PubMed] [Google Scholar]
- 20.Santen RJ. Yue W. Naftolin F. Mor G. Berstein L. The potential of aromatase inhibitors in breast cancer prevention. Endocr Relat Cancer. 1999;6:235–243. doi: 10.1677/erc.0.0060235. [DOI] [PubMed] [Google Scholar]
- 21.Featherstone C. Colley A. Tucker K. Kirk J. Barton MB. Estimating the referral rate for cancer genetic assessment from a systematic review of the evidence. Br J Cancer. 2007;96:391–398. doi: 10.1038/sj.bjc.6603432. [DOI] [PMC free article] [PubMed] [Google Scholar]