Abstract
Introduction:
Tc-99m macro aggregated albumin (MAA) is synonymous for lung perfusion scintigraphy and is part of the study in the evaluation of pulmonary thromboembolism. We wanted to highlight the utilities of Tc-99m MAA other than pulmonary embolism as a pictorial assay.
Materials and Methods:
Patients referred for Tc-99m MAA scintigraphy under various indications were included in this pictorial essay. Commercially available TechneScan LyoMAA cold kit from Mallinckrodt Medical B.V., Holland was used. Acquisition protocols for different indications are described in this article. Different clinical indications (e.g., pulmonary artery stenosis, hepatopulmonary syndrome, FEV1 calculation in lung surgery planning, selective internal radiation therapy planning, venography for deep venous thrombosis, left to right cardiac shunts, etc.) where Tc-99m MAA scintigraphy was asked for; how it helped in different clinical scenarios and how it can be used clinically is explained with unique and interesting case examples and images. We also reviewed the literature to look for certain remote indications of MAA imaging for the sake of completion like – (shunt scintigraphy, peritoneopleural communication, etc.)
Conclusion:
Tc-99m MAA is a very useful radiopharmaceutical, which can be used for many other indications apart from the commonly used indication of lung perfusion scan in pulmonary embolism. It can provide useful clinical information in other indications, which we try to highlight in this article.
Keywords: FEV1 calculation, hepatopulmonary syndrome, Tc-99m macro aggregated albumin scintigraphy, shunt scintigraphy
INTRODUCTION
Lung perfusion scintigraphy with labeled, biodegradable particles (Tc-99m macro aggregated albumin [MAA]) is an important diagnostic tool in the evaluation of pulmonary regional perfusion. Tc-99m MAA is synonymous for lung perfusion scintigraphy and is part of the evaluation of pulmonary thromboembolism (PTE). However, Tc-99m MAA can be used for a variety of clinical indications. Apart from PTE, it can be used for indications such as pulmonary artery stenosis, hepatopulmonary syndrome (HPS), FEV1 calculation in lung surgery planning, selective internal radiation therapy (SIRT) planning, venography for deep venous thrombosis, left to right cardiac shunts, etc., which we aim to highlight in this pictorial essay. We attempted to incorporate relevant images of patients who presented to our department for all the common and rarely used indications of MAA scintigraphy. Indications which we never encountered in our department but having the potential to be used more frequently, are also described.
MATERIALS AND METHODS
Patients referred for various indications of lung perfusion scintigraphy are included in this essay. Commercially available kits of TechneScan LyoMAA from Mallinckrodt Medical B.V, Holland were used for labeling Tc-99m. Each kit contains 2 mg of macro aggregated human serum albumin particles. 95% of the particles in each vial vary between 10 μm and 100 μm in size. Only less than 0.2% are between 100 μm and 150 μm. The number of particles per vial is 4,500,000. Freshly prepared Sodium pertechnetate in a volume of 1-10 ml was added to a vial of TechneScan LyoMAA. Vial is carefully swirled a few times and incubated for 5 min at room temperature. Contact with air was avoided.
Procedure variations
Whole body lung perfusion scan
Whole body images were obtained 10 min after injection of 3 mCi of TechneScan LyoMAA. In children, dose was titrated as per body weight. Anterior and posterior static acquisition was carried out using a dual head large rectangular field of view gamma camera (GE Infinia Hawkeye 4) with low energy, high resolution collimation and a matrix of 256 × 1,024. The photo peak was kept at 140 keV with a 10% window on either side. All scintigrams were interpreted visually by two experienced nuclear medicine physicians. This protocol is used in patients with suspected HPS and also as part of shunt quantification in patients with known or suspected right to left cardiac shunt.
Lung perfusion imaging as part of post-operative FEV1 prediction
Procedure is similar to a normal lung perfusion scan; however, multiple static images of lungs are unnecessary. Anterior and posterior lung images are acquired for 500K counts. Region of interest are drawn depending on whether lobectomy or pneumonectomy is planned. One can choose drawing manual region of interests (ROIs) over automatic ones in those patients where the ROIs do not correspond to the involved lung segments.
Scintigraphy as part of SIRT for liver tumors
In an intervention lab setting, 200-400 MBq of Tc-99m MAA is administered intra-tumorally by selective angiogram under fluoroscopic guidance by an experienced interventional radiologist. Subsequently, patient is shifted to the department for a delayed whole body and single photon emission computed tomography/computed tomography (SPECT/CT) acquisition.
Tc-99m MAA venography
1.5-3 mCi of Tc-MAA is simultaneously injected into dorsal veins of feet. Immediate dynamic whole body scintigraphy is performed in anterior and posterior projections using a dual head large rectangular field of view gamma camera. Static images of the lungs are also essential in the anterior, posterior, lateral and posterior oblique projections. Subsequently, static images of the lower limbs and pelvis are also obtained in anterior and posterior projections.
Tc-99m MAA (LeVeen) shunt scintigraphy
After positioning and immobilization of the patient, abdomen is exposed from xiphisternum to the groin. Angiocath (22G) mounted on a 20cc syringe can be used to enter through the locally anesthetized area into the peritoneal cavity. One must ensure that the catheter is firmly placed in the peritoneal cavity by aspirating a few millilitre of ascitic fluid. After confirmation, remove the needle from a 5cc syringe containing 111 MBq (3 mCi) of Tc-99m MAA that is fitted with the catheter. The tracer is then instilled into the abdominal cavity. After injection, the patient should be instructed to roll from one side to the other, which will facilitate mixing of the radioactive injection with the ascitic fluid. Widespread accumulation of the radioactivity should be visualized in the abdomen initially, confirming an appropriate injection. Static images of the abdomen and chest are obtained at 15, 30, 45 and 60 min after injection. If required, images may be obtained up to 4 h post-injection. Patient can alternately be given a kinked straw and instructed to inhale through to generate a pressure differential between thoracic and intra-peritoneal pressure allowing the ascitic fluid containing radiotracer to enter the superior vena cava (SVC) through the LeVeen shunt. Anterior static images using 128 × 128 matrix can be acquired. This LeVeen shunt is a peritoneovenous shunt (PVS) used in patients with chronic ascites, having a one-way valve that is surgically placed in the peritoneal cavity. This connects into the patient's jugular vein, which then empties into the SVC. When the LeVeen shunt is functional, the injected Tc-99m MAA travels to the lungs and lodges in the capillaries, as with a perfusion lung study. The lungs are usually visualized within 1 h of Tc-99m MAA intra-peritoneal injection. Early visualization of lungs even at 10 min post-injection can be expected.
Procedure of Tc-99m MAA scintigraphy for diagnosis of peritoneopleural communications
The non-absorbable MAA tracer (4 mCi) is instilled intraperitoneally via the dialysis catheter along with the dialysate exchange in order to reproduce the state of increased intraperitoneal pressure and thereby enhance any occult abnormality. Multiview planar imaging is performed for 3-5 min/view or 500,000-1,500,000 counts/image. Anterior views were obtained over the abdomen, pelvis (including genital area) and thorax. Initial imaging to include the entire area of interest is started within 15 min in order to depict the intra-peritoneal distribution of the tracer as well as detect the very early transit of tracer outside the peritoneal cavity. One or more additional images over a 2-h period after tracer administration are usually sufficient to delineate the route and dynamics of a leak when present. Patient is encouraged to remain ambulatory during this period in order to better reproduce the offending pathophysiology. Careful marking of key normal and abnormal anatomic features including sites of entrances of the dialysis catheter into the skin and peritoneal cavity may be quite useful in the interpretation of the scan.
Case examples
Tc-99m MAA lung perfusion scan in pulmonary artery stenosis
Case 1
1-year-old child, a known case of patent ductus arteriosus (PDA) underwent PDA coil closure. Subsequent follow-up angiogram showed left pulmonary artery (LPA) origin stenosis. Patient was referred to assess the significance of LPA stenosis. Lung perfusion scan showed generalized reduced tracer distribution in the left lung segments, showing a differential uptake of left: right lungs to be 29:71%. Patient subsequently underwent LPA stenting for severe LPA stenosis. Follow-up lung perfusion scan showed very minimal improvement in the left lung MAA uptake (differential perfusion postoperatively was left : right lung of 33:67%) [Figures 1 and 2].
Figure 1.
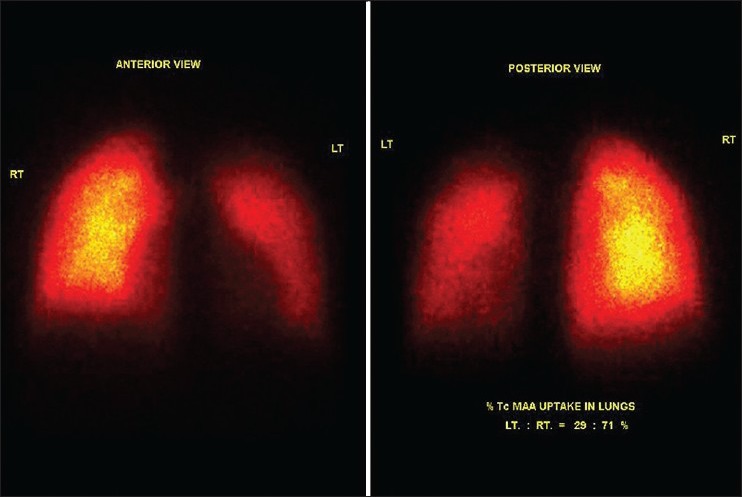
Pre left pulmonary artery stenting scan. Significantly reduced Tc-99m macro aggregated albumin tracer distribution in entire left bronchopulmonary segments. Percentage uptake in left:right lung segments is 29:71%
Figure 2.
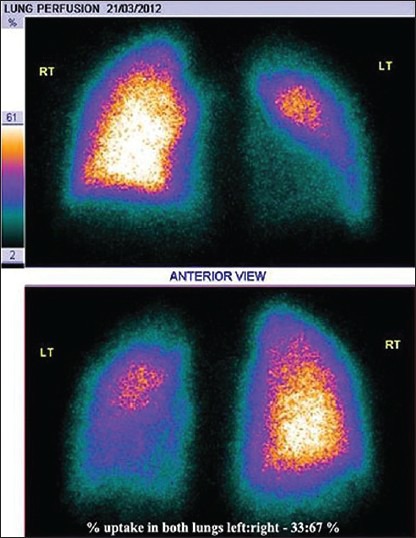
Post left pulmonary artery stenting scan. Very minimal improvement in perfusion of left bronchopulmonary segments. Percentage uptake of left:right lung is 33:67%
Case 2
A 2-year-old child was a known case of congenital cyanotic heart disease. He underwent corrective surgery. On follow-up, he was found to have LPA stenosis. Lung perfusion scan showed differential perfusion of left: right lungs to be 11:89%. Patient underwent LPA stenting for severe LPA stenosis. Follow-up lung perfusion scan showed differential perfusion in left: right lung to be 48:52%. There was a significant improvement in differential perfusion of bilateral lungs post-treatment [Figures 3 and 4].
Figure 3.
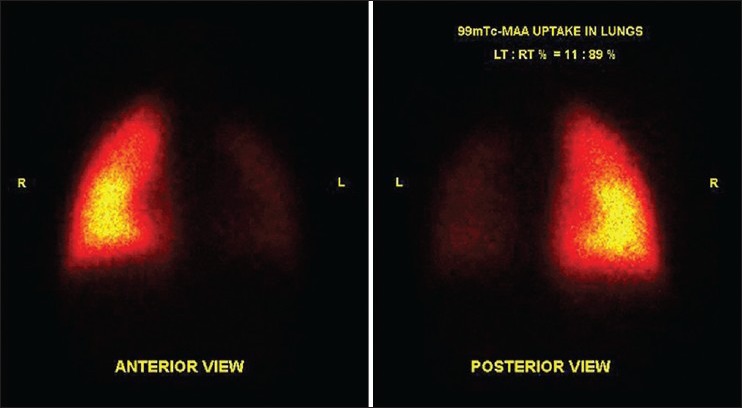
Significantly reduced tracer distribution in entire left lung bronchopulmonary segments. Normal tracer distribution seen in the right lung bronchopulmonary segments. Percentage tracer uptake in left:right lung was 11:89%
Figure 4.
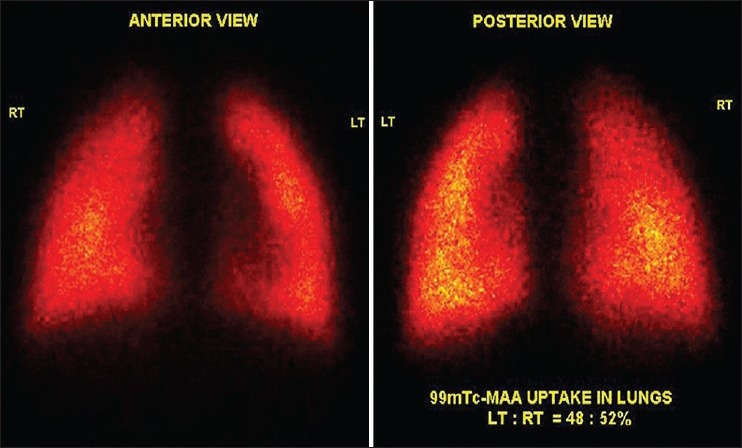
Significant improvement in tracer distribution in the left lung bronchopulmonary segments indicating successful stenting. Percentage tracer uptake in left:right lobe was 48:52%
Tc-99m MAA lung perfusion scan in suspected HPS
Case 3
Patient is a known case of alcohol related chronic liver disease with portal hypertension. Patient was referred as a suspected case of HPS. A whole body MAA scan was done to look for arteriovenous (AV) shunting [Figure 5].
Figure 5.
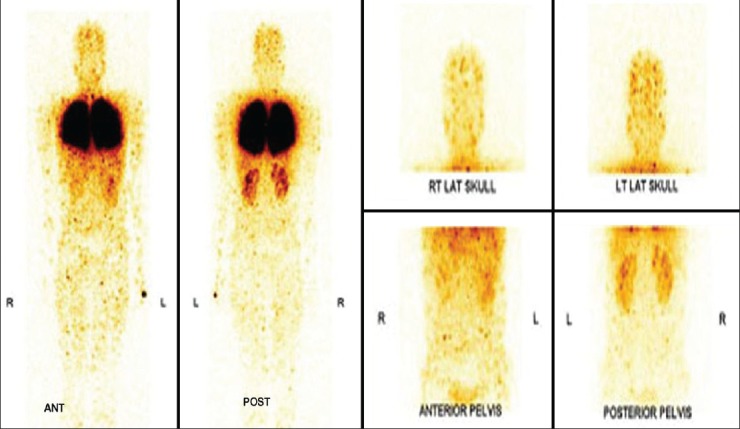
Whole body and static images show normal perfusion to bilateral bronchopulmonary segments. There is abnormal tracer uptake in bilateral kidneys, cutaneous and subcutaneous arterioles suggestive of significant arteriovenous shunting, confirming hepatopulmonary syndrome
Case 4
A 49-year-old male patient who is a known case of cryptogenic cirrhosis suspected to have HPS was referred for MAA scan [Figure 6].
Figure 6.
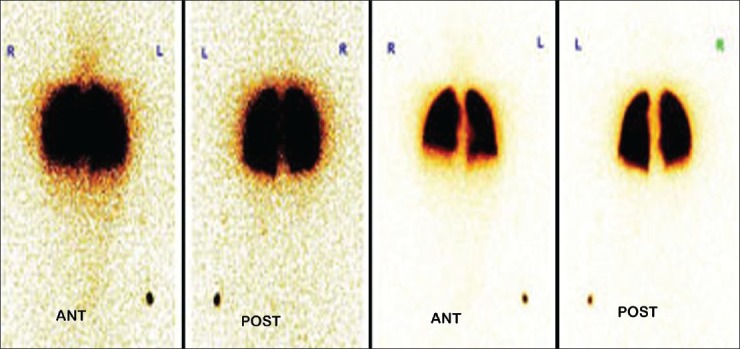
Whole body images show normal perfusion to bilateral bronchopulmonary segments with no evidence of any arteriovenous shunt. Thus scan was negative for hepatopulmonary syndrome
Tc-99m MAA lung perfusion scan for prediction of post-operative FEV1 in patient scheduled for pneumonectomy/lobectomy
Case 5
A 35-year-old female who is a known case of multidrug resistant tuberculosis presented with orthopnea, left sided chest pain and productive cough. Her chest X-ray was suggestive of pyopneumothorax. Intercostal drain insertion and pleural fluid aspiration was done. As patient's condition was not improving by medical management, surgical management was contemplated. She underwent pulmonary function test, which showed adequate FEV1 value. In view of extensive involvement of the left lung parenchyma, cardiothoracic surgeon wanted to predict the post-operative FEV1 by virtue of a lung perfusion scintigraphy. The calculated differential MAA distribution in the left lung was 9% and hence, we predicted a 9% loss of the estimated FEV1 post-pneumonectomy. Patient was thus cleared for surgery [Figures 7 and 8].
Figure 7.
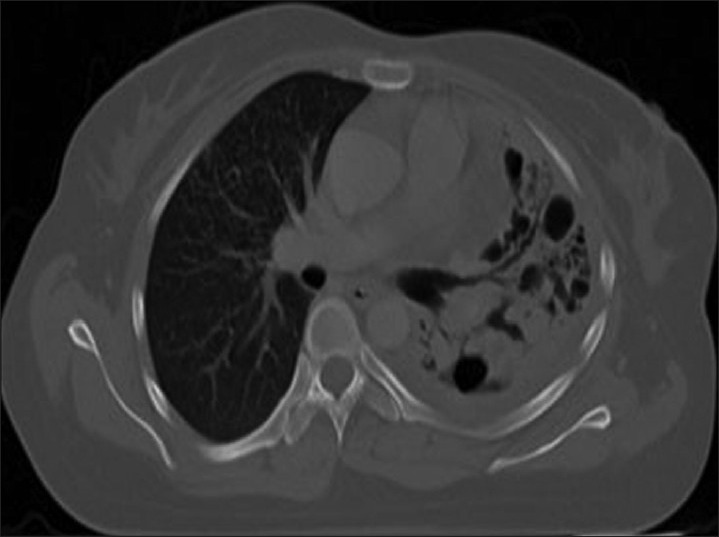
Computed tomography thorax showing consolidation of the left lung with air bronchogram
Figure 8.
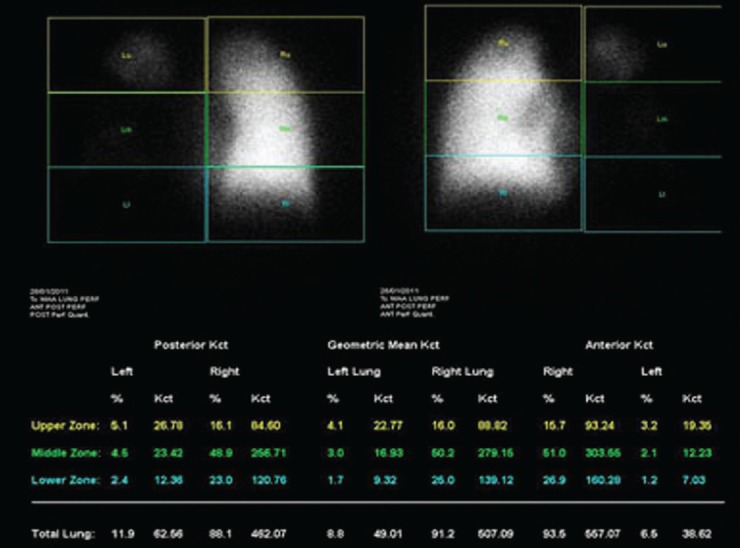
Anterior and posterior images of both lungs show significantly reduced tracer distribution in entire left lung bronchopulmonary segments. Percentage macro aggregated albumin uptake in left:right lung is 9:91%. Thus, there would be a loss of only 9% of calculated FEV1, if patient undergoes left pneumonectomy
Case 6
A 58-year-old male patient was admitted with breathlessness, associated dry cough and fever. He was diagnosed to have bilateral bullous disease of lungs. Bilateral bullectomy was planned. Hence, patient was referred for lung perfusion scan to predict post-operative FEV1 and to look for segmental differential perfusion. However, surgery was later deferred due to associated severe cardiac comorbidities [Figures 9 and 10].
Figure 9.
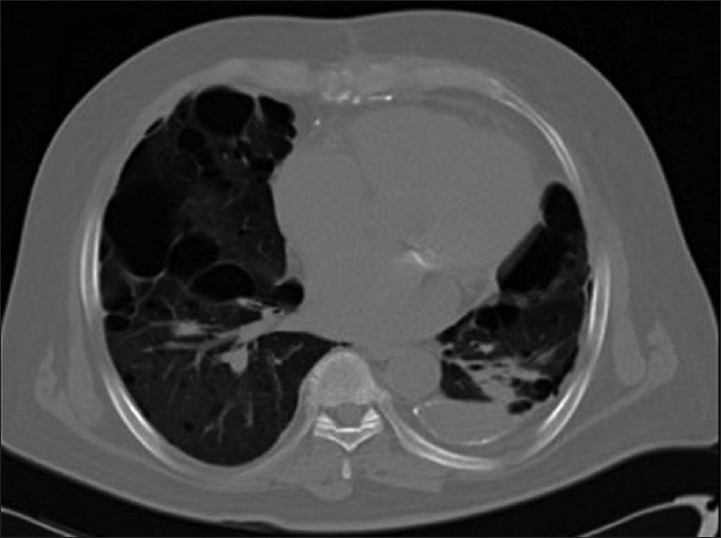
Mixed emphysematous changes noted in computed tomography thorax in bilateral lower lobes. Bulla noted in right lower lobe. Chronic focal pleural fluid collection was seen in left cardiophrenic angle
Figure 10.
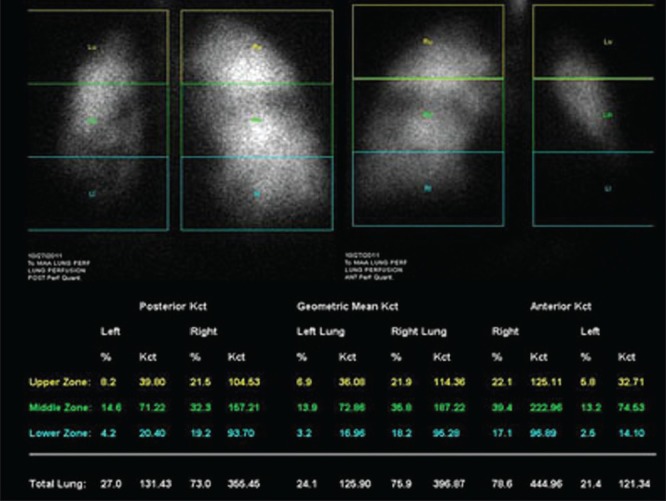
Lung perfusion scan shows significantly reduced tracer distribution in bilateral bronchopulmonary segments. Quantification of segmental perfusion is given in image
Tc-99m MAA lung perfusion scan as part of SIRT for liver tumor
Case 7
A 69-year-old male patient, who is a known case of right hepatocellular Ca with right portal vein thrombosis, was referred for Tc-99m MAA scintigraphy for planning liver radionuclide microspheres therapy. Tc-99m MAA scintigraphy showed significant MAA uptake corresponding to the site of CT detected liver tumor. There was minimal MAA shunting in bilateral lungs. No other extra hepatic sites of abnormal MAA uptake were seen. Patient was thus considered for liver radionuclide microsphere therapy with reduction in administered activity [Figures 11 and 12].
Figure 11.
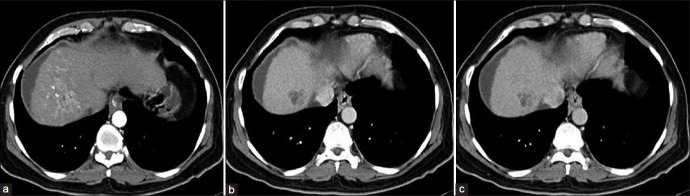
Ill-defined heterogeneously enhancing segment VIII lesion (a), showing washout in portal (b) and delayed phase (c) right branch of portal vein is thrombosed
Figure 12.
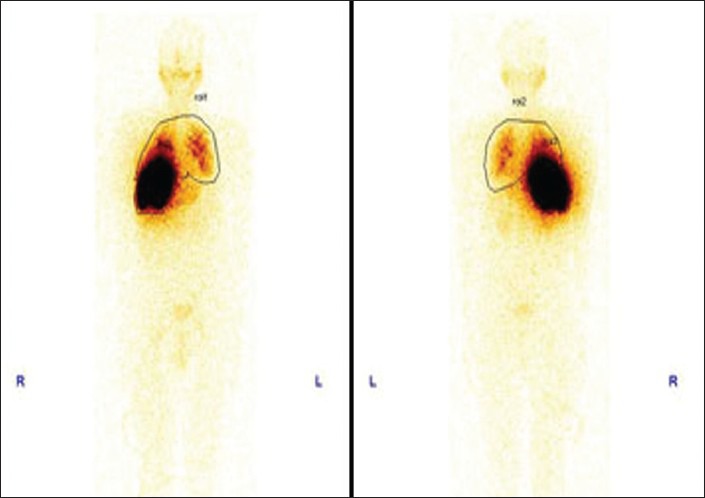
Macro aggregated albumin shunts to lungs calculated by geometric mean 13%. No systemic shunts to other organs like stomach, pancreas, gall bladder or duodenum
Case 8
A 57-year-male patient, known case of large right hepatocellular Ca with extensive portal vein thrombosis was referred for Tc-99m MAA scintigraphy for planning liver radionuclide microspheres therapy. Tc-99m MAA scintigraphy showed significant MAA uptake in the region of the right lobe liver tumor as well as in the left lobe of the liver. Minimal MAA uptake was also seen in bilateral lungs with no other extra hepatic sites of abnormal MAA concentration. On quantification, lung shunting was calculated to be > 20% and lungs would receive radiation exposure exceeding the safety limit. Patient is not an ideal candidate for liver radionuclide microsphere therapy [Figures 13 and 14].
Figure 13.
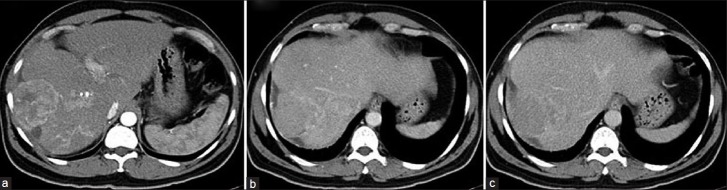
Large hypodense liver lesion with exophytic component seen involving segment VIII and V of the right lobe of the liver. Early arterial enhancement (a), washout in the delayed phase (b and c). Hypoenhancing tumor thrombus involving right and left branch of portal vein extending into main portal vein
Figure 14.
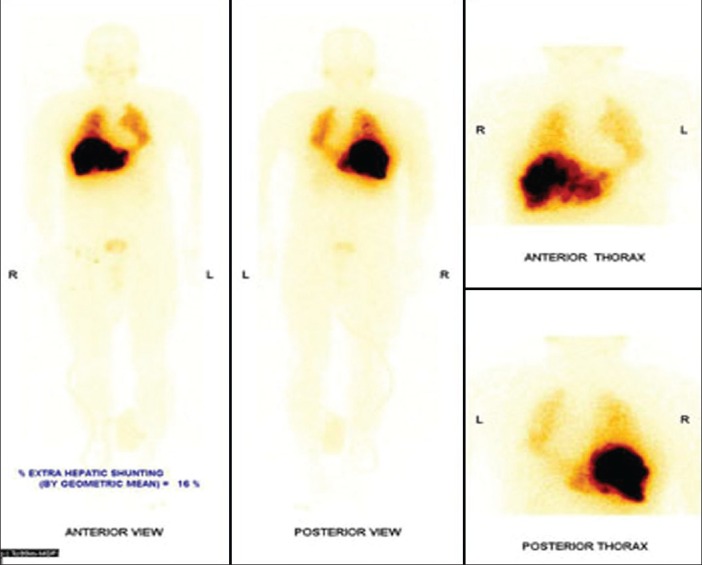
Significant macro aggregated albumin (MAA) uptake seen in the region of the right lobe liver tumor as well as in the left lobe. Significant MAA uptake in bilateral lungs. No other extra hepatic abnormal MAA uptake seen. Percentage of MAA shunt = 21%
Tc-99m MAA venography
Case 9
A 21-year-old male patient with sudden onset dyspnea was referred for ventilation perfusion scan to look for pulmonary embolism. In view of the acute presentation and no risk factors, a simultaneous venography study using Tc MAA was scheduled. Tc-99m MAA was injected simultaneously through bilateral pedal veins as part of pulmonary perfusion scan to look for deep vein thrombosis. Whole body images showed significant collateral vessels with associated anteromedial basal segmental lung perfusion defect in the left lung [Figures 15 and 16]. Later doppler study of bilateral lower limbs confirmed subacute thrombus involving left iliac and left common femoral veins.
Figure 15.
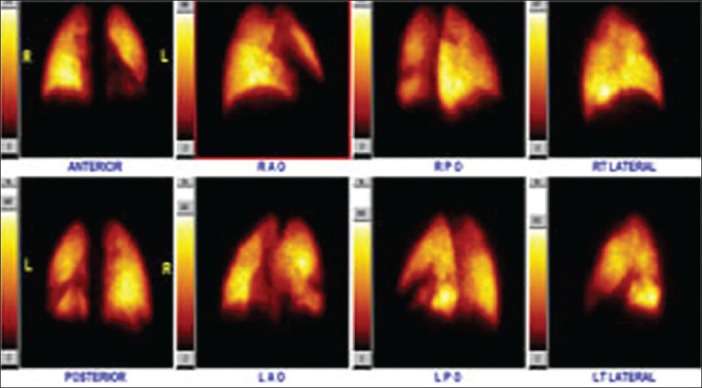
Lung perfusion scan showing left anteromedial basal segmental lung perfusion defect
Figure 16.
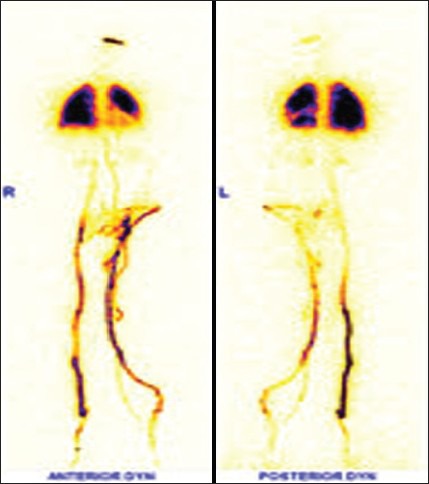
Radionuclide venography of bilateral lower limbs showing significant collateral channels in left lower limb veins
Tc-99m MAA shunt scintigraphy
Case 10
A 27-years-old known cirrhotic female patient, who underwent peritoneal venous shunt (PVS) 6 months ago, was referred for the evaluation of shunt patency. There was no history of malena or hemetemesis. On examination, her abdomen was very tense and mildly tender with positive fluid thrill. Dilated cutaneous veins (caput medusae) were visible around the umbilicus. From this clinical presentation, it was evident that her PVS was not functioning properly. It was confirmed by Tc-99m MAA shunt scintigraphy and site of shunt obstruction was localized by a simple and non-invasive nuclear medicine protocol [Figures 17 and 18].
Figure 17.

LeVeen peritoneo venous shunt with intra-peritoneal and venous tubes. Pressure-sensitive one-way valve connected to a tube traversing the subcutaneous tissue of the chest wall to the neck where it enters the internal jugular vein and terminates in the superior vena cava
Figure 18.
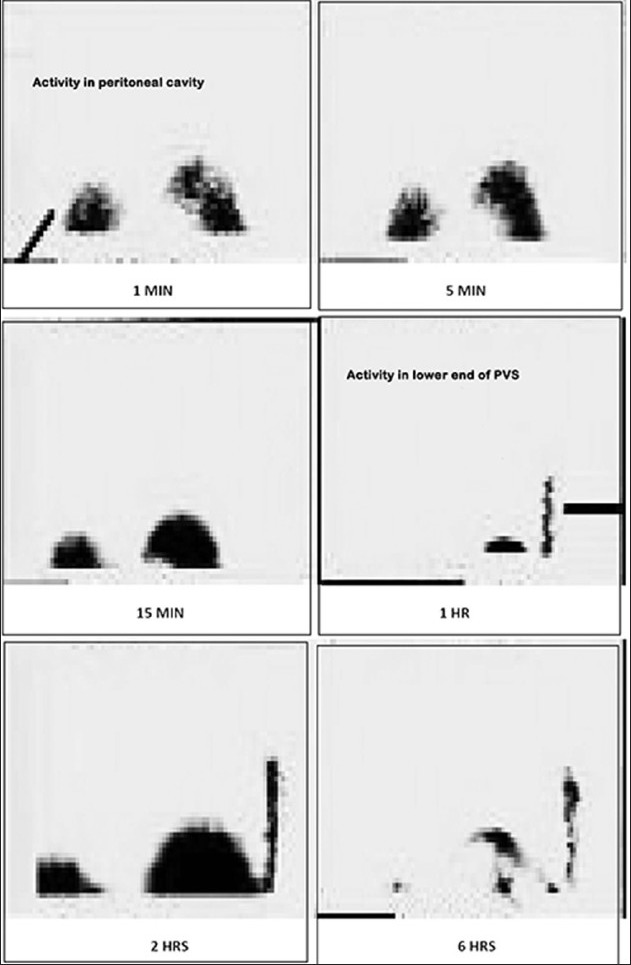
At 1st h after intraperitoneal injection of radiotracer, activity is seen accumulating in the shunt tubing from abdominal end. This intensified afterwards (in 2nd h image) and then gradually decreased (in 6th h image). No activity is seen in the lung and in the venous end of tube consistent with mid-tubal obstruction
Tc-99m MAA scintigraphy in quantification of right to left cardiac shunts
Case 11
A 10-old-year female patient, a known case of atrial septal defect and ventricular septal defect was referred for lung perfusion scintigraphy to quantify the right to left shunt [Figure 19].
Figure 19.
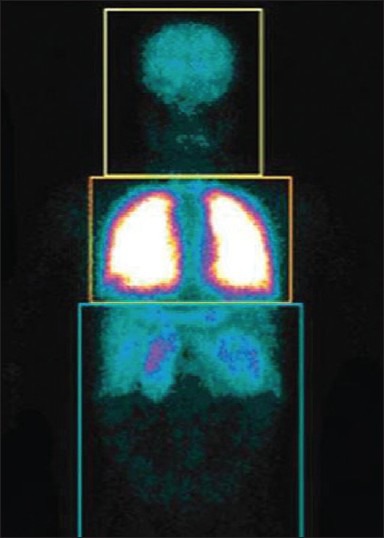
The perfusion is normal and symmetrical in both lungs. Abnormal activity is seen in both kidneys indicating right to left shunt. To quantify right to left shunt, region of interests were drawn around the entire body and around the lung. Right to left shunt is determined by subtracting the count in the pulmonary region from the whole body region. In this case, the right to left shunt was 30%
DISCUSSION
Tc-99m MAA lung perfusion scan in patients with pulmonary artery stenosis
Lung perfusion abnormalities are common in children with a congenital heart defect, especially after surgical intervention. Most of these patients are asymptomatic, even though they have a grossly abnormal lung perfusion scintigram. The distribution of Tc-99m MAA particles between the two lungs is directly proportional to the division of pulmonary artery flow. The increased ability to restore equal lung perfusion either surgically or with transcatheter interventions such as balloon angioplasty of branch pulmonary arteries or coil embolization of systemic to pulmonary artery collateral vessels has emphasized the usefulness of this relatively non-invasive, simple and easily reproducible method for assessing pulmonary blood flow. Lung perfusion scintigram is more accurate than the other methods. For detection of pulmonary blood flow imbalance by inspection of chest X-ray films is possible only when there was grossly unequal pulmonary blood flow. Lung perfusion scintigram is a relatively easy, reliable and non-invasive method for the accurate determination of relative pulmonary blood flow, a value not easily calculated by other means such as chest radiography and two-dimensional echocardiography (2D echo). This method allows the detection of changes in pulmonary blood flow and enables one to plan more invasive studies or special transcatheter or surgical interventions.[1]
Tc-99m MAA lung perfusion scan in patients with suspected HPS
Arterial deoxygenation due to an intrapulmonary shunt is the hallmark of HPS. Severe hypoxemia (PaO2 < 60 mmHg) in the absence of primary lung disease, in combination with liver disease clinically raises the suspicion of HPS. The pulmonary capillaries, which normally measure 8-15 μm, dilate up to 100 μm in diameter.
Tc-99m MAA of 15-150 μm in diameter normally gets trapped almost completely within the pulmonary capillary bed after intravenous injection. These Tc-99m MAA particles readily pass through the dilated pulmonary capillaries in patients with HPS and enter the systemic circulation to be trapped in normal size capillaries of the brain, liver, kidney and other organs, in proportion to their blood supply. Normally less than 6% of Tc-99m MAA particles bypass the lung to lodge in other organs. It is believed that the liver in HPS either produces vasodilators or is incapable of inactivating vasodilators produced elsewhere. Contrast 2D echo with agitated saline is the most preferred initial diagnostic imaging procedure. A perfusion scintigraphy supplements 2D echo by providing both quantification and specificity for a definitive diagnosis of HPS. 2D echo is sensitive test, but lacks specificity and quantification of shunt is also not possible.
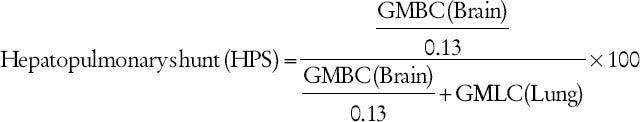
An assumption is made that about 13% of the cardiac output is delivered to the brain:
Geometric mean brain count
Geometric mean lung count.
In normal subjects and in patients with intrinsic lung disease or with cirrhosis, but without shunt, the hepatopulmonary shunt ratio varies from 3% to 6%. A value higher than 6% is considered indicative of HPS. Thus, Tc-99m MAA is an important supplementary investigation for HPS.[2,3,4,5,6,7]
Tc-99m MAA lung perfusion scan for prediction of post-surgery FEV1 in patient undergoing lobectoby/segmental resection
An accurate pre-operative general and pulmonary-specific evaluation is essential as post-operative complications and morbidity of lung resection surgery for any indication are significant. After confirming anatomic resectability, patients must undergo a thorough evaluation to determine their ability to withstand the surgery and the loss of the resected lung. The measurement of FEV1 and diffusion capacity of the lung for carbon monoxide (DLCO) should be performed first. If FEV1 and DLCO are > 60% of predicted, patients are at low risk for complications and can undergo pulmonary resection, including pneumonectomy, without further testing. However, if FEV1 and DLCO are < 60% of predicted, further evaluation by means of a quantitative lung scan is required. If lung scan reveals predicted post-operative values for FEV1 and DLCO of > 40%, patient can undergo lung resection. If the predicted post-operative FEV1 and predicted post-operative DLCO are < 40%, exercise testing is necessary. Tc-99m MAA lung perfusion scintigraphy with quantification is very useful method for determination of post-operative FEV1 and can play an important role in taking the decision of surgery and also deciding the type of resection.[8,9,10,11,12]
Tc-99m MAA lung perfusion scan in planning of radionuclide microsphere therapy for liver tumor
Once a patient has been selected as a candidate for SIRT an initial angiographic evaluation has to be performed as the first step because anatomy of the mesenteric system and the hepatic arterial bed has a high degree of variation in approximately 40% of patients. Dystopic spread of microspheres to other extra hepatic visceral sites such as stomach, duodenum, or pancreas, may be associated with the risk of severe radiation-damage leading to pain, ulceration and possibly perforation, pancreatitis, cholecystitis, skin necrosis and other complications due to radiation to non-target sites. For avoiding dystopic spread, embolization of vessels, such as the gastroduodenal, right gastric, pancreaticoduodenal branches and the cystic artery is required or catheter for treatment can be placed beyond the respective origins of these vessels. A feature of the neoplastic vasculature within tumors is the formation of AV anastomoses or shunts. Shunts allow microspheres to directly enter the venous return by bypassing the terminal arterioles in the tumor. This will deposit the shunted microspheres into the lung, resulting in radiation pneumonitis. Tc-99m MAA can play a significant role for assessing this entity. The angiogram along with Tc-MAA injected into the hepatic artery similar to the application during microsphere treatment is done. Scintigraphy should be performed within 1 h of injection of Tc-MAA to prevent false-positive extra hepatic activity due to free technetium. Due to free technetium, thyroid gland and often the stomach may be seen in Tc-MAA images, which usually seem confusing and a pathologic uptake in the stomach should be ruled out in such cases before the treatment. Alternatively, 600 mg perchlorate per os 30 min before angiography can be given to prevent the uptake of Tc-99m pertechnetate in the thyroid and stomach. Following infusion of 200-400 MBq Tc-MAA in the hepatic arterial branches, a whole-body scan in anterior and posterior projections is acquired and percentage of lung shunting is quantified. The percentage of lung shunting can be determined from the total counts within regions of interest over both lobes of the lung and the liver, using the geometrical mean of anterior and posterior images. Depending on the shunt volume, a reduction in the total administered dose to the liver is necessary. It has been established that highest tolerable dose to the lungs is up to 30 Gy with a single injection and up to 50 Gy for multiple injections. A more practical way is a decision based on shunt quantification. If the lung shunting is more than 10%, the amount of microspheres delivered to the patient is to be reduced or SIRT is even impossible if there is a shunt of more than 20% of the administered dose. SPECT/CT provides valuable additional information because of improved localization.[13]
Tc-99m MAA venography
Tc-99m MAA venography can reveal venous collaterals in patients with deep vein thrombosis. Approximate quantitation of the shunt can also be done, but the clinical significance of such quantification is unknown. Because of availability of better techniques such as Venous Doppler study, radionuclide venography is not in much use nowadays. Only advantage is it can be combined with lung perfusion study in patient suspected for pulmonary embolism by injecting tracer in bilateral lower limbs.
Tc-99m MAA shunt scintigraphy
The LeVeen PVS provides unidirectional flow of sterile ascitic fluid from the abdomen to the vascular system. The shunt is unidirectional and allows flow from the abdomen to the vascular system in thorax, when intraperitoneal pressure is approximately 3 cm H2O greater than intrathoracic and central venous pressures. It is used in patients with intractable ascites refractory to medical management. It can provide symptomatic relief in patients not responding to medical management. Important complication of this shunt is blockade of tube. Tc-99m MAA shunt scintigraphy is the study of choice in assessing shunt patency because it is a very sensitive and non-invasive test.[14,15]
Tc-99m MAA scintigraphy in quantification of right to left cardiac shunts
Right to left shunt may occur in many types of congenital heart disease with elevated right ventricular pressure, such as Tetrology of Fallot (TOF), transposition of great arteries, truncus arteriosus and ebstein anomaly. In case of right to left shunt, some of the MAA escape from the pulmonary circulation and lodge in the systemic capillaries. By estimating the counts in the lungs and in the systemic circulation, it is possible to estimate the size of the right to left shunt. Quantitative Tc-99m MAA whole body scintigraphy can be of use for this indication in quantifying the shunt fraction.
More indications of Tc-99m MAA scintigraphy
Extensive literature review showed few more indications of 99mTc-MAA scintigraphy (20-23), although we never encountered such indications in our department.
Tc-99m MAA scintigraphy in peritoneal pleural communication
CAPD is an effective therapy for end-stage renal disease. Hydrothorax is an uncommon complication of CAPD, is possibly secondary to peritoneopleural communications. However, the real mechanism is still unclear. Peritoneal scintigraphy is a useful diagnostic tool in the identification of peritoneopleural communication.[16]
99mTc-MAA hysterosalpingoscintigraphy (HSSG) in the evaluation of fallopian tube patency and function
HSSG is performed after instillation of 4 mCi (148 MBq) of Tc-99m MAA in posterior vaginal fornix. Serial static images are acquired in the supine position at 1 h, 2 h, 3 h and if needed, at 24 h and fallopian tube patency is evaluated. Thus, HSSG can play an important role in the evaluation of infertility.[17]
Tc-99m MAA rhinoscintigraphy
One droplet (~50 μCi) of Tc-99m MAA is dripped on the floor of the nasal meatus approximately 1 cm behind the anterior end of the inferior turbinate with a 27 g syringe. Detectors are set laterally and images are acquired in the supine position. 30-s dynamic images are obtained during 20 min. Then, a 30-s additional static image is taken. The distance between the point where the radiopharmaceutical was dripped and the point where the particles reached the nasal cavity was measured on a straight line with a system computer. Then, to determine the nasal mucociliary transport rate in mm/min, this length is divided by the time which is elapsed. Rhinoscintigraphy is a reliable, easily reproducible and harmless method, so it may be used for follow-up examinations in patients who have had surgery of the nose and paranasal sinuses and for drug therapy of rhinopathic conditions.[18]
99mTc-MAA in radio guided occult lesion localization and excision of breast lesion
1 mCi of Tc-99m MAA is injected directly or close to the breast lesion (<2 cm). The injections are guided by stereotactic mammographic guidance for micro calcifications, focal distortions of parenchyma and lumps. Ultrasound guidance (USG) guidance is used when nodules are observed on USG. Gamma probe is used to check the position of the hot spot and to establish the margins of the resection. After removal, resection cavity is checked for residual activity greater than background. Radio guided surgery is an important tool in removal of non-palpable breast lesion, is a simple, fast and feasible method that can be implemented in clinical routine of patients with non-palpable breast lesions.[19]
Tc-99m MAA lung perfusion scintigraphy in assessing the effect of unilateral endobronchial valve insertion
Effect of endobronchial valve insertion on lung perfusion in chronic obstructive pulmonary disease patients with heterogeneous emphysema is not known. Serial changes in regional perfusion can be assessed using Tc-99m MAA lung perfusion.[20] A larger trial is currently underway for the same.
CONCLUSION
Tc-99m MAA is a very useful radiopharmaceutical, which can be used for many other indications apart from the commonly used indication of lung perfusion scan in pulmonary embolism. It can provide useful clinical information in other indications, which we tried to highlight in this article.
ACKNOWLEDGMENT
The authors would like to thank Journal of the College of Physicians and Surgeons Pakistan for providing images of LeVeen shunt patency evaluation.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Tamir A, Melloul M, Berant M, Horev G, Lubin E, Blieden LC, et al. Lung perfusion scans in patients with congenital heart defects. J Am Coll Cardiol. 1992;19:383–8. doi: 10.1016/0735-1097(92)90495-9. [DOI] [PubMed] [Google Scholar]
- 2.Abrams GA, Nanda NC, Dubovsky EV, Krowka MJ, Fallon MB. Use of macroaggregated albumin lung perfusion scan to diagnose hepatopulmonary syndrome: A new approach. Gastroenterology. 1998;114:305–10. doi: 10.1016/s0016-5085(98)70481-0. [DOI] [PubMed] [Google Scholar]
- 3.Abrams GA, Jaffe CC, Hoffer PB, Binder HJ, Fallon MB. Diagnostic utility of contrast echocardiography and lung perfusion scan in patients with hepatopulmonary syndrome. Gastroenterology. 1995;109:1283–8. doi: 10.1016/0016-5085(95)90589-8. [DOI] [PubMed] [Google Scholar]
- 4.Krishnamurthy GT, Krishnamurthy S. 2nd ed. Springer; 2009. Nuclear Hepatology: A Textbook of Hepatobiliary Disease. Publication Date: July 9, 2009, ISBN-10: 3642006477. [Google Scholar]
- 5.Genovesi MG, Tierney DF, Taplin GV, Eisenberg H. An intravenous radionuclide method to evaluate hypoxemia caused by abnormal alveolar vessels. Limitation of conventional techniques. Am Rev Respir Dis. 1976;114:59–65. doi: 10.1164/arrd.1976.114.1.59. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe JD, Tashkin DP, Holly FE, Brachman MB, Genovesi MG. Hypoxemia of cirrhosis: Detection of abnormal small pulmonary vascular channels by a quantitative radionuclide method. Am J Med. 1977;63:746–54. doi: 10.1016/0002-9343(77)90161-9. [DOI] [PubMed] [Google Scholar]
- 7.Krowka MJ, Cortese DA. Hepatopulmonary syndrome: An evolving perspective in the era of liver transplantation. Hepatology. 1990;11:138–42. doi: 10.1002/hep.1840110123. [DOI] [PubMed] [Google Scholar]
- 8.Datta D, Lahiri B. Preoperative evaluation of patients undergoing lung resection surgery. Chest. 2003;123:2096–103. doi: 10.1378/chest.123.6.2096. [DOI] [PubMed] [Google Scholar]
- 9.Kristersson S, Lindell SE, Svanberg L. Prediction of pulmonary function loss due to pneumonectomy using 133 Xe-radiospirometry. Chest. 1972;62:694–8. doi: 10.1378/chest.62.6.694. [DOI] [PubMed] [Google Scholar]
- 10.Olsen GN, Block AJ, Tobias JA. Prediction of postpneumonectomy pulmonary function using quantitative macroaggregate lung scanning. Chest. 1974;66:13–6. doi: 10.1378/chest.66.1.13. [DOI] [PubMed] [Google Scholar]
- 11.Wernly JA, DeMeester TR, Kirchner PT, Myerowitz PD, Oxford DE, Golomb HM. Clinical value of quantitative ventilation-perfusion lung scans in the surgical management of bronchogenic carcinoma. J Thorac Cardiovasc Surg. 1980;80:535–43. [PubMed] [Google Scholar]
- 12.Corris PA, Ellis DA, Hawkins T, Gibson GJ. Use of radionuclide scanning in the preoperative estimation of pulmonary function after pneumonectomy. Thorax. 1987;42:285–91. doi: 10.1136/thx.42.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmadzadehfar H, Biersack HJ, Ezziddin S. Radioembolization of liver tumors with yttrium-90 microspheres. Semin Nucl Med. 2010;40:105–21. doi: 10.1053/j.semnuclmed.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Adil AR, Waqar A. Evaluation of LeVeen-shunt patency using Tc-99m labelled macroaggregated albumin. J Coll Physicians Surg Pak. 2005;15:821–2. [PubMed] [Google Scholar]
- 15.Algeo JH, Jr, Powell M, Couacaud J. LeVeen shunt visualization without function using technetium-99m macroaggregated albumin. Clin Nucl Med. 1987;12:741–3. doi: 10.1097/00003072-198709000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Yao-Nan Yuan, Yu-Ming Fan, Chuang-Hsin Chiu, Wen-Shang Huang, Shiou-Chi Cherng, Cherng Yi-Cheng, Yuh Feng Lin, Ching Yuan Chen. Diagnosis of peritoneopleural communication with 99mTc-MAA scintigraphy in a patient with continuous ambulatory peritoneal dialysis: A case report and literature review. Ann Nucl Med Sci. 2004;17:51–55. [Google Scholar]
- 17.Akhtar K, Sabih DE, Laghari NA, Mateen A, Sabih Z, Haq AU, et al. Role of hysterosalpingoscintigraphy in the workup of infertility. J Coll Physicians Surg Pak. 2006;16:760–3. [PubMed] [Google Scholar]
- 18.Polat C, Dostbil Z. Evaluation of the nasal mucociliary transport rate by rhinoscintigraphy before and after surgery in patients with deviated nasal septum. Eur Arch Otorhinolaryngol. 2010;267:529–35. doi: 10.1007/s00405-009-1116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Esser S, Hobbelink MG, Peeters PH, Buskens E, van der Ploeg IM, Mali WP, et al. The efficacy of ‘radio guided occult lesion localization’ (ROLL) versus ‘wire-guided localization’ (WGL) in breast conserving surgery for non-palpable breast cancer: A randomized clinical trial-ROLL study. BMC Surg. 2008;8:9. doi: 10.1186/1471-2482-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung SC, Peters MJ, Chen S, Emmett L, Ing AJ. Effect of unilateral endobronchial valve insertion on pulmonary ventilation and perfusion: A pilot study. Respirology. 2010;15:1079–83. doi: 10.1111/j.1440-1843.2010.01815.x. [DOI] [PubMed] [Google Scholar]


