Abstract
Lytic bone metastases are rare in prostate cancer. We here present 18 fluorine fluorodeoxyglucose (18F-FDG) positron emission tomography computed tomography (PET-CT) images of a 67-year-old male patient with lytic metastases from prostate cancer. Repeat 18F-FDG PET-CT done 6 months later showed response to medical castration therapy. While the role of 18F-FDG PET-CT for sclerotic bone metastases in prostate cancer remains controversial, it appears to be useful for detection and response assessment of lytic prostate cancer metastases.
Keywords: Bone, lytic, metastasis, prostate cancer, 18 fluorine fluorodeoxyglucose positron emission tomography-computed tomography
INTRODUCTION
Bone metastases are very common in prostate cancer with most of them being sclerotic in nature. Lytic bone metastases are very rare in prostate cancer. 18 fluorine fluorodeoxyglucose (18F-FDG) positron emission tomography computed tomography (PET-CT) has low sensitivity in detection of sclerotic metastatic lesions of prostate cancer. However, in the detection of lytic prostate cancer metastasis, 18F-FDG PET-CT is useful. In this context, we report a case of a patient having lytic skeletal metastases from prostate cancer where 18F-FDG PET-CT was found to useful for detection and response assessment.
CASE REPORT
A 67-year-old male patient presented with low grade fever of 3 months duration. During routine work-up, multiple lytic lesions were demonstrated on the radiograph of pelvis, suspicious for metastases. The patient underwent 18F-FDG PET-CT for localization of the primary malignancy. 18F-FDG PET-CT images revealed multiple lytic lesions involving almost entire skeleton, showing increased 18F-FDG uptake (highest maximum standardized uptake value-6.9 [SUVmax]) [Figure 1a and c]. The prostate gland was enlarged and showed increased 18F-FDG uptake along the periphery (SUVmax-5.5) [Figure 1e]. Also noted were enlarged 18F-FDG avid pelvic (SUVmax-10.6) [Figure 1g] and retroperitoneal (SUVmax-6.4) [Figure 1i] lymph nodes. Based on the 18F-FDG PET-CT findings a diagnosis of carcinoma prostate with nodal and lytic skeletal metastases was made. The diagnosis was confirmed with transrectal ultrasound guided biopsy from prostate, which revealed prostatic adenocarcinoma with perineural invasion (Gleason score-9/10). The serum prostate specific antigen (PSA) was elevated (370 ng/ml; normal: 0-4 ng/ml).
Figure 1.
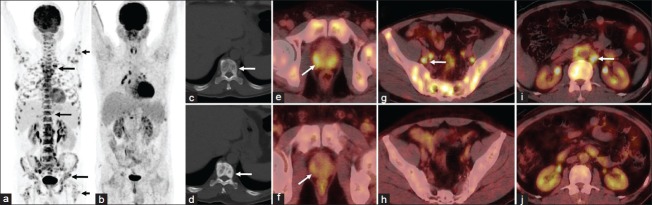
Baseline 18 fluorine fluorodeoxyglucose (18F-FDG) positron emission tomography-computed tomography (PET-CT) images revealed multiple 18F-FDG avid lytic lesions involving almost entire skeleton (a, c, arrows). The prostate gland was enlarged and showed increased FDG uptake along the periphery (e, arrow) with enlarged FDG avid pelvic (g, arrow) and retroperitoneal (i, arrow) lymph nodes. Post-therapy 18F-FDG PET-CT revealed sclerotic non-FDG avid lesions replacing most of the lytic lesions (b, d, arrow) with reduction in size of the prostate gland and disappearance of peripheral FDG uptake (f, arrow). The pelvic and retroperitoneal lymph nodes also have disappeared (h, j)
As lytic metastases are uncommon in prostate cancer, the patient underwent bone marrow biopsy to rule out any other malignant pathology. Bone marrow biopsy revealed metastatic carcinoma, positive for cytokeratin and PSA but negative for thyroid transcription factor-1, confirming the diagnosis. The patient was advised orchiectomy, which he refused. The patient was then put on medical castration therapy with combined androgen blockade. He was given luteinizing hormone releasing hormone agonist goserelin (10.8 mg subcutaneous injection every 3 months) along with non-steroidal anti-androgen bicalutamide (50 mg once daily orally).
Six months later the patient was referred for response assessment 18F-FDG PET-CT. 18F-FDG PET-CT revealed that most of the lytic lesions were replaced with sclerotic lesions with no significant 18F-FDG uptake [Figure 1b and d], except for lytic lesions in eighth dorsal vertebra, which showed mild 18F-FDG uptake (SUVmax-3.2). Also noted was a reduction in size of the prostate gland and disappearance of peripheral 18F-FDG uptake (SUVmax-1.1) [Figure 1f]. The pelvic and retroperitoneal lymph nodes also have disappeared [Figure 1h and j]. Thus, a diagnosis of minimal residual disease was made on 18F-FDG PET-CT. This correlated with serum PSA levels, which showed drastic reduction (0.14 ng/ml; normal: 0-4 ng/ml).
DISCUSSION
While bone metastases are very common in prostate cancer, lytic metastases are rare.[1] They probably result from overproduction of parathyroid hormone related peptide by prostate cancer cells in vivo.[2] 18F-FDG PET has low sensitivity for detection of sclerotic metastases in prostate cancer and is inferior to bone scintigraphy for this purpose.[3] In contrast, 18F-FDG PET-CT is superior to bone scintigraphy for detection of lytic prostate cancer metastasis.[4] The 18F-FDG uptake in prostate cancer cells is modulated by androgens.[5] The utility of 18F-FDG PET-CT for monitoring response to anti-androgen therapy is still widely debated, but it is generally considered to be useful for differentiating active from healed lesions.[6,7] Unfortunately, the low pre-therapy uptake of 18F-FDG hampers response evaluation.[8] In the present case, 18F-FDG PET-CT helped in making the primary diagnosis and demonstrated nodal and skeletal metastases. In addition, it helped in monitoring response to medical castration therapy by showing resolution of prostatic 18F-FDG uptake, resolution of nodal lesions and sclerotic changes in almost all of skeletal lesions.[9] Therefore, it appears that 18F-FDG PET-CT will be especially useful in prostate cancer patients with lytic skeletal metastasis.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Jadvar H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J Nucl Med. 2011;52:81–9. doi: 10.2967/jnumed.110.077941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabbani SA, Gladu J, Harakidas P, Jamison B, Goltzman D. Over-production of parathyroid hormone-related peptide results in increased osteolytic skeletal metastasis by prostate cancer cells in vivo. Int J Cancer. 1999;80:257–64. doi: 10.1002/(sici)1097-0215(19990118)80:2<257::aid-ijc15>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Shreve PD, Grossman HB, Gross MD, Wahl RL. Metastatic prostate cancer: Initial findings of PET with 2-deoxy-2- F-18 fluoro-D-glucose. Radiology. 1996;199:751–6. doi: 10.1148/radiology.199.3.8638000. [DOI] [PubMed] [Google Scholar]
- 4.Ozcan Kara P, Kara T, Kara Gedik G, Sari O, Sahin O. Comparison of bone scintigraphy and 18F-FDG PET-CT in a prostate cancer patient with osteolytic bone metastases. Rev Esp Med Nucl. 2011;30:94–6. doi: 10.1016/j.remn.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Jadvar H, Xiankui L, Shahinian A, Park R, Tohme M, Pinski J, et al. Glucose metabolism of human prostate cancer mouse xenografts. Mol Imaging. 2005;4:91–7. doi: 10.1162/15353500200505118. [DOI] [PubMed] [Google Scholar]
- 6.Oyama N, Akino H, Suzuki Y, Kanamaru H, Ishida H, Tanase K, et al. FDG PET for evaluating the change of glucose metabolism in prostate cancer after androgen ablation. Nucl Med Commun. 2001;22:963–9. doi: 10.1097/00006231-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Morris MJ, Akhurst T, Osman I, Nunez R, Macapinlac H, Siedlecki K, et al. Fluorinated deoxyglucose positron emission tomography imaging in progressive metastatic prostate cancer. Urology. 2002;59:913–8. doi: 10.1016/s0090-4295(02)01509-1. [DOI] [PubMed] [Google Scholar]
- 8.Yu EY, Muzi M, Hackenbracht JA, Rezvani BB, Link JM, Montgomery RB, et al. C11-acetate and F-18 FDG PET for men with prostate cancer bone metastases: Relative findings and response to therapy. Clin Nucl Med. 2011;36:192–8. doi: 10.1097/RLU.0b013e318208f140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellamy EA, Nicholas D, Ward M, Coombes RC, Powles TJ, Husband JE. Comparison of computed tomography and conventional radiology in the assessment of treatment response of lytic bony metastases in patients with carcinoma of the breast. Clin Radiol. 1987;38:351–5. doi: 10.1016/s0009-9260(87)80207-6. [DOI] [PubMed] [Google Scholar]


