Sir,
Primary cardiac tumors are highly F-18 fluorodeoxyglucose (FDG) avid, and positron emission tomography/computed tomography (PET/CT) can be useful in initial staging. We report a case of right atrial sarcoma in which F-18 FDG PET/CT was used in initial evaluation and subsequent detection of the residual disease after chemotherapy. A 37-year-old patient presented with dyspnea (New York Heart Association Class II), facial edema, asthenia and weight loss. His chest X-ray showed cardiomegaly while contrast enhanced CT of thorax revealed a lobulated heterogeneously enhancing space occupying lesion in right atrium. Mass was seen to involve pericardium. Cytological examination from CT guided fine-needle aspiration cytology revealed atypical spindle cells with hyperchromatic nuclei suggesting the diagnosis of sarcoma. Patient was subjected to PET/CT (Discovery STE-16, GE Healthcare, Milwaukee, USA) 64 min after intravenous administration of 10.2 mCi of F-18 FDG. The reconstructed images showed highly avid F-18 FDG in the periphery of the mass [Figure 1a and c] with a SUVmax of 5.4. No other abnormal F-18 FDG uptake was noticed ruling out metastases. Patient was given chemotherapy consisting of mesna, doxorubucin, ifosfamide and dacarbazine, as he refused for surgery. Clinically he responded to chemotherapy with relieve of symptoms. After completion six cycles of chemotherapy he was again reassessed for residual disease with F-18 FDG PET/CT. Study was performed 72 min after intravenous injection of 10.8 mCi of F-18 FDG. PET/CT images showed F-18 FDG uptake with a SUVmax of 4.6 (decrease of only 11.5% when compared to pre-therapy SUVmax) in the right atrial wall [Figure 1b and d] in the location of previous tumoural involvement suggesting the residual disease with possible progression if left untreated. Hence, an alternative chemotherapy regimen was started. Unfortunately patient did not respond to alternate chemotherapy regimen, progressed and died 7 months later.
Figure 1.
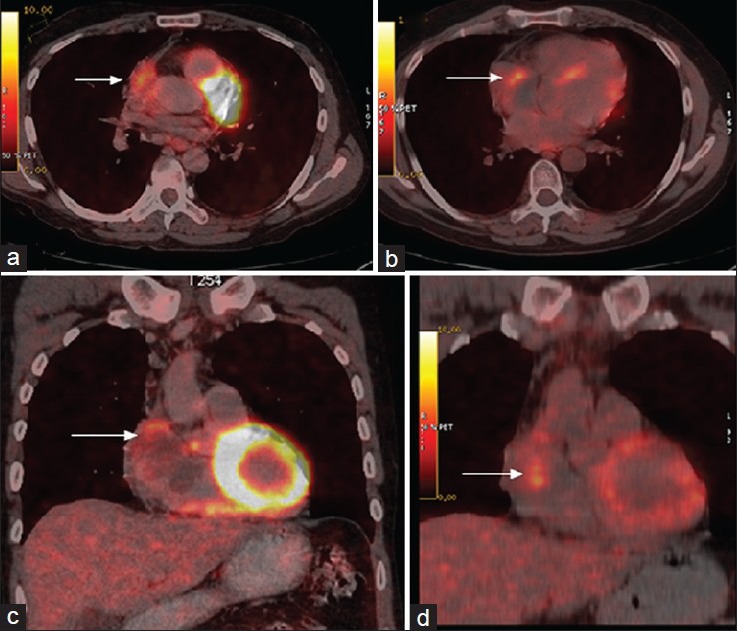
F-18 fluorodeoxyglucose (FDG) positron emission tomography/computed tomography transaxial and coronal pre-treatment slices (a and c) showing FDG uptake in right atrial wall with extension into the pericardium and post-treatment images (b and d) showing significant residual FDG uptake in right atrial wall
Primary cardiac tumors are uncommon, reported frequency being just 0.02%.[1] Amongst sarcomas, angiosarcomas have a frequency of less than 1%, while a primary angiosarcoma of the heart and the great vessels accounts for only 3% of all angiosarcomas.[2] Surgical resection remains the best treatment option in a non-metastatic disease.[2] However, if surgery is contraindicated, chemotherapy and radiotherapy are given but with a varied response.[3] A sarcoma in a patient above the age of 16 is likely to be an angiosarcoma and in a patient less than 16 years it is more likely to be a rhabdomyosarcoma.[4] Clinical presentation varies depending on location, size and extent of tumor. Usual work up of these patients includes standard X-ray, ECHO, contrast-enhanced computed tomography, magnetic resonance imaging (MRI) and angiography. Few case reports have demonstrated the utility F-18 FDG PET to decide upon the malignant nature of disease and to stage the disease before starting the appropriate treatment.[5,6] In our patient, increased F-18 FDG uptake was helpful in confirming the malignant nature of the tumor and also excluded distant metastases. Conventional imaging modalities like CT and MRI have limited use in assessment of response and detecting residual disease, as these cannot differentiate fibrous tissue from residual disease. Nuclear imaging using myocardial perfusion imaging and gallium-67 is less useful in such cases due to variable avidity and limited spatial resolution.[4] F-18 FDG PET/CT appears to be the most promising imaging modality to assess the response to chemotherapy. Only few case reports have been published demonstrating F-18 FDG uptake in sarcomas of heart. But findings of F18-FDG uptake pattern of our case F-18 FDG uptake in the tumor and extension of uptake into the adjacent pericardium were similar to the findings demonstrated by Higashiyama et al.[6] However, to the best of our knowledge, till date no published report demonstrated the utility of F-18 FDG PET in assessment of residual disease in cardiac sarcomas. F-18 FDG PET/CT in our patient was useful in determining residual disease.
REFERENCES
- 1.Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77:107. doi: 10.1016/s0002-9149(97)89149-7. [DOI] [PubMed] [Google Scholar]
- 2.Truong PT, Jones SO, Martens B, Alexander C, Paquette M, Joe H, et al. Treatment and outcomes in adult patients with primary cardiac sarcoma: The British Columbia cancer agency experience. Ann Surg Oncol. 2009;16:3358–65. doi: 10.1245/s10434-009-0734-8. [DOI] [PubMed] [Google Scholar]
- 3.Kakizaki S, Takagi H, Hosaka Y. Cardiac angiosarcoma responding to multidisciplinary treatment. Int J Cardiol. 1997;62:273–5. doi: 10.1016/s0167-5273(97)00259-3. [DOI] [PubMed] [Google Scholar]
- 4.Burke AP, Cowan D, Virmani R. Primary sarcomas of the heart. Cancer. 1992;69:387–95. doi: 10.1002/1097-0142(19920115)69:2<387::aid-cncr2820690219>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Hori Y, Funabashi N, Miyauchi H, Nakagawa K, Shimura H, Miyazaki M, et al. Angiosarcoma in the right atria demonstrated by fusion images of multislice computed tomography and positron emission tomography using F-18 Fluoro-Deoxyglucose. Int J Cardiol. 2007;123:e15–7. doi: 10.1016/j.ijcard.2006.11.093. [DOI] [PubMed] [Google Scholar]
- 6.Higashiyama S, Kawabe J, Hayashi T, Kurooka H, Oe A, Kawamura E, et al. Effectiveness of preoperative PET examination of huge angiosarcoma of the heart. Clin Nucl Med. 2009;34:99–102. doi: 10.1097/RLU.0b013e318192c3e3. [DOI] [PubMed] [Google Scholar]


