Abstract
Rationale
Autologous bone marrow- or cardiac-derived stem cell therapy for heart disease has demonstrated safety and efficacy in clinical trials but functional improvements have been limited. Finding the optimal stem cell type best suited for cardiac regeneration is key toward improving clinical outcomes.
Objective
To determine the mechanism by which novel bone-derived stem cells support the injured heart.
Methods and Results
Cortical bone stem cells (CBSCs) and cardiac-derived stem cells (CDCs) were isolated from EGFP+ transgenic mice and were shown to express c-kit and Sca-1 as well as 8 paracrine factors involved in cardioprotection, angiogenesis and stem cell function. Wild-type C57BL/6 mice underwent sham operation (n=21) or myocardial infarction (MI) with injection of CBSCs (n=67), CDCs (n=36) or saline (n=60). Cardiac function was monitored using echocardiography. Only 2/8 paracrine factors were detected in EGFP+ CBSCs in vivo (basic fibroblast growth factor and vascular endothelial growth factor) and this expression was associated with increased neovascularization of the infarct border zone. CBSC therapy improved survival, cardiac function, regional strain, attenuated remodeling, and decreased infarct size relative to CDC- or saline-treated MI controls. By 6 weeks, EGFP+ cardiomyocytes, vascular smooth muscle and endothelial cells could be identified in CBSC- but not in CDC-treated animals. EGFP+ CBSC-derived isolated myocytes were smaller and more frequently mononucleated, but were functionally indistinguishable from EGFP- myocytes.
Conclusions
CBSCs improve survival, cardiac function, and attenuate remodeling through two mechanisms:1) secretion of pro-angiogenic factors that stimulate endogenous neovascularization, and 2) differentiation into functional adult myocytes and vascular cells.
Keywords: Stem cells, myocardial infarction, differentiation, paracrine signaling, neovascularization
Introduction
Ischemic injury of the heart, including myocardial infarction (MI), is a major health problem that leads to structural and functional remodeling1 and often culminates in heart failure.2 Novel therapies to repair or replace damaged cardiac tissue are needed to improve the prognosis of MI patients. Stem cell therapy has the potential to repair hearts after ischemic injury. A variety of adult stem cell types that might repair the injured heart have been tested in animal models. These studies have shown that transplantation of autologous cardiac-3-6 or bone marrow-derived7, 8 stem cells, induced pluripotent stem cells,9-11 and direct reprogramming of endogenous non-stem cells into cardiogenic phenotypes12, 13 can improve cardiac function after injury. Early stage clinical trials have largely focused on autologous stem cells due to their ease of isolation and lack of immunogenicity. These trials have demonstrated the ability of both bone marrow-14-16 and cardiac-derived17-19 cells to offer moderate functional benefits when transplanted after cardiac injury. While the outcomes of these trials continue to improve, the overall beneficial effects of autologous stem cell therapies are still relatively modest and the fundamental mechanisms of stem cell mediated repair are largely unknown and controversial.
The mechanisms of stem cell-mediated cardiac repair are critical unanswered questions in the field. Many preclinical studies in animal models have shown that differentiation6-8 of injected cells into new cardiac myocytes is one potential mechanism of this repair. Secretion of paracrine factors that enhance cardioprotection of the endogenous myocardium, neovascularization, and recruitment of endogenous stem cells that promote repair are other major mechanisms.4, 5, 20, 21 The goal of the present study was to define the contributions of differentiation of transplanted bone-derived stem cells into new cardiac tissue (cardiac myocytes and blood vessels) versus stem cell-mediated induction of endogenous cardiac repair via secretion of paracrine factors.
Well-characterized sources of stem cells shown to enhance cardiac repair of the diseased heart should lead to effective cell therapies. Certain stem cell types may have a greater capacity to transdifferentiate, while others may produce more paracrine factors and have a greater potential to stimulate neovascularization and other endogenous repair mechanisms. In this study, we examined eight specific paracrine factors that have been previously suggested to play some role in stem cell mediated repair of the heart: angiopoietin-1 (Ang-1), basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), insulin-like growth factor-1 (IGF-1), platelet-derived growth factor (PDGF), stem cell factor (SCF), stromal-derived factor-1 (SDF-1), and vascular-endothelial growth factor (VEGF). HGF and IGF-1 are thought to be cardioprotective: HGF has cytoprotective, anti-apoptotic and pro-angiogenic effects,22-24 while IGF-1 can inhibit apoptosis and may stimulate growth and proliferation of stem cells.5, 23-26, SCF, and SDF-1 are thought to stimulate stem cell function: SCF, the ligand for the c-kit receptor,27 may stimulate stem cell homing,28 while SDF-1 is a chemotactic ligand that induces stem cell proliferation and homing to the site of injury.24, 25, 29 Ang-1, bFGF, PDGF and VEGF all promote angiogenesis: Ang-1 induces vascular cell migration and enhances stability of newly-formed vasculature,24, 28 bFGF induces proliferation of endothelial and smooth muscle cells,23-25, 28, 30 PDGF stimulates smooth muscle cell proliferation,24, 25, 28 and VEGF induces endothelial cell proliferation and tube formation.23-25, 28-30
Although a solid consensus regarding stem cell repair mechanisms is lacking, most cardiac stem cell researchers currently believe that bone marrow-derived stem cells act primarily through paracrine stimulation of new blood vessel formation or through differentiation into vascular cells rather than transforming to myocytes, while cardiac-derived stem cells (CDCs) may differentiate into adult myocytes.31 Although some studies have demonstrated that bone marrow-derived stem cells can regenerate adult myocytes7, 8, others have suggested that these new myocytes are mostly formed through stimulation of endogenous cardiac stem cells rather than through direct differentiation of the transplanted cells.25, 32 Our goal was to define if and by what mechanisms, bone-derived stem cells induce repair of the injured heart.
It has long been hypothesized that both the heart and bone marrow contain stem cell niches where cells with the capability to differentiate down the cardiac or mesenchymal lineage exist in a dedifferentiated state.7, 33-35 In these environments, the stem cell's pluripotent state is thought to be supported by complex signaling interactions with surrounding cells, such as those found in the stromal lining of the bone marrow cavity. Investigators looking for a source of multipotent stem cells developed a new isolation technique using cortical bone tissue, rather than the bone marrow, to isolate cells that might be in a more primitive state.36 These cells, which can be easily obtained through routine bone biopsy procedures, were negative for most markers of the hematopoietic lineage and expressed the pluripotency marker Sca-1. Under specific culture conditions these cells differentiate in vitro into osteoblasts, chondrocytes, and adipocytes36 but no one has yet tested their cardiogenic potential in the injured heart. In this study, we report for the first time that injection of cortical bone-derived stem cells (CBSCs)into the heart after MI improved survival and cardiac function, and CBSCs demonstrated greater improvements across all parameters compared to the more widely studied CDCs. CBSCs differentiated into mature cardiac tissue, while CDCs did not, and CBSCs demonstrated a greater capacity for paracrine-mediated endogenous repair to produce these effects. Our study suggests that CBSCs are a source of stem cells that are more abundant and more easily isolated than CDCs, and that CBSCs have a greater capacity to repair hearts damaged by ischemic injury.
Methods
Please refer to the Supplemental Methods section for more detailed experimental methods.
Results
Cortical bone stem cell characterization
Cortical bone stem cells (CBSCs) or cardiac-derived stem cells (CDCs) were analyzed for expression of c-kit and Sca-1 mRNA abundance using quantitative real-time PCR (qPCR) (Online Figure I). C-kit and Sca-1 protein expression was detected using both immunostaining (Online Figure II) and flow cytometry (Online Figure III). CBSCs expressed greater levels of both transcripts than did CDCs: over 3-fold higher levels of c-kit and 2-fold higher levels of Sca-1 (Online Figure I). Both stem cells types demonstrated positive membrane immunostaining for c-kit and Sca-1 (Online Figure II). Flow cytometry analysis demonstrated that the majority of CBSCs and CDCs expressed c-kit (CD117), Sca-1, and β1-Integrin (CD29). Additionally both cell types lacked expression of the hematopoietic stem cell marker CD34, the common leukocyte antigen CD45, and other common markers of the hematopoietic lineage that can be detected by a cocktail of antibodies (Lin) against CD5, CD11b, CD45R, antigen 7-4, Gr-1, Ly6G/C, and Terr-119 (Online Figure III).
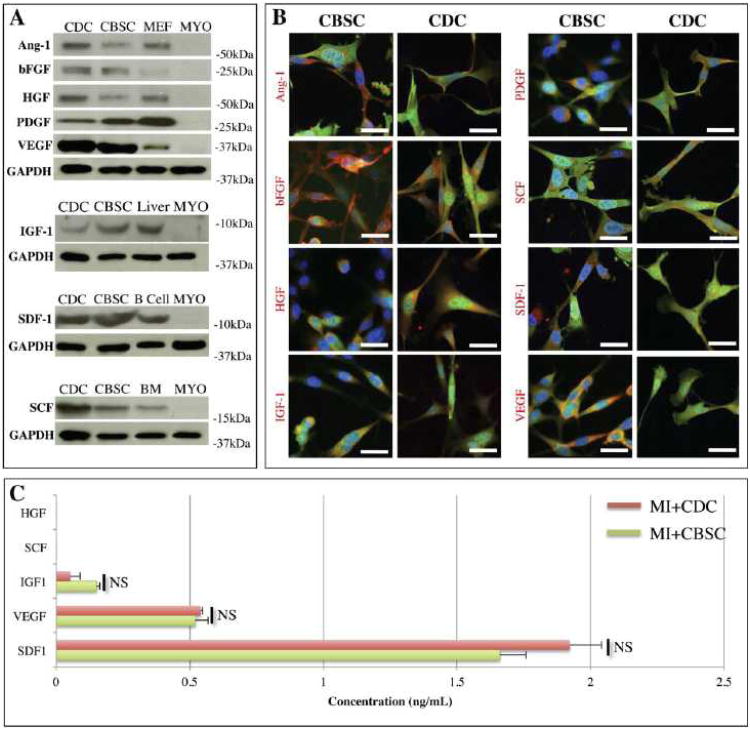
We next studied if the stem cells, cultured in vitro, expressed or secreted paracrine factors that are thought to be involved in cardioprotection, neovascularization, or recruitment of endogenous cardiac stem cells.24, 25, 29 Eight specific factors produced by CBSCs and CDCs were analyzed: Ang-1, bFGF, HGF, IGF-1, PDGF, SCF, SDF-1, and VEGF. Protein expression of all eight factors was detected by Western analysis performed on CBSC or CDC lysates collected in vitro (Figure 1A). Positive expression of these factors in vitro was confirmed by immunostaining (Figure 1B). Enzyme-linked immunosorbent assays of stem cell-conditioned media demonstrated that IGF-1, VEGF, and SDF-1 were all secreted by proliferating CBSC and CDCs in culture (Figure 1C), and no significant difference between the amount of paracrine factors secreted by each cell type could be detected. Neither HGF nor SCF were secreted in detectable amounts by either stem cell type, even though both factors were seen at the protein level by both Western analysis and immunostaining. These results show that both CBSCs and CDCs produce factors known to be associated with beneficial cardiac remodeling after MI.22, 23,25
Figure 1. In vitro characterization of stem cells.
A) CBSC or CDC lysates were analyzed by Western analysis. Positive controls include mouse endothelial fibroblasts (MEF), liver, bone marrow (BM), and B lymphocytes (B Cell). Myocyte (MYO) lysates were used as negative controls for all samples. B) CBSCs (green) were fixed in vitro and immunostained against each paracrine factor (red). Nuclei are labeled with DAPI (blue) and scale bars = 20 μm. C) CBSCs or CDCs were allowed to proliferate over 72 hours and their culture media was analyzed by ELISA for the presence of soluble HGF, IGF, SCF, SDF-1, and VEGF. Samples were analyzed in triplicate and background signal was subtracted using unconditioned media blanks. NS = No Significant difference (p > 0.05).
CBSCs differentiate in vitro in co-culture with neonatal rat ventricular myocytes
Both bone marrow-derived32, 37 and CDCs38 have previously been shown to differentiate in vitro in co-culture with neonatal rat ventricular myocytes. To quantify the rate of in vitro differentiation into cells expressing cardiac myocyte proteins, CBSCs cocultured with neonatal rat ventricular myocytes were fixed and stained for α-sarcomeric actin (Online Figure IV A and B) or connexin43 (Online Figure IV C and D). Staining revealed that 11.5±0.9% (n=5) of EGFP+ cells expressed α-sarcomeric actin and 7.5±1.7% (n=5) appeared coupled via connexin43 gap junctions to their neighboring cells. A very small fraction of EGFP+ cells (<1%) were observed to contract spontaneously after 72 hours in co-culture. Thus, like bone marrow- or CDCs, CBSCs, in-vitro, demonstrate some capacity to differentiate into cells expressing cardiac specific proteins in vitro.
CBSC transplants improved survival and cardiac function, attenuated adverse left ventricular remodeling and reduced infarct size
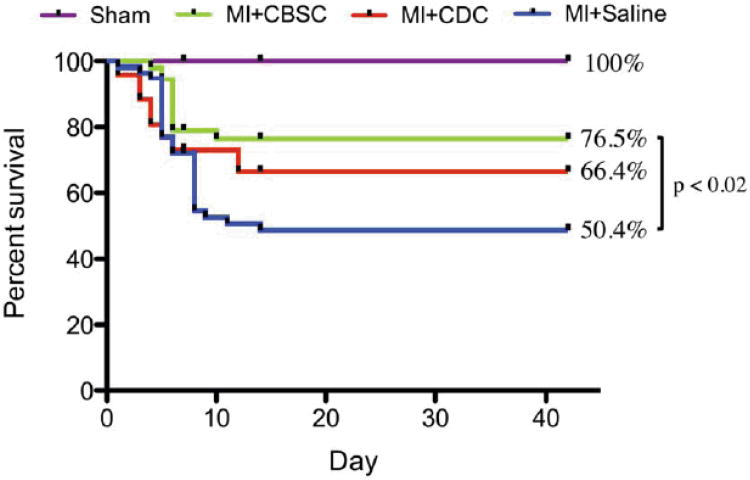
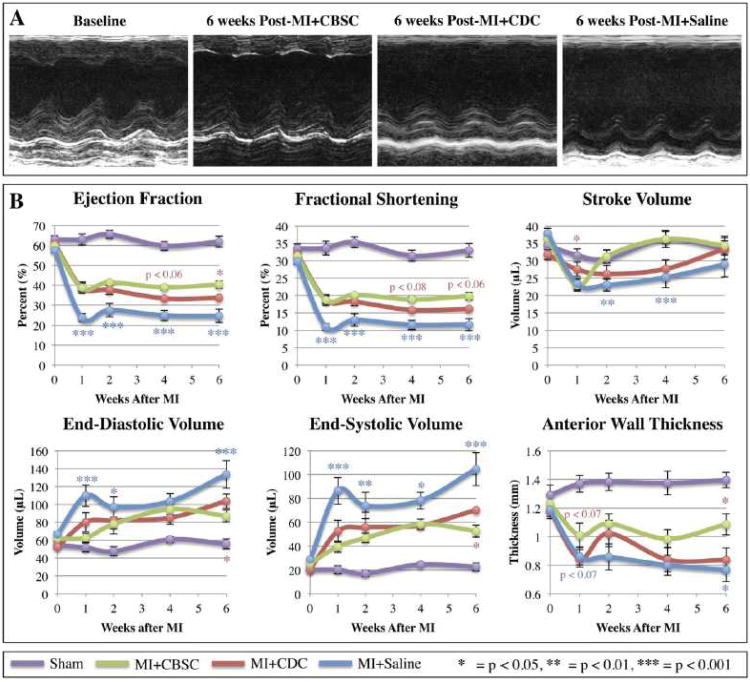
The effects of CBSCs versus CDCs on post-MI structural and functional remodeling were studied. The MI procedure reduced 6-week survival to 50.4% in animals receiving sham saline injections. Animals receiving MI and CBSC therapy demonstrated a 76.5% 6-week survival, which was greater than CDC-treated animals and significantly greater than the saline-treated controls (Figure 2). Animals receiving CDC therapy did demonstrate an improvement in survival relative to saline-treated MI controls (66.4%), but this improvement was not statistically significant. Both stem cell therapies improved cardiac function and attenuated adverse cardiac remodeling characteristic of MIs in this model system (Figure 3), and these changes were most pronounced in the MI+CBSC group. Animals receiving MI+CBSC had significantly improved ejection fraction and fractional shortening compared to the MI+Saline control animals as early as 1 week post-MI, and these changes were sustained through 6 weeks post-MI. Improvements in ejection fraction and fractional shortening were greater in CBSC treated animals versus CDC treated animals. Both stem cell treatment groups had an initial decline in stroke volume (SV), but SV returned to normal by 2 weeks post-MI and remained significantly improved relative to MI+Saline control animals. Animals in the MI+Saline group demonstrated significant increases in end-diastolic and systolic volumes with thinning of the infarct-affected anterior wall (Figure 3). By contrast, MI animals treated with CBSCs or CDCs had significantly smaller post-MI diastolic and systolic volumes at all time points studied, and they demonstrated attenuated thinning of the anterior wall relative to saline-injected controls, with CBSC treated animals demonstrating the most profound attenuation across all parameters (Figure 3).
Figure 2. Six-week survival.
Mice underwent sham, MI+Saline, MI+CDC or MI+CBSC surgery. Data was analyzed using a Kaplan-Meier regression and significance was determined using the Log-Rank test.
Figure 3. Cardiac function measured by echocardiography.
Animals underwent sham, MI+Saline, MI+CBSC or MI+CDC surgeries and received follow-up serial echocardiography at 1, 2, 4 and 6 weeks post-MI. A) Representative M-mode tracings from animals at baseline, 6 weeks post-MI+CBSC, 6 weeks post-MI+CDC, or 6 weeks post-MI+Saline. B) Structural and functional parameters derived from echocardiography measurements are shown. */**/***= CBSC v. Saline, */**/*** = CBSC v. CDC
Five mice were randomly selected from each group (MI+Saline, MI+CDC, or MI+CBSC) to undergo acute infarct size analysis at 24 hours post-MI, and no significant difference between the area at risk (AAR) or ischemic area (IA) was detected between animals in any group (Online Figure V). These results demonstrate that acute infarct size was similar in all study animals. Therefore, the structural and functional improvements in the MI+CBSC and MI+CDC groups can be attributed to the effects of cell transplantation alone and are not the result of a stem cell mediated reduction in initial infarct size. After 6 weeks post-MI, chronic infarct size was analyzed by measuring the area of infarcted tissues relative to total myocardial area on hematoxylin and eosin (H&E)-stained cross sections from saline-, CDC-, or CBSC-injected hearts. Animals receiving either CDC or CBSC therapy had significantly smaller infarcted areas relative to saline-treated controls, with CBSC-treated animals demonstrating the most significantly reduced infarct sizes, suggesting that infarct size or expansion were reduced 6 weeks after CBSC therapy.
Contractile myocardium was detected at CBSC injection sites
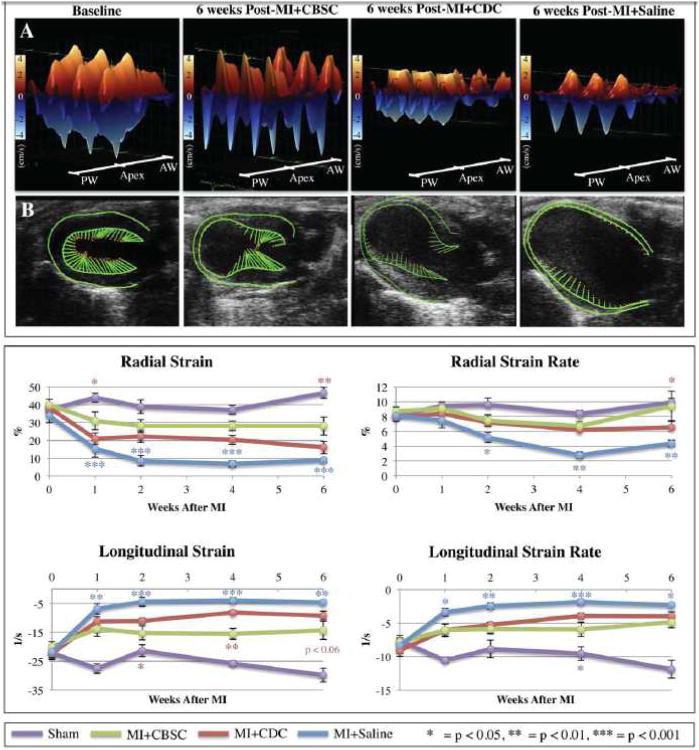
Strain analysis was performed on long-axis B-mode images to determine if regions injected with CBSCs developed contractile activity. Figure 4A shows representative three-dimensional wall velocity diagrams for 3 consecutive cardiac cycles taken from animals at baseline, 6 weeks post-MI+Saline, and 6 weeks post-MI+CBSC. Online Figure VI outlines how 3D wall velocity diagrams are constructed relative to the acquired parasternal long-axis B-mode echocardiogram of the left ventricle. Points along the left ventricular (LV) endocardial surface are plotted along the x-axis, from the base of the posterior wall to the apex to the base of the anterior wall. At baseline, all hearts demonstrated uniform and synchronous contraction and relaxation across the LV endocardium (Figure 4A). In the MI+Saline control group after 6 weeks, there was a dramatic reduction in wall velocity across the endocardium of the infarct-related anterior wall (Figure 4A). CDC-treated animals demonstrated some improvements in wall velocity at the infarct border zone, while CBSC-treated MI animals demonstrated even greater improvements in wall velocity at the infarct border zone segments where CBSCs were injected (Figure 4A).
Figure 4. Left ventricular endomyocardial strain.
A) Three-dimensional regional wall velocity diagrams showing contraction (orange/positive values) or relaxation (blue/negative values) of 3 consecutive cardiac cycles. B) Vector diagrams showing the direction and magnitude of endocardial contraction at mid-systole. C) Global averages of strain and strain rate measured in the radial or longitudinal axes across the LV endocardium. */**/***= CBSC v. Saline, */**/*** = CBSC v. CDC
Figure 4B shows vector diagrams taken from long-axis B-mode echocardiograms, and Online Videos I-IV shows live video loops of three consecutive cardiac cycles from each heart. The normal hearts demonstrate uniform, synchronous contractions and relaxations throughout the cardiac cycle (Online Video I). Animals with MI injury showed pronounced LV chamber dilation, wall thinning, and hypokinesis of the infarcted wall, as evidenced by the lack of vector activity in this region (Online Video IV). In animals receiving stem cell therapy, the LV chamber was less dilated, with attenuated wall thinning and increased contractile activity in the border zone segments that received cell therapy. While CDC-treated animals did show some improvements in wall velocity and strain at the border zone segments (Online Video III), CBSC-treated animals had much greater wall velocities and contraction vectors nearly equal in magnitude to baseline control hearts at the infarct border zone segments where CBSCs were injected (Online Video II).
Global averages of LV endocardial strain and strain rate are plotted in Figure 4C. Strain, which measures change in length relative to the initial length (Strain = Final Length [L]/Initial Length [L0]) was measured either in the radial (from the center of the ventricle cavity outward) or longitudinal axis (from the apex to the base). The rate of change in strain (Strain Rate = Strain/Time) was also calculated. In both axes, strain and strain rate were significantly reduced following MI. Significant improvements in both strain and strain rate in both the radial and longitudinal axes were observed in MI animals receiving CBSC or CDC therapy relative to MI+Saline controls, and CBSC-treated animals showed improved strain relative to CDC animals across all parameters at all time points. These data demonstrate that CBSC-treated hearts had significantly improved global and regional contractility by 6 weeks post-MI.
Expression of pro-angiogenic paracrine factors in vivo by stem cells induces new blood vessel formation
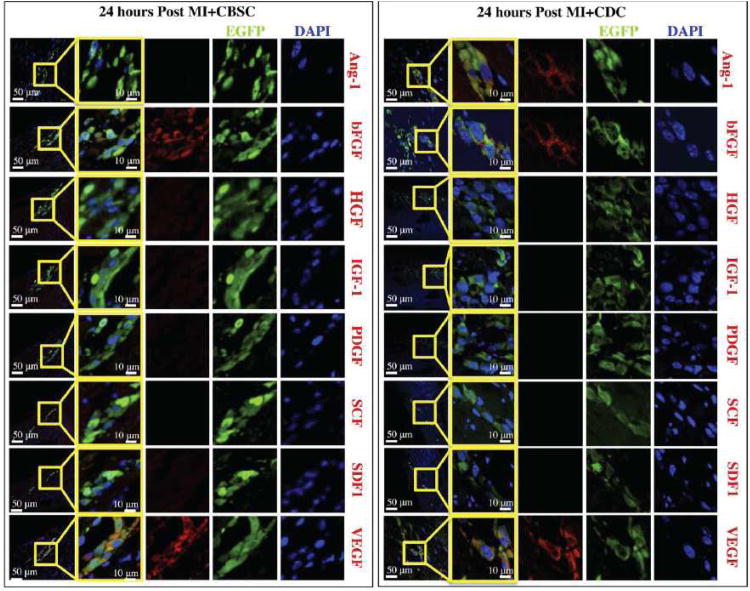
The environment in the infarct border zone is dramatically different from that in the tissue culture dish. Therefore, paracrine factor expression and production are likely to change after transplantation of CBSCs into the infarcted heart. At 24 hours post-MI, hearts receiving CBSC transplants were immunostained for the 8 paracrine factors studied in vitro. Injection sites containing EGFP+ CBSCs were identified and found to express bFGF and VEGF, but Ang-1, IGF-1, HGF, or PDGF were not detected at any injection sites examined (Figure 5). Injection sites containing EGFP+ CDCs were found to express Ang-1 in addition to bFGF and VEGF, but we could not detect the 5 other factors. These studies suggest that, when injected into the MI border zone, CBSCs express only bFGF and VEGF (and CDCs express only Ang-1, bFGF, and VEGF). Despite expressing an additional paracrine factor after 24 hours, expression of all paracrine factors by CDCs was not sustained (Online Table II). No amounts of Ang-1, bFGF, or VEGF could be detected in any CDC injection sites after 24 hours post-MI (data not shown).
Figure 5. Characterization of paracrine factors secreted by stem cells after 24 hours post-MI in vivo.
Animals receiving MI+CBSCs or MI+CDCs were sacrificed 24 hours post-MI for analysis of paracrine factor production. EGFP+ CBSCs stained positive for bFGF, and VEGF (shown in red), but negative for HGF, IGF-1, PDGF, SCF, and SDF-1. EGFP+ CDCs stained positive for Ang-1 in addition to bFGF and VEGF, but also stained negative for HGF, IGF-1, PDGF, SCF, and SDF-1. Nuclei are labeled with DAPI (blue) and injected CBSCs are green.
VEGF expression was detected in CBSC injection sites as late as 2 weeks post-MI (Online Figure VII). The fluorescence intensity tracings of each color channel (green = EGFP, red = VEGF, blue = DAPI) across a confocal line scan of the cell selected in Online Figure VIIA demonstrates the colocalization of the VEGF and EGFP signals (Online Figure VIIB). A scatterplot of pixel intensities (Online Figure VIIC) in the red channel versus the green channel was generated, and the diagonal deflection documents colocalized red/green pixels (56.2% of green and red pixels in this line scan were colocalized). A control scatterplot of the blue (DAPI) versus green channel (EGFP) shows only 13.6% of blue/green pixels were colocalized (Online Figure VIID). Paracrine factors involved in cardioprotection or homing and recruitment of endogenous stem cells (HGF, IGF-1, SCF and SDF-1) were not detected at any time point in vivo. An overview of all in vitro and in vivo paracrine factor analyses for CBSCs can be found in Online Table I, while a summary of CDC paracrine factor analyses is shown in Online Table II.
To determine whether the in vivo expression of the CBSC-secreted pro-angiogenic factors bFGF and VEGF (and Ang-1 secreted by CDCs) resulted in increased neovascularization, blood vessel density was measured in the infarct zones from MI animals treated with saline, CDCs or CBSCs (Online Figure VIII). MI animals receiving CBSC treatment had 15.13±3.04 von Willebrand factor (vWF)-positive blood vessels (purple) per 20× field of view and MI animals receiving CDC therapy had 10.13±1.13 vWF+ vessels per field, while saline-treated controls had only 4.95±1.30 vWF+ vessels per field. These data support the idea that secretion of pro-angiogenic paracrine factors in vivo by both stem cell types, and especially by CBSCs, induced increased neovascularization. Increased blood supply to the injured heart could have contributed to the functional benefits we observed following CBSC transplant. The majority of these blood vessels did not contain EGFP+ cells, suggesting that they were derived via endogenous repair rather than from the injected cells.
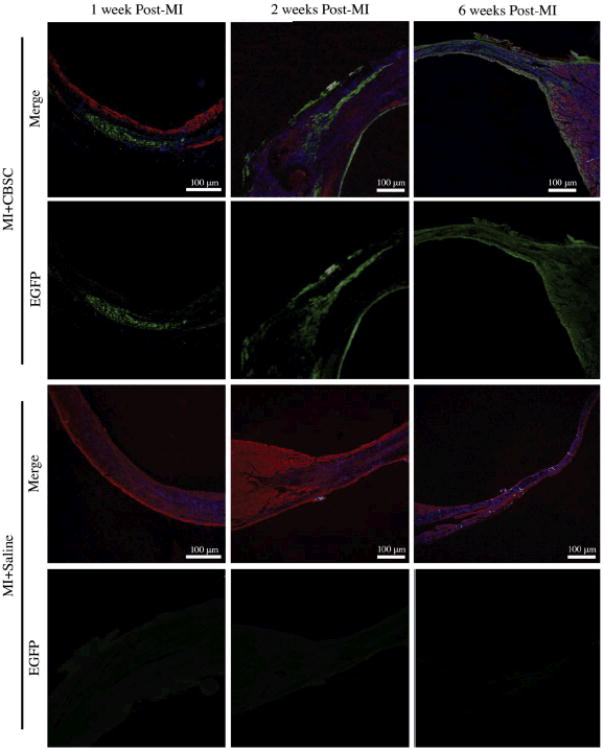
EGFP+ CBSCs expand over time as cells proliferate and CBSCs transdifferentiate into five distinct phenotypes
CBSC injection sites were examined 1, 2 and 6 weeks after MI (Figure 6). Injection sites were identified after immunostaining against α-sarcomeric actin (red) and EGFP (green). Histological samples from CBSC- and saline-injected hearts were stained simultaneously, and the absence of EGFP signal in the saline-injected controls was confirmed, documenting a very low level of autofluorescence under the conditions employed. All tissue sections were first examined under low magnification to identify injection sites and then high magnification confocal images were analyzed. At 1-week post-MI, the majority of EGFP+ cells were found in discrete groups of small, round, poorly differentiated cells. By 2 weeks post-MI, the area of the heart with EGFP+ cells had expanded and the majority of these EGFP+ cells were enlarged and had an elongated appearance. By 6 weeks Post-MI, injected stem cells had spread throughout the infarct zone and into the infarct border zone and discrete injection sites were no longer visible. Six weeks after injection, the EGFP+ stem cells were spread throughout the border zone and infarct zone.
Figure 6. Expansion of EGFP+ CBSCs as stem cells engraft, align, and elongate over 6 weeks.
Samples were immunostained against α-sarcomeric actin (red), nuclei are labeled with DAPI (blue) and injected EGPF+ CBSCs are green.
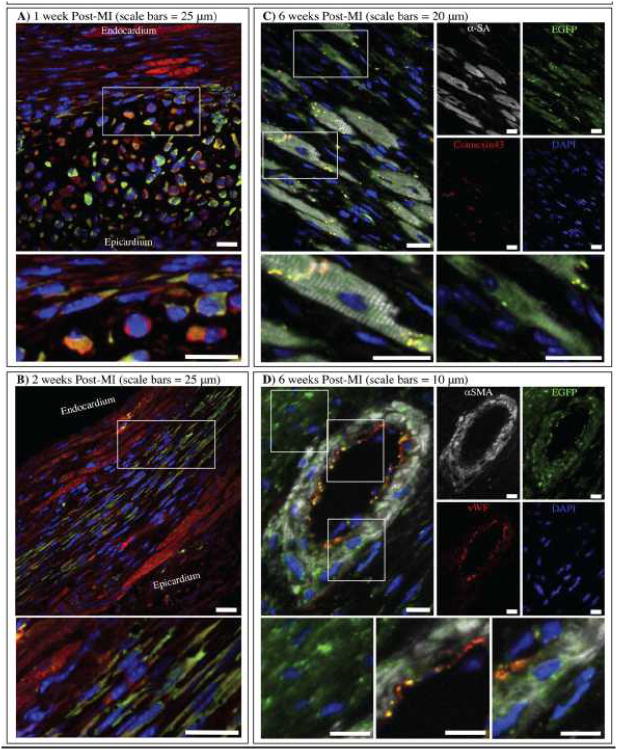
Time-related changes in CBSC phenotype were studied. After 1 week, most EGFP+ cells were still small (10-20 μm) and round in shape (similar to the morphology of the injected cells), but the majority had begun to express unorganized cytosolic α-sarcomeric actin (Figure 7A). A small number of cells that were in close contact with viable endogenous myocytes began to elongate and align in the axis of contraction, as defined by the sarcomeric organization of surviving myocytes in the region. By 2 weeks post-MI, as the injection sites had begun to expand, the majority of cells were elongated, aligned along the axis of contraction, and were expressing additional cytosolic α-sarcomeric actin (Figure 7B). As at 1 week, EGFP+ cells that had engrafted closest to viable endogenous myocytes appeared the largest and most mature, with even greater amounts of α-sarcomeric actin visible in their cytoplasm. Although actin expression appeared to be increased by 2 weeks post-MI, it was still relatively unorganized, without the characteristic sarcomeric striations of adult myocytes. Individual color channel images for Figure 7A and B and staining controls for 1 and 2 week post-MI+Saline are shown in Online Figure IX.
Figure 7. Cortical bone stem cells grow and differentiate over 6 weeks.
A) 1 week post-MI and B) 2 weeks post-MI samples were immunostained for α-sarcomeric actin (red) and EGFP (green). 6 weeks post-MI samples were immunostained for C) α-sarcomeric actin (white), connexin43 (red) and EGFP (green) or D) α-smooth muscle actin (white), von Willebrand Factor (red) and EGFP (green). Nuclei were labeled with DAPI (blue) in all images.
By 6 weeks Post-MI, newly formed EGFP+ myocardium was visible at the border zone and five distinct EGFP+ stem cell phenotypes were identified: 1) cardiac myocytes with α-sarcomeric actin in organized sarcomeres were found to be coupled to neighboring cells via connexin43+ gap-junctions (Figure 7C), 2) cells that express unorganized α-sarcomeric actin (Figure 7C), 3) vascular smooth muscle cells that stain positive for α-smooth muscle actin (Figure 7D), 4) vascular endothelial cells that stain positive for von Willebrand factor (Figure 7D), and 5) EGFP+ cells that are smaller (<20 μm) and lack any expression of adult cardiomyocyte or vascular cell proteins (Figure 7D). Online Figure X shows an additional low-magnification image of the CBSC-treated border zone at 6 weeks post-MI in which a newly formed region of EGFP+ myocardium can be seen adjacent to a region EGFP- endogenous myocardium. Online Figure XI shows a low magnification view of the blood vessel shown in Figure 7D, in which the EGFP+ vessel is clearly surrounded by regions of EGFP- myocardium. Individual confocal color channel images and staining controls for Figure 7C are shown in Online Figure XII. Individual color channel images and staining controls for Figure 7D are shown in Online Figure XIII. These results support the idea that CBSCs can differentiate into the major cell types of the adult heart.
Cardiac-derived stem cells expand and proliferate over time but adopt a less mature adult myocyte phenotype
Online Figure XIV shows images from the hearts of CDC-treated MI animals studied at 1, 2, and 6 weeks post-MI. EGFP+ CDC injection sites were identified and stained for α-sarcomeric actin (red) in Online Figure XIVA, or for α-sarcomeric actin (white) and connexin43 (red) in Online Figure XIVB. Similar to what we observed with CBSCs, by 1 week post-MI CDCs initially appear small (10-20 μm) and round and they had begun to express unorganized α-sarcomeric actin. By 2 weeks, the cells have begun to align and elongate like CBSCs and they continue to express unorganized α-sarcomeric actin. By 6 weeks post-MI, however, no EGFP+ cells with organized sarcomeres were found. Cells continue to grow larger and are still aligned, and some had even begun to express connexin43 gap junctions (Figure S-XIVB, right panel), but cytosolic α-sarcomeric actin was not yet striated and mature gap junctional plaques were not identified. These data suggest that under our conditions, CDCs adopted a less mature cardiomyocyte phenotype over the 6-week course of this study.
CBSC-derived EGFP+ myocytes isolated 6 weeks after injection demonstrated mature contractile properties
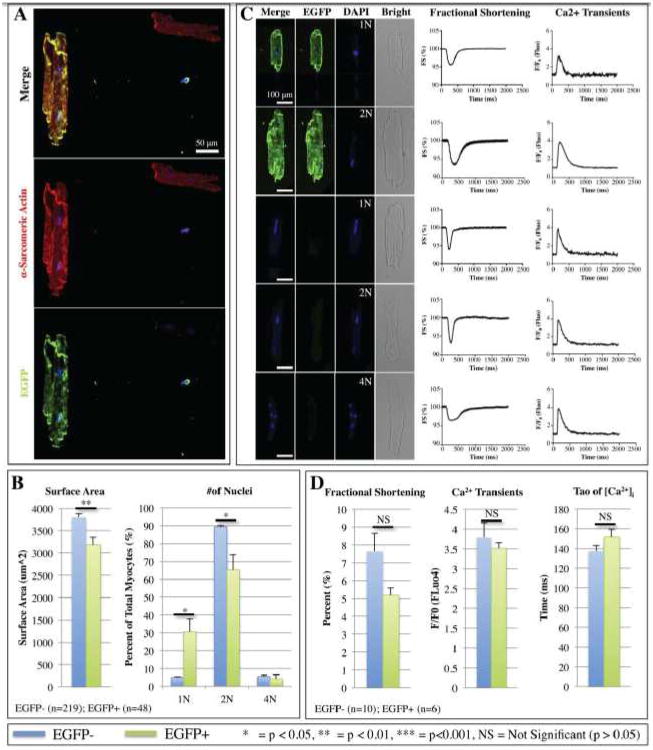
Some animals that received MI and CBSC therapy were sacrificed 6 weeks after injury and myocytes from their left ventricles were isolated and studied in vitro. Isolated myocytes were also immunostained for α-sarcomeric actin (red), EGFP (green) and nuclei were labeled with DAPI (blue) in order analyze myocyte size and the number of nuclei per cell (Figure 8). Of all the myocytes in the left ventricle, an estimated 0.84% expressed EGFP, suggesting that these cells had been derived from transplanted CBSCs. In order to estimate how many new myocytes were formed from EGFP+ CBSCs (assuming that 0.84% of isolated myocytes were EGFP+), the total number and volume of myocytes in the heart was approximated. Our lab has previously estimated the total number of ventricular myocytes in the feline heart39 by measuring the average volume of the heart and the average volume of a myocyte. The mean weight of the mouse ventricles 6 weeks post-MI+CBSC = 205.3±10.8 mg and the average volume of a mouse myocyte can be approximated using average myocyte dimensions (100 × 20 × 10 um = 20,000 um3 = 2×10-8 mL). If the density of myocardial tissues = 1.06 mg/mm3,40 then the average volume of hearts at 6 weeks post-MI+CBSC = 205.3/1.06 = 193.7 mm3 = 0.1937 mL. Myocytes are known to make up about 80% of the heart by volume,41 so the volume of the heart constituting myocytes = 0.8 × 0.1937 = 0.1550 mL. Thus, the total number of new EGFP+ myocytes in the average mouse heart at 6 weeks post-MI+CBSC = 0.1550 mL/2×10-8 mL = 7.75×106 total myocytes/heart. If 0.84% of myocytes analyzed were positive for EGFP, then 0.0084 × 7.75×106 = 65,100 new EGFP+ myocytes were formed per heart. At the time of surgery, 40,000 cells were initially injected. To form this number of new myocytes the injected CBSCs must have proliferated and committed to the cardiac lineage. As shown in Figure 7, only a fraction of the EGFP+/α-sarcomeric actin+ cells were striated. In Online Figure SXV, we measured the relative numbers of striated (muture) and non-striated/unorganized (less mature) EGFP+/α-sarcomeric actin+ cells in 6 week post MI CBSC hearts. This histological analysis showed that there were approximately equal numbers of striated and nonstriated EGFP+/α-sarcomeric actin+ new myocytes. Collectively these analyses suggest that the number of CBSC-derived new myocytes (about 130,000) is about 3X greater than the number of cells originally injected (40,000).
Figure 8. Isolated myocyte size, number of nuclei, and cell physiology were analyzed 6 weeks post-MI+CBSC.
A) Myocytes were immunostained against α-sarcomeric actin (red), EGFP (green), and DAPI (blue). B) Average surface area of EGFP+ versus EGFP- myocytes and percent of total EGFP+ or EGFP- cells that were mono-, bi-, or tetranucleated. C) Representative fractional shortening and Ca2+ transients of EGFP+ myocytes with 1 or 2 nuclei, or EGFP- myocytes with 1, 2, or 4 nuclei. Scale bars = 100 μm. D) Mean fractional shortening, peak Ca2+ F/F0, and the time constant of decay (τ) of Ca2+ transients for all EGFP+ versus EGFP- myocytes.
EGFP+ isolated myocytes were identified, and the size and number of nuclei per cell were analyzed and compared to EGFP- myocytes isolated from the same hearts. Figure 8A shows a representative EGFP+ myocyte with two nuclei. When compared to EGFP- controls, EGFP+ myocytes had smaller average cross-sectional surface area (Figure 8B). Over 90% of EGFP- myocytes were binucleated, while only 5.4% of EGFP- cells were mononucleated and 4.1% were tetranucleated. In contrast, there were significantly more mononucleated and significantly fewer binucleated EGFP+ myocytes, with 1/3 EGFP+myocytes having only one nucleus (Figure 8B). There was no significant difference in tetranucleation between EGFP+ and EGFP- myocytes.
Isolated EGFP+ and EGFP- myocyte fractional shortening (FS) and Ca2+ transients were measured. Figure 8C shows representative EGFP+ and EGFP- myocytes under fluorescence or bright field histology with their corresponding FS or Ca2+ transients. Mean FS, peak Ca2+ (F/F0) and time constant of decay of the Ca2+ transients were measured (Figure 8D). EGFP+ myocytes isolated 6 weeks after MI had contractions and Ca2+ transients that were indistinguishable from EGFP- controls, documenting that they had assumed a mature adult phenotype.
Discussion
This study explored the potential beneficial effects of transplanting a novel population of c-kit+/Sca-1+ cells from the stem cell niches within the bone, rather than from bone marrow, into the border zone of a myocardial infarction, and the effects of this novel cell type were compared to a widely studied population of c-kit+/Sca-1+ cardiac-derived stem cells. Our results show that CBSCs have beneficial effects on the structure and function of the heart after MI. Animals with MI that received a CBSC transplant had improved 6-week survival, cardiac function, and attenuation of adverse left ventricular remodeling compared with both saline-injected MI controls and CDC-treated animals. Strain analysis demonstrated that CBSC-treated MI animals not only had improved global function, but their hearts also had greater contractile function at the MI border zone relative to saline- and CDC-injected hearts. Strain analysis allows for precise imaging at the infarct border zone, where stem cells were specifically administered to achieve optimal effect.42 At border zone segments where new myocardium was detected using histological techniques, strain analysis of CBSC-treated mice showed greater contraction (Figure 4A-B), suggesting that the stem cells enhanced contractile function in the region where they were injected. Histological analysis showed that, while CDCs adopted less mature adult phenotypes, EGFP+ stem cell-derived adult cardiac myocytes with normally striated α-sarcomeric actin networks and connexin43+ gap junctions were present throughout the border zone in CBSC treated mice (Figure 7C). CBSC-derived new cardiac myocytes that were isolated from hearts 6 weeks after MI had normal contractions and Ca2+ transients. These findings support the idea that newly formed cardiac muscle was derived from injected CBSCs and these cells make a contribution to the improved contractile function of the injured heart.
CBSCs secrete pro-angiogenic factors and promote neovascularization
A major goal of the present study was to explore paracrine effects of injected CBSCs. Many studies have looked at stem cell paracrine factors in vitro and drawn conclusions without studying the changes in expression of these factors after injection into the heart.21 Other groups have looked at the changing paracrine factor expression by stem cells after injection, but they have either studied total myocardial expression of these factors43 (rather than expression specifically by stem cells) or they have only examined specific stem cell expression of a single factor.28 Ours is the first study to both establish in vitro expression of multiple paracrine factors and examine the temporal changes in expression of all of these factors specifically by the stem cells after transplantation.21-24, 27, 28, 30, 44 We specifically explored neovascularization and the presence of paracrine factors in regions with injected CBSCs. Our studies showed that CBSCs produced the pro-angiogenic factors bFGF and VEGF (both in vitro and in vivo). These factors are known to be involved in vascular cell proliferation and induction of angiogenesis,22-25, 28-30 and there was evidence of increased neovascularization in the infarct zone of hearts treated with stem cells in this study (Figure S-IV). Although CDCs initially produced Ang-1 in addition to bFGF and VEGF, these factors were not observed past 24 hours post-MI and the neovascularization of the border zone in CDC treated animals was less robust than CBSC-treated animals by 6 weeks post-MI. Additionally, our studies showed that, while CDCs did not transdifferentiate into mature cardiac myocytes or blood vessels by 6 weeks post-MI, CBSCs transdifferentiated into adult vascular cells, including smooth muscle and endothelial cells, although this was observed relatively infrequently. While there was strong evidence of myocyte transdifferentiation (EGFP+ cardiac myocytes were identified in 5/8 hearts analyzed 6 weeks post-MI), and many cells from hearts at earlier time points could be identified in intermediate stages of myocyte differentiation (in 6/6 hearts analyzed after 1 week post-MI+CBSC and in 6/6 analyzed fixed at 2 weeks post-MI+CBSC), the same could not be said for cells of the vascular lineage (EGFP+ vascular cells were only identified in 2/8 hearts analyzed at 6 weeks post-MI and no intermediate EGFP+ lumen or tube like structures were found at 1 or 2 weeks post-MI). These findings suggest that paracrine-induced new blood vessel formation via endogenous repair appears to be the primary mechanism for the enhanced angiogenesis observed in CBSC-injected hearts. The in vivo paracrine factor analysis showed those factors involved in cardioprotection (HGF, IGF) or stimulation of endogenous stem cells (SCF, SDF-1) that were expressed in CBSCs in vitro were not found in CBSCs after they were injected into the MI border zone. These results suggest that part of the beneficial effect of CBSCs on MI hearts is paracrine-mediated enhancement of angiogenesis.
CBSCs differentiate into new cardiac myocytes
In the present study we used CBSCs and CDCs from an EGFP+ mouse so that we could easily trace the fate of the injected cells. Using time course in vivo histological analysis with this stably expressed fluorophore, we were able to demonstrate that CBSCs differentiated over time down the myocyte lineage by 6 weeks post-MI while CDCs did not adopt a fully mature cardiac myocyte phenotype. As discussed above, we found evidence for enhanced revascularization of the MI border and infarct zone in CBSC injected hearts. However, the new blood vessels were rarely comprised of EGFP+ cells, suggesting that CBSCs enhanced endogenous repair. Our experiments demonstrate that EGFP+ CBSCs express cardiac proteins soon after injection into the MI heart, and by 6 weeks post-MI these cells have organized sarcomeres, are connected to their neighbors via gap junctions, and contract with Ca2+ transients that are similar to those of EGFP-myocytes isolated from the same hearts.
Our studies suggest that the transition from an injected CBSC to a fully functional cardiac myocyte takes between 2-6 weeks under our conditions. After one week most the EGFP+ cells had immature characteristics and expressed unorganized cardiac contractile proteins. Two weeks after MI areas with EGFP+ cells were larger, suggesting cell proliferation, and the cells had begun to elongate along the axis of EGFP- myocytes that had survived the infarct. More EGFP+ cells expressed cardiac contractile proteins but no organized sarcomeres were found. By 6 weeks post-MI there were many regions containing EGFP+ myocytes with organized sarcomeres and these cells were well integrated in the myocardium via gap junctions. The contribution of these new myocytes to the improved cardiac contractile performance of the post-MI heart cannot be proven with the techniques used in these experiments. However, regional strain measurements from sites of CBSC injection documented increased contractile performance in the regions where we isolated EGFP+ myocytes with robust contractile activity. These findings support the idea that CBSC-mediated new myocyte formation is at least partially responsible for improvements in cardiac structure and function in MI hearts with CBSC treatments.
Our major evidence for CBSC-derived new myocyte formation is that we found EGFP+ cardiac myocytes in the MI border zone of hearts 6 weeks after CBSC injections. There is always concern that injected cells might have fused with existing myocytes,45 but little or no evidence for fusion in these types of experiments has been observed,3, 46 and complex studies using multiple stem cell labeling strategies have largely contradicted the fusion hypothesis.8 We also have evidence that the EGFP+ myocytes we found were newly formed from smaller CBSC progeny. Our experiments showed that EGFP+ isolated myocytes were smaller and a higher percentage of these cells were mononucleated, consistent with a maturing adult cardiac myocyte. We estimated the number of new, CBSC-derived EGFP+ myocytes by isolating myocytes from hearts six weeks after CBSC injection into the MI border zone. We estimated that the number of new myocytes significantly exceeds the number of injected CBSCs, consistent with proliferation of either CBSCs or newly formed myocyte progeny. Importantly, our approach may have significantly underestimated the number of new myocytes because it is more difficult to isolate viable myocytes from infarct zones with fibrotic regions than from undamaged regions of the same heart. The present data are also consistent with previously published reports from our laboratory in which newly formed myocytes were detected in the growing heart during adolescence.39 These results are also similar to observations made in several studies by the Anversa laboratory, in which newly formed isolated myocytes were observed to be smaller than spared endogenous myocytes that lacked the stem cell marker.3, 5, 8 Collectively our results suggest that CBSCs survive in the MI border zone and within weeks they begin to form new cardiac tissue. The survival and differentiation of CBSCs was associated with improved cardiac structure and function and enhanced survival.
Other CBSC cardioprotective effects
Our experiments show improved cardiac structure and function in the first two weeks after MI, before injected cells had enhanced vascularity and local contractility. The mechanisms of these early beneficial effects are not clear. One possibility is that injected cells thicken and stabilize the infarcted wall, thereby reducing wall stress and slowing dilation. These results are consistent with early stage clinical trials with wall stabilizing agents.47-50 The cardiac functional data of MI animals treated with CDCs and CBSCs also provide some insight into the wall stabilizing effect of cell therapy. By 1 week post-MI, both the MI+CDC and MI+CBSC groups had similar cardiac functional parameters (ejection fraction and fractional shortening). However, with time the functional improvements in the MI+CBSC group were sustained while the function in MI+CDC animals continued to decline. The initial functional benefit may be attributed to a wall stabilizing effect that would likely be the same between both cell types, while the sustained functional improvement seen only in the MI+CBSC group could be the result of enhanced paracrine mediated revascularization and maturation of CBSCs into new cardiac tissue.
CBSCs versus CDCs and other stem cell types
Overall our data suggests that a bone derived c-kit+/Sca-1+ stem cell population can support the injured heart through direct transdifferentiation into adult cardiomyocytes and vascular cells as well as through secretion of pro-angiogenic paracrine factors. CBSCs proved somewhat more effective at repairing the injured heart than CDCs, in part because CBSCs showed the capacity to transdifferentiate and form cells with a mature adult phenotypes in this animal model. While we did not observe full differentiation of CDCs within the 6-week time frame of this study, it is possible that they could differentiate if allowed more time (their phenotypic changes over 6 weeks did mimic those of CBSCs in the first 2 weeks post-MI). Difficulty of maintaining the undifferentiated state of CDCs in vitro during cell expansion also likely contributed to the less robust cardiogenic potential of the CDCs used in the present experiments. We believe that cortical bone provides an easy-to-access, more abundant source of stem cells that are potentially more pluripotent than has previously been isolated from the bone marrow. Additionally, these stem cells can be isolated from cortical bone in high numbers without the need for arduous sorting processes such as FACS or magnetic bead sorting that are required to isolate the relatively rare populations of c-kit+ cells from the bone marrow or myocardium. We are cautious when making comparisons to previous studies with other cell types because the effectiveness of different cell therapies is likely to be influenced by a host of factors, including the isolation approach, methods of cell expansion and purification, and the animal model in which the cells are tested.
Previously, there has been much skepticism that cells from outside the heart are capable of cardiomyogenesis,51, 52 and many researchers have argued that new myocytes in stem cell treated hearts are derived from paracrine stimulation of endogenous stem cells.25, 32 Our study shows that bone-derived stem cells can directly form new myocytes, independently of endogenous CDCs, and these new myocytes can be isolated and their contractile properties and Ca2+ transients are indistinguishable from endogenous myocytes. Transplanted CDCs in our model failed to produce myocytes with a mature adult phenotypes. Additionally, our stem cells have shown a wide potential to support the injured heart through several mechanisms without the need of modifications that have been used in other studies.22, 23, 53, 54
In summary, our studies show that CBSCs can survive the hostile environment of the post-MI heart without modification, secrete factors that enhance endogenous angiogenesis-mediated repair, and differentiate into new cardiac tissue. These beneficial effects culminate in a heart with less structural remodeling and improved cardiac pump function.
Supplementary Material
Novelty and Significance.
What Is Known?
Ischemic heart disease leads to cardiac dysfunction and heart failure by inducing the death of cardiac myocytes.
Stem cells isolated from heart tissue or bone marrow partially improve heart function when transplanted into animals and humans with ischemic heart damage.
Mechanisms underlying the beneficial effects of stem cell are not clearly defined and are thought to include differentiation of injected stem cells into new cardiac tissue as well paracrine activation of endogenous repair.
What New Information Does This Article Contribute?
Cortical bone (rather than the bone marrow) stem cells (CBSCs) injected into the border zone of an induced myocardial infarction (MI) have salutary effects on cardiac structure, function and survival.
CBSCs enhance angiogenesis in the MI border zone by secreting factors that trigger endogenous blood vessel formation.
CBSCs injected into the MI border zone survive, proliferate and differentiate into new, functionally mature heart muscle cells that enhance cardiac function in the regions of cell injection.
Cell therapy to treat damaged hearts has proven safe in humans, but the functional and survival benefits of these therapies have been modest and the mechanisms underlying putative benefits remain largely unknown. Hence, there is still a need to identify stem cells that can survive the hostile environment of the ischemic heart and can differentiate into new cardiac tissue and/or elicit endogenous cardiac repair. The present study examined the ability of a novel population of stem cells derived from the cortical bone (CBSCs) to repair the mouse heart after MI. CBSCs were ckit+ and Sca1+ but did not express hematopoietic lineage markers. CBSCs survived and engrafted into the post MI heart and improved survival and cardiac function, and they attenuated adverse cardiac remodeling. CBSCs were shown to have beneficial effects on cardiac structure and function after MI by: 1) direct differentiation into functionally mature cardiac myocytes and vascular structures, and 2) secretion of paracrine factors that induce enhanced formation of endogenous vascular repair. These results show that these novel CBSCs can survive when injected into the ischemic heart and have the potential to differentiate into cardiac myocytes with mature function and to secrete factors that promote endogenous repair.
Acknowledgments
None
Sources Of Funding: S.R.H. received NIH grants R01HL089312, T32HL091804, P01HL091799, and R37HL033921. C.A.M. received an AHA predoctoral fellowship.
Nonstandard Abbreviations and Acronyms
- MI
Myocardial infarction
- CDCs
cortical bone-derived stem cells
- CDCs
cardiac-derived stem cells
- Qpcr
quantitative real-time polymerase chain reaction
- Lin
hematopoietic lineage antibody cocktail
- Ang-1
angiopoietin-1
- Bfgf
basic fibroblast growth factor
- HGF
hepatocyte growth factor
- IGF-1
insulin-like growth factor-1
- PDFG
platelet-derived growth factor
- SCF
stem cell factor
- SDF-1
stromal-derived factor-1
- VEGF
vascular-endothelial growth factor
- SV
stroke volume
- AAR
area at risk
- IA
ischemic area
- H&E
hematoxylin and eosin
- LV
left ventricle
- Vwf
von Willebrand Factor
- EGFP
enhanced green fluorescent protein
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 2.Gomez AM, Guatimosim S, Dilly KW, Vassort G, Lederer WJ. Heart failure after myocardial infarction: Altered excitation-contraction coupling. Circulation. 2001;104:688–693. doi: 10.1161/hc3201.092285. [DOI] [PubMed] [Google Scholar]
- 3.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 4.Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, Dai S, Li C, Chen N, Peng Y, Dawn B, Hunt G, Leri A, Kajstura J, Tiwari S, Shirk G, Anversa P, Bolli R. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rota M, Padin-Iruegas ME, Misao Y, De Angelis A, Maestroni S, Ferreira-Martins J, Fiumana E, Rastaldo R, Arcarese ML, Mitchell TS, Boni A, Bolli R, Urbanek K, Hosoda T, Anversa P, Leri A, Kajstura J. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–116. doi: 10.1161/CIRCRESAHA.108.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 7.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 8.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, Iaffaldano G, Padin-Iruegas ME, Gonzalez A, Rizzi R, Small N, Muraski J, Alvarez R, Chen X, Urbanek K, Bolli R, Houser SR, Leri A, Sussman MA, Anversa P. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A. 2007;104:17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, Hasenfuss G, Martin U. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507–517. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zwi L, Caspi O, Arbel G, Huber I, Gepstein A, Park IH, Gepstein L. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation. 2009;120:1513–1523. doi: 10.1161/CIRCULATIONAHA.109.868885. [DOI] [PubMed] [Google Scholar]
- 12.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 15.Yousef M, Schannwell CM, Kostering M, Zeus T, Brehm M, Strauer BE. The balance study: Clinical benefit and long-term outcome after intracoronary autologous bone marrow cell transplantation in patients with acute myocardial infarction. J Am Coll Cardiol. 2009;53:2262–2269. doi: 10.1016/j.jacc.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 16.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 17.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: The scipio trial: Surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (caduceus): A prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng L, Hu Q, Wang X, Mansoor A, Lee J, Feygin J, Zhang G, Suntharalingam P, Boozer S, Mhashilkar A, Panetta CJ, Swingen C, Deans R, From AH, Bache RJ, Verfaillie CM, Zhang J. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation. 2007;115:1866–1875. doi: 10.1161/CIRCULATIONAHA.106.659730. [DOI] [PubMed] [Google Scholar]
- 21.Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H, Marban L, Marban E. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59:942–953. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 23.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 24.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams AR, Hare JM. Mesenchymal stem cells: Biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 27.Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, Hsu RY, Birkett NC, Okino KH, Murdock DC, et al. Stem cell factor is encoded at the sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]
- 28.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 29.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 30.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 31.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 32.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: A paradigm shift in cardiac biology. Circulation. 2006;113:1451–1463. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- 34.Kucia M, Dawn B, Hunt G, Guo Y, Wysoczynski M, Majka M, Ratajczak J, Rezzoug F, Ildstad ST, Bolli R, Ratajczak MZ. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ Res. 2004;95:1191–1199. doi: 10.1161/01.RES.0000150856.47324.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J, Anversa P, Leri A. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A. 2006;103:9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu H, Guo ZK, Jiang XX, Li H, Wang XY, Yao HY, Zhang Y, Mao N. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc. 2010;5:550–560. doi: 10.1038/nprot.2009.238. [DOI] [PubMed] [Google Scholar]
- 37.Kubo H, Berretta RM, Jaleel N, Angert D, Houser SR. C-kit+ bone marrow stem cells differentiate into functional cardiac myocytes. Clin Transl Sci. 2009;2:26–32. doi: 10.1111/j.1752-8062.2008.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 39.Chen X, Wilson RM, Kubo H, Berretta RM, Harris DM, Zhang X, Jaleel N, MacDonnell SM, Bearzi C, Tillmanns J, Trofimova I, Hosoda T, Mosna F, Cribbs L, Leri A, Kajstura J, Anversa P, Houser SR. Adolescent feline heart contains a population of small, proliferative ventricular myocytes with immature physiological properties. Circ Res. 2007;100:536–544. doi: 10.1161/01.RES.0000259560.39234.99. [DOI] [PubMed] [Google Scholar]
- 40.Anversa P, Loud AV, Levicky V, Guideri G. Left ventricular failure induced by myocardial infarction. I. Myocyte hypertrophy. Am J Physiol. 1985;248:H876–882. doi: 10.1152/ajpheart.1985.248.6.H876. [DOI] [PubMed] [Google Scholar]
- 41.Legato MJ. Cellular mechanisms of normal growth in the mammalian heart. Ii. A quantitative and qualitative comparison between the right and left ventricular myocytes in the dog from birth to five months of age. Circ Res. 1979;44:263–279. doi: 10.1161/01.res.44.2.263. [DOI] [PubMed] [Google Scholar]
- 42.Duran JM, Taghavi S, Berretta RM, Makarewich CA, Sharp T, Iii, Starosta T, Udeshi F, George JC, Kubo H, Houser SR. A characterization and targeting of the infarct border zone in a swine model of myocardial infarction. Clin Transl Sci. 2012;5:416–421. doi: 10.1111/j.1752-8062.2012.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 44.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (sfrp2) is the key akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 46.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, Cohen S, Leor J. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117:1388–1396. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 48.Segers VF, Lee RT. Biomaterials to enhance stem cell function in the heart. Circ Res. 2011;109:910–922. doi: 10.1161/CIRCRESAHA.111.249052. [DOI] [PubMed] [Google Scholar]
- 49.Kleinman HK, Martin GR. Matrigel: Basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Kofidis T, Lebl DR, Martinez EC, Hoyt G, Tanaka M, Robbins RC. Novel injectable bioartificial tissue facilitates targeted, less invasive, large-scale tissue restoration on the beating heart after myocardial injury. Circulation. 2005;112:I173–177. doi: 10.1161/CIRCULATIONAHA.104.526178. [DOI] [PubMed] [Google Scholar]
- 51.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 52.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 53.Mohsin S, Khan M, Toko H, Bailey B, Cottage CT, Wallach K, Nag D, Lee A, Siddiqi S, Lan F, Fischer KM, Gude N, Quijada P, Avitabile D, Truffa S, Collins B, Dembitsky W, Wu JC, Sussman MA. Human cardiac progenitor cells engineered with pim-i kinase enhance myocardial repair. J Am Coll Cardiol. 2012;60:1278–1287. doi: 10.1016/j.jacc.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quijada P, Toko H, Fischer KM, Bailey B, Reilly P, Hunt KD, Gude NA, Avitabile D, Sussman MA. Preservation of myocardial structure is enhanced by pim-1 engineering of bone marrow cells. Circ Res. 2012;111:77–86. doi: 10.1161/CIRCRESAHA.112.265207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.