Abstract
Pregnancy, breastfeeding, and oral contraceptive pill use interrupt menstrual cycles and reduce endometrial and ovarian cancer risk. This suggests the importance of turnover within Mullerian tissues, where the accumulation of mutations in p53 and PTEN has been correlated with number of cycles. The most common type of endometrial cancer (Type I) is endometrioid and molecular abnormalities include mutations in PTEN, KRAS and β-catenin. The Type I precursor is Endometrial lntraepithelial Neoplasia which displays PTEN defects. Type II endometrial cancer (whose precursors are less clear) includes serous and clear cell tumors and the most common alteration is p53 mutation. For ovarian cancer, histopathologic types parallel endometrial cancer and include serous, mucinous, endometrioid, and clear cell; some molecular features are also shared. The most frequent type of ovarian cancer is high grade serous that often displays p53 mutation and its precursor lesions may originate from normal-appearing fallopian tube epithelium that contains a p53 “signature”. Mutations in KRAS, BRAF and PTEN are described in mucinous, endometrioid and low grade serous cancers and these may originate from ovarian cortical inclusion cysts. A consideration of molecular and other pathogenetic features, like epidemiology and histopathology, may provide a bener understanding of endometrial and ovarian cancer.
Keywords: Ovarian cancer, Endometrial cancer, p53, PTEN, KRAS, Precursor lesions, Endometrial intraepithelial neoplasia, Fallopian tube, Cortical inclusion cysts
I. Introduction
Pathogenesis is a term that implies understanding a disease at many levels, thus requiring knowledge of susceptible populations, etiologic factors, histopathology at the gross and microscopic level, and delineation of precursor lesions. Understanding a disease at these levels should not be abrogated when we focus on “molecular pathogenesis”. Rather, by overlaying an understanding of the process at a biochemical or genetic level, the greatest understanding of the origins of a disease is achieved and has the best chance of translating into methods for prevention or treatment. Thus, in our consideration of the Molecular Pathogenesis of Endometrial and Ovarian Cancer, epidemiologic and histopathologic aspects will be first examined. We are considering endometrial and ovarian cancer together because these gynecologic cancers have many intriguing similarities, which together with some important differences, will be revealed in this chapter.
2. Epidemiologic “Pathogenesis”
2.1 Age and Geographic Distribution
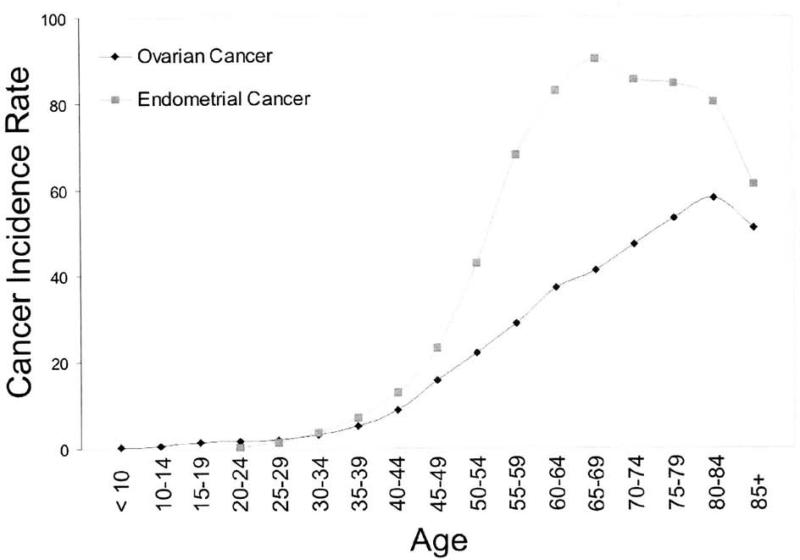
In the U.S., endometrial cancer is the most common gynecologic cancer accounting for 40,100 new cases and 7,470 deaths [1]. Ovarian cancer accounts for fewer cases (21,650) but more deaths (15,520) [1]. Ovarian cancer is the leading cause of death from a gynecologic cancer and fifth leading cause of cancer deaths overall in the U.S. Endometrial and Ovarian Cancer share similar patterns of distribution by age and geography. Both cancers rise sharply in occurrence during the perimenopausal years and peak after the menopause (Fig I). However, while endometrial cancer rates drop rather sharply after age 65, ovarian cancer rates continue to rise well into a woman's eighties. Worldwide much higher rates of endometrial and ovarian cancer are observed in industrialized and Northern European populations and lower rates in third world countries (Table 1). The correlation between these rates is significant (Pearson correlation = 0.809. p<0.001) and associations with per capita fat consumption have been described for both [2-4].
Figure 1.
Age-Specific Incidence Rates of Endometrial and Ovarian Cancers1.
1Age-Specific Cancer Incidence Rates are per 100,000 and are age-adjusted to the 2000 US Standard Population. Rates include all races and pertain to invasive cancers only. Adapted from Ries et al. [199] http://seer.cancer.gov/csr/1975_2005/.
Table 1.
Age-Adjusted Incidence Rates1 for Endometrial and Ovarian Cancers in Different Regions of the World.
| Region | Endometrium | Ovary |
|---|---|---|
| World | 6.5 | 6.6 |
| More developed countries | 13.6 | 10.2 |
| Less developed countries | 3 | 5 |
| Eastern Africa | 3.2 | 5.8 |
| Middle Africa | 2.5 | 3.3 |
| Northern Africa | 2.4 | 2.6 |
| Southern Africa | 3.5 | 52 |
| Western Africa | 2.2 | 4.6 |
| Caribbean | 8.8 | 4.3 |
| Central America | 4.5 | 7.2 |
| South America | 6.7 | 7.7 |
| Northern America | 22 | 10.7 |
| Eastern Asia | 2.5 | 3.7 |
| South-Eastern Asia | 4.2 | 7.2 |
| South Central Asia | 2.3 | 5.3 |
| Western Asia | 5.8 | 5.3 |
| Eastern Europe | 11.8 | 10.2 |
| Northern Europe | 12.2 | 13.3 |
| Southern Europe | 11.8 | 9.7 |
| Western Europe | 12.5 | 11.3 |
| Australia/New Zealand | 10.6 | 9.4 |
| Melanesia | 6.7 | 6.6 |
| Micronesia | 7.4 | 6 |
| Polynesia | 11.8 | 7.7 |
Age-Adjusted Incidence Rates are per 100,000 and are adjusted to the World Standard. Incidence rates are highly correlated (Pearson correlation = 0.81, p<0.001). Data from Ferlay et al. [161], IARC website (http://www-dep.iarc.fr/).
2.2. Risk Factors
As reviewed in Table 2, many shared risk factors for endometrial and ovanan cancer relate to reproductive factors including trends for decreasing risk associated with increasing number of pregnancies, longer duration of breastfeeding, and more years of oral contraceptive (OC) pill use. A late age at menarche decreases risk for both cancers and a late age at menopause increases risk for endometrial cancer, but less clearly so for ovarian. These events can be fit into a composite variable that estimates years of ovulation or, when average cycle length is included, number of ovulatory cycles. Increasing ovulatory cycles clearly correlates with increased risk for ovarian cancer. Though less well studied, ovulatory cycles also appears to correlate with endometrial cancer risk [5].
Table 2.
Risk factors for Endometrial, Breast, and Ovarian Cancers1
| Factor | Endometrium | Ovary | Comment | References |
|---|---|---|---|---|
|
Menstrual, reproductive, and medical events
| ||||
| Menarche | ↓ | ↓ | Late age | [162-164] |
| Cycle Length | ↑ | ↑ | Short or irregular cycles | [162, 164-167] |
| Menopause | ↑ | ↑ | Late age | [2, 168] |
| Early Age at First Birth | ↓ | ↓ | Late last birth also decreases endometrial and ovarian cancer risk | [167, 169] |
| Parity | ↓ | ↓ | [167, 169] | |
| Breastfeeding | ↓ | ↓ | [170-174] | |
| Oral Contraceptives | ↓ | ↓ | Non-sequentials decrease endometrial cancer risk | [106, 175-178] |
| Ovulatory cycles | ↑ | ↑ | [5, 179, 180] | |
| Menopausal Hormones | ↑ | ↑ | Unopposed important lo endometrial cancer risk | [181-186] |
| Body Mass Index | ↑ | ↑ | [15, 16, 187, 188] | |
| PCOS | ↑ | ↑ | [17-19] | |
|
Genetic/ familial syndromes | ||||
| Cowden's Syndrome | ↑ | Ø | [24] | |
| BRCA1,2 | ↑ | ↑ | [21, 189] | |
| HNPCC | ↑ | ↑ | [22, 23] | |
|
Other environmental factors | ||||
| Smoking | ↓ | ↑ | Ovarian cancer risk is restricted to mucinous histology | |
| [35, 36, 190-193] | ||||
| Talc use | ? | ↑ | [37-40] | |
| Tubal Ligation | ↓ | ↓ | [31-34, 194] | |
| Intrauterine Device Use | ↓ | ↓ | Ovarian cancer association is possible | [27, 29, 30, 195, 196] |
A positive association is indicated by ↑, negative association by ↓, and no association as Ø. A ‘?’ represents too little data to determine whether an association exists.
The link between ovulatory cycles, endometrial, and ovarian cancer illustrates the potential relevance of a consideration of risk factors to molecular pathogenesis. For ovarian cancer, the monthly disruption and repair of the surface epithelium of the ovary is proposed to lead to genetic damage due to the accumulation of mutations of the tumor suppressor, p53, in the ovarian or fallopian tube epithelium [6-9]; but this mechanism would not explain the association with endometrial cancer. One possibility is that “incessant ovulation” largely equates with “incessant menstruation” involving repeated disruption and re-growth of the uterine lining. A greater number of cycles of endometrial regeneration may increase the likelihood of random genetic mutations because DNA replication errors occur during cell division, and thus are more likely to occur in tissues undergoing many cell divisions. Estimates of the rate of sporadic mutagenesis in human cells, on the order of 10−7 mutations per gene per cell division [10] suggest that the number of cells with “first hits” on a multistep carcinogenesis pathway [11, 12] may number in the hundreds for every gram (109 cells/gram) of proliferative tissue. It is no surprise, then, to find that sporadic mutations of another tumor suppressor gene, PTEN, are observed in almost half (43%) of histologically normal endometria of naturally cycling premenopausal women [13].
Another risk factor for endometrial and ovarian cancer is menopausal hormone use, especially of estrogen only forms unopposed by a progestin, although the magnitude of this effect is greater in endometrial cancer. Medical conditions which may relate to risk for both types of cancers include high body mass index and polycystic ovarian syndrome (PCOS). High body mass index (BMI) is a well-established risk factor for Type 1 endometrial cancer and has been linked to elevated levels of estrogen, particularly in post-menopausal women where adipose tissue is the main site of estrogen production from androgen precursors [14]. High BMI also appears to be associated with increased ovarian cancer risk through similar hormonal mechanisms, although the link is not as well established [15, 16]. PCOS, a condition characterized by ovarian hyperandrogenism, chronic anovulation and progesterone deficiency, has also been associated with both ovarian and endometrial cancer [17-19].
Several familial syndromes are associated with increased risk of endometrial and ovarian cancer. The most well-known germ line mutations that predispose to ovarian cancer involve the BRCA1 and BRCA2 genes. It has been estimated that mutations in BRCA1/2 might account for up to 10% of all ovarian cancer cases [20]. The risk of endometrial cancer does not appear to he significantly elevated in BRCA1/2 mutation carriers in general, however among the subgroup of women who used tamoxifen for treatment or primary prevention of breast cancer, risk of endometrial cancer was significantly elevated (Relative Risk = 11.6, p=0.004) [21]. A familial autosomal dominant syndrome characterized by defective DNA mismatch repair, Hereditary Nonpolyposis Colorectal Cancer (HNPCC), is associated with a 40-60% and 8-15% increased lifetime risk of endometrial and ovarian cancer, respectively [22, 23]. Another autosomal dominant disorder that has been suggested to predispose to endometrial cancer development is Cowden's Syndrome, in which 13 - 80% of patients have a germ line mutation of PTEN [24, 25]. However Cowden's Syndrome likely accounts for a very small propottion of endometrial cancer cases when viewed against somatic PTEN mutations observed in sporadic cases [26]. Patients with Cowden's Syndrome may also present with benign ovarian cysts and teratomas but generally not malignant ovarian neoplasms [24].
There are other shared risk factors between endometrial and ovarian cancer that are surprising and raise intriguing questions related to pathogenesis. IUD use, even hormonally inert types, clearly decrease the risk for endometrial cancer [27, 28] and some studies suggest these may also decrease the risk for ovarian cancer [29, 30]. Similarly, tubal ligation is a strong protective risk factor for ovarian cancer that may also decrease the risk for endometrial cancer [31-34]. Current smoking is associated with reduced risk of endometrial cancer, particularly in postmenopausal women, possibly due to the anti-estrogenic effects of smoking such as reduced body weight, lower age at menopause and differences in estrogen metabolism [35]. Current smoking has also been associated with decreased risk of clear cell ovarian tumors, however no association was observed with serous and endometrioid tumors and smoking doubled the risk of mucinous ovarian cancers [36]. Talc use, another factor found to consistently increase the risk for ovarian cancer [37-40] has not yet been investigated for endometrial cancer.
In summary, this brief review of epidemiologic risk factors reveals a surprising number of similarities in endometrial and ovarian cancers which suggests that there may also be shared mechanisms in their molecular pathogenesis.
3. Histopathology
3.1 Endometrial cancer
The histopathologic categories of endometrial cancer, identified by their unique microscopic features, include endometrioid (most common), serous and clear cell as well as the rarer subtypes mucinous, squamous, transitional cell, carcinosarcoma and undifferentiated tumors (Table 3). Although endometrial cancers exhibit a variety of histologic features, >95% can be classified into two clinicopathologic groups, endometrioid (Type I) and non-endometrioid (Type II) [41, 42].
Table 3.
| Type | Histologic description | Comments |
|---|---|---|
| Endometrioid | Resemblance to benign endometrial epithelium. Composed of tubular glands, lined by stratified or pseudostratified columnar cells. Nuclear pleomorphism is often mild to moderate. | |
| Serous | Forms complex papillary fronds covered by stratified, highly atypical epithelial cells that often display marked nuclear pleomorphism, and numerous mitoses. Exfoliation of cells (“hobnail”) and psammoma body formation (calcium deposition on intracytoplasmic filaments of degenerating cells) may be observed. | Similar histology to that seen in the ovary. Typically behaves aggressively with tendency for myometrial invasion, extensive lymphatic invasion and early dissemination. This subtype is regarded as high-grade by definition |
| Clear Cell | Clear, glycogen-filled hobnail-like cells with highly pleomorphic nuclei. Cells often grow in tubular or papillary arrangements. However, unlike serous carcinoma, papillae often have hyalinized cores. | Generally presents as advanced stage and has a poor prognosis |
| Mucinous | Composed of endocervical-type columnar cells that contain mucin-rich cytoplasm. | Tumors are most often low grade. Mixed endometrioid and mucinous adenocarcinomas are relatively common. |
| Squamous | Patterns range from individual cell keratinization to the formation of large keratin masses. | Pure squamous neoplasms are rare however focal squamous areas are identified in a portion of endometrioid carcinomas. |
| Transitional Cell | Typically displays nested or papillary urothelial morphology including longitudinal nuclear grooves | Pure primary tumors are rare. |
| Carcinosarcoma | Display an admixture of carcinomatous and sarcomatous components. The epithelial component is usually high grade and can be of endometrioid (most common), serous, clear cell, mucinous, undifferentiated or squamous type. The sarcomatous component may resemble endometrial (homologous) or non-endometrial (heterologous) stroma. | Also known as Malignant Mesodermal (Mullerian) Mixed Tumor. |
| Undifferentiated | Lack a distinctive appearance therefore unable to classify into specific category of tumor. Often composed of diffuse sheets and nests that display extensive necrosis. | Associated with poor prognosis |
3.1.1 Endometrial Adenocarcinoma, Endometrioid Type (Type I)
The majority (70-80%) of endometrial cancers are classified as Type I tumors. Most Type I tumors are described as endometrioid and exhibit a resemblance to benign endometrium. Other histologic variants include mucinous tumors that resemble the endocervix and adenosquamous tumors that display keratinization (43]. Type I tumors are influenced by endocrine modulation (estrogen unopposed by progesterone) and generally follow an indolent clinical course. These tumors are better differentiated with mild to moderate nuclear pleiomorphism and show less myometrial invasion and low potential for lymphatic spread [44].
3.1.2 Endometrial Adenocarcinoma, Non-Endometrioid Type (Type II)
Type II tumors are typically characterized by serous or clear cell histologies, or the very poorly differentiated phenotypes of carcinosarcoma or undifferentiated carcinoma [45]. They are not associated with clinical evidence of estrogen stimulation, exhibit low sensitivity to progestin and typically arise in the setting of an atrophic endometrium. They have a high degree of nuclear pleomorphism [46, 47], exhibit deeper myometrial invasion, are at higher risk of lymphatic spread and are associated with a more aggressive clinical course [48, 49].
3.2 Ovarian Cancer
Ovarian malignancies may arise from germ cell, stromal, or epithelial compartments. Approximately 25% of ovarian tumors, including benign neoplasms, are of germ cell origin and the most common is the mature teratoma (dermoid) which accounts for nearly 1/3 of benign ovarian neoplasms [50]. Only 2-3% of germ cell tumors are malignant and these include rare types such as dysgerminomas, endodermal sinus tumors and embryonal carcinomas [50]. Ovarian stromal tumors account for 6% of all ovarian tumors and include neoplasms derived from the sex cords and specialized stroma of the developing gonad [50]. Stromal tumors may arise from female-type (granulosa, theca) and male-type cells (Sertoli, Leydig) as well as other indifferent sex cord derivatives. Some of these tumors are hormonally active; benign thecomas are known for their estrogen production while Sertoli-Leydig cell tumors recapitulate testicular structures and may have virilizing properties [51]. The most common stromal tumor is the granulosa cell tumor which accounts for approximately 10% of ovarian cancers.
By far the most common types of malignant ovarian cancers are epithelial which tend to parallel the same types arising in the endometrium (Table 4). Four major histologic subtypes of epithelial ovarian cancer have been described, with each resembling different types of epithelia in the female reproductive tract [52]. Features associated with fallopian tube, endocervical or endometrial epithelia are observed in serous, mucinous and endometrioid forms of ovarian cancer, respectively. Clear cell tumors are the fourth major histological subtype and are identified by clear, peg-like cells that resemble the lining of the endometrial glands during pregnancy. The majority of malignant ovarian tumors fall into the invasive serous category followed by endometrioid, clear cell and mucinous types.
Table 4.
Histopathologic Categories of Epithelial Ovarian Cancer [adapted from reference 50].
| Type | Histologic description | Comments |
|---|---|---|
| Serous | Low and high grade subtypes distinguished by nuclear cytology correspond to different clinicopathologic subsets. External surfaces may be covered with papillary fronds and papillae that may become fused and form slit-like spaces. Psammoma bodies are often seen. | |
| Endometrioid | Resembles endometrial epithelia. Exhibits distinctive tubular glands lined by a pseudostratified epithelium with little or no intracellular mucin. | Histologically identical to endometrial endometrioid carcinoma. Often seen in association with endometriosis Concomitant endometrial carcinoma present in one-quarter of cases. |
| Mucinous | Epithelial lining of glands and cysts may be intestinal (goblet cells scattered among mucin-free cells) or endocervical/ mullerian type (mucin-filled, columnar cells with basal nuclei). Many or all of the cells contain abundant intracytoplasmic mucin. | Many intestinal type tumors now recognized as metastatic from appendix or large bowel. |
| Clear Cell | Cells are hobnail-like with glycogen-rich, clear cytoplasm and highly pleomorphic nuclei. Multiple papillae often encountered. | Histologically identical to clear cell carcinoma of the endometrium. |
| Squamous | Cell keratinization is common. | Pure squamous cell neoplasms are rare. Squamous elements are often seen in endometrioid tumors |
| Transitional Cell | Characterized by nests and columns of ‘transitional type’ epithelial cells that resemble the lining of the urinary bladder. | Includes malignant Brenner tumors and Transitional Cell Carcinoma. |
| Malignant Mesodermal (Mullerian) Mixed Tumors | Tumors contain both epithelial and mesenchymal elements. The epithelial component most often resembles endometrioid or serous carcinoma, but occasionally mucinous, squamous or clear cell elements are observed. | Most tumors are identical to those that are more frequently encountered in the endometrium. |
| Undifferentiated | Lack a distinct appearance and may exhibit a variety of growth patterns such as solid masses and irregular nests of epithelial cells separated by stroma that may be desmoplastic. Psammoma bodies, glands, papillae and mucinous pools may be present. |
A distinction made for ovarian cancer that does not have an exact parallel in endometrial cancer is the designation of “borderline” or “low malignant potential” ovarian tumor types that may spread beyond the ovary yet have an indolent course. While all of the tumor types described in Table 4 have a malignant counterpart, generally the low malignant potential tumors have serous or mucinous histopathology while low malignant potential endometrioid and clear cell tumors are rarely observed [50].
An anempt has been made to apply a dichotomous low vs high grade stratification to cancer of the ovary [53, 54], as the underlying genotypic findings and associated clinical outcomes fall into two distinct groups. This model is a starting point to make diagnostic classifications that are concordant with ovarian cancer pathogenesis, but is not as useful as the Type I and II distinction developed for endometrial cancer. Thus, there is a low grade category of tumors that tend to arise in a stepwise manner from borderline tumors, and may have serous or mucinous histologies. These tumors generally exhibit lower rates of cell proliferation, a gradual increase in chromosomal instability and a less aggressive clinical course. The most common type is low grade serous carcinoma and 25% of all serous carcinomas fall into this category. In contrast, high grade tumors are generally serous histology and exhibit widespread chromosome instability, tend to develop rapidly, metastasize early and are associated with a poor prognosis.
4. Molecular Pathogenesis – General Aspects
Features that describe the molecular pathogenesis in a cancer include large scale genomic changes as well as mutations or alterations in specific genes or pathways. The main mechanisms responsible for large scale genomic changes in tumor cells are microsatellite instability (MSI) or chromosome instability (changes in DNA copy number) [55]. MSI is often a direct result of defective mismatch repair mechanisms and can be identified by replication errors in repeated units of 1-4 DNA base pairs (microsatellites) that are distributed throughout the genome [56]. In endometrial cancer, MSI most often occurs from epigenetic silencing and inactivation of the MutL Homolog 1 (MLH1) gene through hypermethylation of CpG islands in its promoter region [57]. This form of genetic instability increases the mutation rate and can accelerate the acquisition of further generic damage that may lead to carcinogenic transformation.
Chromosome instability reters to chromosomal modifications such as gains, losses or rearrangements that may lead to oncogene activation or rumor suppressor inactivation. Chromosome instability can be detected cytogenetically using techniques like conventional karyotyping, in which metaphase spreads of human chromosomes are analyzed, or fluorescence in situ hybridization (FISH) in which specific chromosomes or loci are marked by fluorescent probes. Comparative genomic hybridization (CGH) assesses genomic imbalance and provides a measure of gene amplication and deletion.
Allelic imbalance (AI) is another type of chromosome instability where one allele of a gene is lost or amplified. Loss of heterozygosity (LOH) is a common form of AI and refers to the situation where one chromosome has a normal allele of a gene and the other has a mutant or deleted allele. If one allele is already inactivated then only a second inactivating hit may be required, which has particular relevance for tumor suppressor genes [55]. A method commonly applied to estimate genome-wide AI is the Single Nucleotide Polymorphism (SNP) array which measures the number of SNP markers with allelic imbalance divided by the total number SNP markers.
Inactivating mutations of specific genes generally involve base substitutions, deletions or insertions of only a few nudeotides and consequently these are often detected by direct sequencing of the gene of interest in the genomic DNA. As DNA sequencing can be labor intensive, immunohistochemical stains have been developed that allow the detection of well-studied genes, such as somatic PTEN mutation in endometrial cancer, so that gene mutation can be inferred based on positive or negative staining in formalin-fixed paraffin-embedded tissues.
The spectrum of genes affected in cancer can be wide and varied and some genes and pathways are involved in a variety of cancers. This is true for endometrial and ovarian cancer where several common genes and pathways have been described. A large amount of research has focused on p53 after it was discovered that the tumor suppressor gene TP53 is frequently mutated in a high proportion of human cancers [58]. Activation of p53 normally occurs in response to DNA damage, aberrant proliferative growth signals, and carcinogenic factors such as exposure to UV radiation [59]. Activated p53 carries out several functions, of which the most comprehensively understood are its ability to cause cell cycle arrest at the G2/M DNA damage checkpoint and to induce apoptosis [60, 61].
Mutations in KRAS and BRAF that cause aberrant activation have been identified in both endometrial and ovarian cancer and appear to play a central role in carcinogenesis by conducting signals that enhance cell proliferation during tumor development [62, 63). RAS, a small GTP binding protein, activates the core unit of a cascade composed of RAF, mitogen/extracellular signal-regulated kinase (MEK1/2) and MAP Kinase (MAPK or ERK) as well as the PI3K/ AKT pathway [64-66].
Inactivating mutations of the tumor suppressor gene, PTEN, are detected in both endometrial and ovarian cancer, PTEN is an inhibitor of PI3K/ AKT signaling and acts to control the rate of cell division and promote apoptosis [67]. Loss of PTEN may occur through a variery of mechanisms, however the most common is inactivation of both alleles through mutation or deletion in combination with LOH at chromosome 10q23 to generate a protein deficient state with a complete loss of function phenotype [68, 69].
Gain of function mutations of the CTNNB1 gene (β-catenin) are identified in endometrial and ovarian cancer [70-73], especially those with squamous differentiation. These mutations stabilize the β-catenin protein in the cell cytoplasm and nucleus which leads to activation of the lymphoid enhancing binding factor (LEF) and T cell-specific transcription factor (TCF) pathways that promote transcription of target genes involved in tumorigenesis such as C-MYC and COX2 [74, 75].
5. Molecular Pathogenesis of Endometrial Cancer
5.1 Type I Endometrial Cancer
Type I endometrial cancers display a wide range of genetic alterations which differ in their temporal sequence and cumulative combinations between patients. MSI is reported in approximately 20% of Type I endometrial cancers of all grades [76-79], however since the majority of endometrioid carcinomas do not exhibit MSI this is not a necessary, or even predominant, feature of endometrial carcinogenesis [43]. More common changes include specific alterations such as PTEN inactivation and aberrant activation of KRAS and β-catenin.
The most common genetic alteration in Type I endometrial cancer is PTEN inactivation. The proportion of endometrial cancers that demonstrate PTEN inactivation varies by case selection, with the highest rates (83%) observed in sporadic cases associated with a coexisting or prior premalignant lesion [80]. Furthermore, the functional role of PTEN in Type I endometrial cancer development has been demonstrated in PTEN knockout mice where 20% developed endometrial cancer [81].
Mutations in KRAS causing aberrant activation have been implicated in 10-30% of Type I endometrial cancers [82-84]. Gain of function mutations in exon 3 of the CTNNB1 gene (β-catenin) are also observed in 25-38% of Type I cancers [70, 71, 85]. Interestingly, MSI, PTEN and KRAS mutations frequently coexist with in the same tumor, however these molecular alterations are not usually seen in combination with β-catenin mutation (86). Hence, it has been suggested that Type I endometrial cancers with β-catenin mutations may develop via a unique pathway that includes a change in differentiation state towards a squamous morphology [87. 88].
In contrast to the more common genetic alterations discussed above, aberrant accumulation of inactive p53 protein is observed in only 5% of Type I endometrial cancers [82]. Furthermore, the mechanism of p53 inactivation differs between Types I and II tumors; in Type I tumors aberrant p53 protein accumulation results from changes in upstream regulatory proteins, such as MDM2 and p14 ARF, while Type II tumors often have p53 truncation mutations [89-91] as discussed below.
5.2 Type II Endometrial Cancer
Type II endometrial cancers demonstrate genetic instability at the chromosome level resulting in a high level of aneuploidy while maintaining intact MMR [46, 47, 92]. The primary genetic defect is mutation of the p53 gene, observed in 75-100% of tumors [44, 93]. One preliminary study has reported that levels of aberrant p53 expression in clear cell carcinoma may be intermediate between those reported in papillary serous carcinoma and endometrioid tumors [94]. Both papillary serous and clear cell carcinoma have low expression of estrogen and progesterone receptors [44]. Amplification or overexpression of HER2 has also been reported in 20% of Type II endometrial cancers [95-98]. In contrast with Type I tumors, inactivation of PTEN and RAS activation is not observed [44].
5.3 Precursor Lesions of Endometrial Cancer
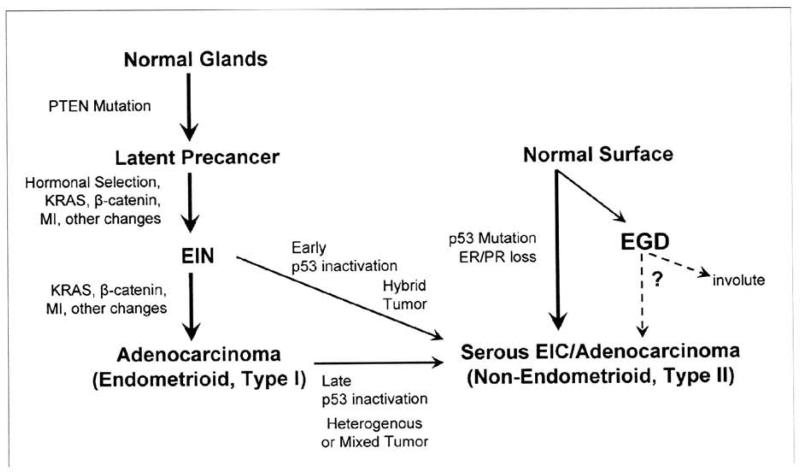
The immediate precursor lesion for Type I endometrial cancer is known as Endometrial Intraepithelial Neoplasia (EIN ) which in turn is presumed to arise from “latent precancers”, or otherwise normal appearing endometrial glands that exhibit somatically acquired mutations in PTEN (Fig 2). PTEN mutations can be detected by immunohistochemical staining in paraffin embedded tissues [13] but are not associated with cytologic or architectural abnormalities and therefore cannot be detected using routine diagnostic methods [43]. The frequency of occurrence of PTEN deficient latent precancers is extremely common, occurring in up to 43% of normal premenopausal women [13]. This high prevalence suggests that the rate of progression from the latent precancer stage to an adenocarcinoma is relatively low, since lifetime endometrial cancer risk is only 2.6% [99].
Figure 2. Multiple Pathways to Endometrial Carcinoma.
Inactivation of PTEN occurs early in Type I endometrial carcinomas, prior to any detectable histologic change (“latent precancer”). Non-genetic hormonal selection factors, such as increased estrogen unopposed by progesterone, may modulate cancer risk through their actions on preclinical latent precencers, which may undergo involution or expansion with additional mutation. Further mutations, sometimes accelerated by a microsatellite instability phenotype (MI), define stepwise progression events to EIN and then adenocarcinoma. Serous (Type II) tumors are initially observed as short-lived preinvasive precursors designated as serous intraepithelial carcinoma (EIC). Endometrial glandular dysplasia (EGD) is a newly described lesion with p53 inactivation and a histology that is intermediate between normal and serous EIC. Progression and involution rates of EGO lesions over time are unknown and it remains to be determined how often EGD lesions are actual precursors of Type II cancers. Rarely, individual examples of Type I tumors may acquire an early or late p53 inactivation event, causing a hybrid or heterogeneous tumor, respectively [adapted from reference 43]
There is strong evidence to support the transition from EIN to a Type I endometrial adenocarcinoma. First, one third of patients diagnosed with EIN have a concurrent occult carcinoma, and those that are initially cancer free have a 45-fold increased risk of endometrial cancer as compared with patients diagnosed with endometrial hyperplasia without EIN [100]. Second, a similar repertoire of genetic alterations have been identified in EIN and adenocarcinomas and, within the same patient, specific PTEN, MSI and X chromosome inactivation patterns are observed in both areas of EIN and adenocarcinoma [78, 101]. While EIN and adenocarcinomas share many characteristics, adenocarcinomas differ in that they exhibit a greater cumulative mutational load as would be expected during carcinogenic transformation. For example, inactivation of PTEN is found in 55% of EIN lesions and 83% of adenocarcinomas which followed the same EIN lesion [80, 102]. Similarly, the proportion of altered microsatellite alleles increases from EIN to carcinoma [78, 101]. Interestingly, no markers have been identified that are uniquely present in adenocarcinomas compared with EIN [103].
Hormonal mechanisms have been integrated into the model of Type I endometrial carcinogenesis. The influence of estrogens can be implicated at the very earliest stages, acting upon PTEN defective latent precancers and EIN lesions. Both have been shown to maintain high levels of nuclear estrogen and progesterone receptors [13] and under conditions of estrogen stimulation it is hypothesized that PTEN defective cells would have a selective proliferative advantage since PTEN would not carry out its normal role to limit the rate of cell division [103].
With the knowledge that PTEN-null clones are present at a high frequency in “ normal” women, and that these (83%) are usually retained for at least one years duration [13], this provides a genetically predisposed cell population that is a target for, and effector of, hormonal risk modulation. This approach has been applied in a recent study that directly observed the effects of risk-modifying factors on latent precancers in the endometrium using PTEN inactivation within normal appearing tissues as the biomarker [104]. The risk-modifying factors examined were use of hormonally-inert mechanical intrauterine devices (IUDs) or low dose combined oral contraceptive (OC) pills, which confer a 40% and 50% reduction in risk, respectively, compared with controls [28, 105, 106]. It was shown that women with a history of IUD or OC pill use had a lower rate of latent endometrial precancers detected by PTEN immunohistochemistry and the magnitude of latent precancer decline was proportionate to the extent of diminished endometrial cancer risk as previously determined by epidemiological studies [104]. This data therefore supports a model in which long-term outcome (e.g. endometrial cancer risk) can be altered by interventions at an early susceptible moment.
Less is known about early events in Type II endometrial carcinogenesis as compared with Type I. This results in part from the rarity of papillary serous endometrial cancers compared with their endometrioid counterparts and the probable rapid emergence of a papillary serous carcinoma from an apparently normal state with a small window for clinical detection of early disease [43]. Two putative precursor lesions have been proposed for papillary serous disease, serous intraep ithelial carcinoma (serous EIC) [107, 108] and endometrial glandular dysplasia (EGD) [109] (Fig 2).
Serous EIC is a noninvasive form of papillary serous adenocarcinoma [110]. EIC is observed in approximately 90% of uteri with invasive papillary serous carcinoma and usually presents as an extension from the co-existing invasive carcinoma [107]. EIC exhibits p53 mutation and cytologic features similar to that observed in serous adenocarcinoma [43]. Serous EIC has rarely been reported as the initial diagnostic manifestation of a non-invasive papillary serous adenocarcinoma [111]. Rather, instances where isolated EIC can metastasize to peritoneal and abdominal sites have been reported [112] and in this case serous EIC should not be classified as “premalignant” but rather would be an immediate precursor to invasive disease.
EGO has been identified in uteri of up to 53% of patients with invasive or noninvasive papillary serous carcinoma [43]. EGO displays a histology and genotype that is intermediate between normal endometrium and serous carcinoma. Specifically, it lacks the cytologic atypia of serous EIC, has moderate abnormalities of p53 and lower mitotic activity [109]. Further molecular analysis identified progressive allelic loss within individual patients from EGO to serous carcinoma [113]. Although this lesion has promise as a putative precursor lesion, the frequency of occurrence is unknown outside of a cancer context and the histologic phenotype is not readily identifiable as it can be easily confused with reactive changes [43]. Further studies are needed to learn more about these putative precursor lesions for Type II endometrial carcinoma and to determine the potential clinical implications for patient management.
6. Molecular Pathogenesis of Ovarian Cancer
6.1 Serous low grade tumors
The most common molecular genetic changes that distinguish serous low grade from serous high grade ovarian tumors are alterations in the MAPK signaling cascade, specifically mutations in KRAS or BRAF. Activating mutations in KRAS have been observed in 30-50% of serous borderline and low grade serous carcinomas, but rarely in high grade serous cancers [54, 114, 115]. BRAF mutations are found in 28 and 30% of borderline or low-grade serous carcinomas, respectively [115]. Neither KRAS nor BRAF mutations are found in high grade serous carcinomas. Interestingly, KRAS and BRAF mutations are mutually exclusive and rarely found with in the same tumor, which supports the hypothesis that mutation of only one is sufficient to activate the MAPK pathway [115].
Studies of allelic imbalance (AI) showed a progressive increase in the degree of AI from a borderline tumor to a low grade serous carcinoma [54]. This contrasts with high grade serous carcinomas which show a high level of AI (and correlated chromosomal instability), even at their earliest clinical stages [53]. In addition, serous borderline and low grade tumors exhibit a similar low level of chromosomal imbalance (measured from CGH analys is) as compared with high grade serous carcinoma [116, 117). MSI does not appear to be a characteristic of serous borderline tumors [118] and p53 mutation is rarely detected [53]. In combination, these data support the likely progression of serous borderline tumors to low grade serous carcinoma and an apparent separate pathway to development for serous high grade tumors.
6.2 Serous high grade carcinoma
The most common genetic alteration in serous high grade carcinoma is p53 mutation, found in 50-80% of advanced stage, high grade, serous carcinomas [119]. In addition to p53 mutation, these tumors demonstrate amplication/ overexpression of HER2 in up to 70% of cases which is rare in other tumor subtypes [120, 121].
Amplification of either PIK3CA or AKT2 was observed in 27% of high grade serous carcinomas but not serous borderline tumors [122]. Furthermore, application of a Single Nucleotide Polymorphism (SNP) array identified widespread DNA copy number changes in high grade serous ovarian carcinomas that were not apparent in low grade serous tumors, such as alterations in loci harboring candidate the oncogenes cyclin El (CCNE1), AKT2, Notch3 and PIK3CA [123].
Serous high grade tumors are characterized by an increased cell proliferation (Ki-67) index [124] as well as high level genomic instability measured as chromosome and allelic imbalance [54, 116, 117]. These tumors rarely have KRAS and BRAF mutations which suggests that they develop via a pathway that is unrelated to RASfRAF/MEKIMAPK signaling [125].
It has been difficult to identify morphologically recognizable precursor lesions for high grade serous carcinoma as these tumors usually present at an advanced stage. Two hypotheses have been proposed to explain their origin: these tumors develop ‘de novo’ from the ovarian surface epithelium or its Mullerian inclusion cysts [126] or, alternatively, the fallopian tube fimbria may be the site of origin of a population of malignant tubal cells that exfoliate and grow rapidly upon seeding ofthe ovary [127, 128] (discussed further in Pathway III below).
6.3 Mucinous tumors
The most common molecular feature of mucinous tumors is a high prevalence of KRAS mutations, generally found in codon 12. Mutation frequencies of 13%, 33-63% and 50% have been described for cystadenomas, borderline tumors and carcinomas, respectively [129-131]. Furthermore, KRAS mutations were identified in both intestinal and endocervical types of benign and borderline mucinous tumors [130, 132, 133].
Another molecular marker that was recently identified in borderline and invasive mucinous ovarian neoplasms is CEACAM6, however it remains to be determined whether this is a general marker for mucinous differentiation or a potential key player in carcinogenic transformation [134]. Other molecular genetic changes such as MSI and p53 mutation, have rarely been reported in mucinous tumors [53, 135].
It has been hypothesized that both serous low grade and mucinous low grade tumors develop in a stepwise manner from well-recognized precursors, namely borderline tumors that may in turn develop from cystadenomas and adenofibromas [136, 137]. Molecular evidence that supports this hypothesis is the identification ofthe same KRAS mutation in benign, borderline and malignant portions of the same tumor [132, 133, 138] and the gradual increase in chromosome instability observed in benign, borderline and malignant tumors [53]. The majority of benign epithelial ovarian tumors are of the serous and mucinous subtype [136] and it is generally accepted that the ovarian surface epithelium or its Mullerian inclusion cysts are a common site for the initiation of serous low grade and mucinous ovarian carcinogenesis [53, 139].
6.4 Endometrioid and clear cell carcinoma
Endometrioid and clear cell carcinomas are unique from serous and mucinous tumors in that a proportion (13-50%) exhibit MSI [72, 140, 141]. Specific molecular alterations that characterize endometrioid ovarian tumors are mutations of β-catenin [72, 73] and PTEN [142] in one third and 20% of cases, respectively. These mutations are detected in well differentiated, stage 1 tumors and thought to be an early event [53]. KRAS mutations have also been identified in endometrioid tumors albeit at a lower frequency (<10%) [143].
Ovarian clear cell carcinomas are relatively rare and consequently little is known about molecular mechanisms involved in tumor development [53, 144]. Based on preliminary studies, PTEN mutation has been repotted in 8-40% of cases [145, 146] and mutation of the TGF-β receptor II was detected in three cases [147]. Overexpression of hepatocyte nuclear factor-1 β at the mRNA and protein level has also been observed in clear cell but not other tumor subtypes [148, 149]. Mutations in p53 have rarely been detected in endometrioid and clear cell tumors [53].
In contrast to the putative pathway to development of mucinous or low grade serous carcinoma, benign and borderline endometrioid and clear cell tumors are rarely reported [52]. Rather, it has been proposed that both endometrioid and clear cell carcinomas may arise from endometriosis implanted on the ovary [53, 150, 151]. Evidence is strongest for the endometriosis to ovarian endometrioid carcinoma progression as similar molecular alterations, specifically LOH at 10q23 (the location of PTEN) and PTEN mutation, were identified in endometriosis, atypical endometriosis and ovarian endometrioid carcinoma in the same patients [142, 145, 152). In addition, functional evidence linking endometriosis and endometrioid ovarian cancer is provided by a mouse model where tissue-specific expression of active mutant Kras resulted in pe lvic endometriosis, and expression of active mutant Kras in combination with Pten inactivation caused metastatic endometrioid ovarian carcinoma that resembled human disease [153].
Clear cell ovarian cardnoma is frequently observed in association with endometriosis, however molecular evidence to support a stepwise progression is not available due to the lack of specific markers for this tumor type [53]. Although there is good evidence that endometriosis is a precursor for at least some ovarian endometrioid and clear cell tumors, further biological evidence is needed to support a causative link in human studies.
6.5 Precursor lesions of ovarian cancer
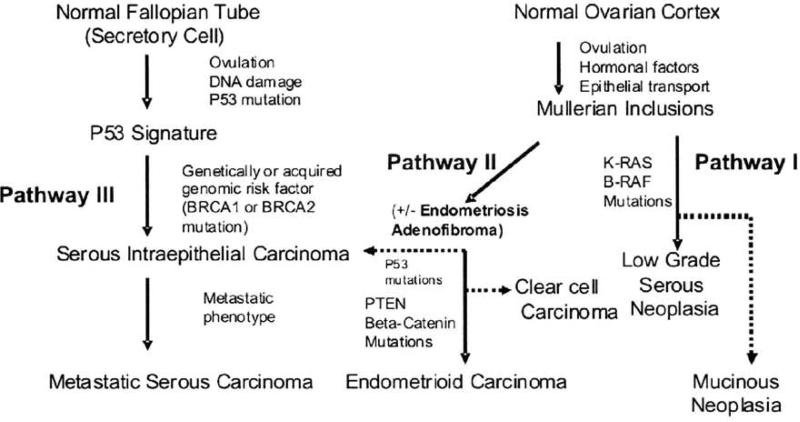
Based on the data currently available, we propose a model to summarize the pathogenesis of the distinct subtypes of ovarian cancer that accounts for the different putative cells of origin for this disease (Fig 3).
Figure 3. Relationship between Potential Precursor Lesions and Histologic Types of Ovarian Cancer.
Three pathways for ovarian cancer are proposed: Pathway 1 begins in the ovary in a majority of cases within Mullerian inclusion cysts (MICs). MICs may form when the ovarian surface or other epithelium (such as salpingeal) becomes entrapped after ovulation or by surface adhesion. MICs have been the classic explanation for the origin of benign serous and mucinous cystadenomas, some of which may progress through borderline counterparts, serous borderline or mucinous borderline, into carcinomas. Pathway I may apply to some low grade invasive serous carcinomas. Pathway II describes the origin of endometrioid and clear cell carcinoma from endometriosis derived from transpo1t and implantation of endometrial cells from the uterus. Counterparts of each type may occur in the uterine lining. Pathway III involves the origin of metastatic (high grade) serous carcinomas and is best characterized in the distal fallopian tube [adapted from 128].
6.5.1 Pathway I (Serous low grade and Mucinous)
begins in the ovary or within Mullerian inclusion cysts (MICs) in the majority of cases. MICs may form when the ovarian surface or other epithelium (such as salpingeal) becomes entrapped after ovulation or by surface adhesion. MICs have been the classic explanation for the origin of benign serous and mucinous cystadenomas, some of which may progress through borderline serous and mucinous counterparts and onto carcinomas.
6.5.2 Pathway II (Endometrioid and Clear Cell)
describes the origin of ovarian cancer from endometriosis derived from transport and implantation of endometrial cells from the uterus [154, 155]. An origin of endometrioid differentiation directly from MICs is also possible since many endometrioid carcinomas are not clearly associated with endometriosis.
6.5.3 Pathway III (Serous high grade)
may arise anywhere in the pelvis, but the fallopian tube is now the best documentted, and most frequent, site of origin. Such tumors are typically detected after bulky peritoneal and ovarian surface involvement has already emerged. For decades, the initial disease focus in the distal fallopian tube has been missed because of its small size and lack of thorough pathologic examination at that site [156]. Epithelial cells prominent at the distal (fimbriated) end of the tubes may undergo repetitive cyclic changes during ovulatory cycles and be subject to genotoxic stresses that lead to DNA damage and p53 mutation accompanied by high cell proliferation rates and poor prognosis [7]. Women who carry BRCA mutations may be especially vulnerable to develop tubal intraepithelial carcinoma which may give rise to high grade serous cancers. Data that supports this hypothesis includes the involvement of the fallopian tubes in a high proportion (42-100%) of BRCA1/2 cancers and frequent identilication of hyperplasia and/or potential pre-neoplastic lesions (p53 positive cells) in prophylactic salpingectomy specimens [8, 157]. Furthermore, a microarray-based study demonstrated that normal fallopian tube had a more similar RNA expression prolile to serous carcinoma as compared to normal ovarian epithelial, endometrial and colon specimens [158]. A recent epidemiological study also found that serous ovarian and fallopian tubes cancers exhibited similarities in risk factor profiles [159]. Fallopian tube cancers are morphologically indistinguishable from high grade serous ovarian cancers and likely should be considered the same disease from a pathologic, clinical and epidemiologic standpoint.
In summary, the evidence base developed in the last 5 years supporting a tubal origin for serous high grade ovarian cancers is now much stronger than the cumulative data of several decades investigating ovarian inclusion cysts as the site of origin. Although it is likely that both tubal and non-tubal sites of origin for high grade serous carcinoma occur, the fallopian tube appears to be more common. Further studies are needed to identify the molecular pathways that may be involved in the carcinogenic transformation ofthe putative cells of origin for high grade serous ovarian carcinoma.
7. Conclusion
This review has highlighted similarities in endometrial and ovarian cancer that includes highly correlated incidence rates, similar risk factor profiles and several common genes and pathways involved in their molecular pathogenesis. Analyses of different histologic subtypes identifies common microscopic features in serous and endometrioid types, regardless of the organ of origin. Furthermore, serous tumors are characterized by defects in p53 and endometrioid tumors are associated with mutations in PTEN or β-catenin regardless of the primary organ site. On the other hand, similar mutations may be associated with distinct histopathologies, for example in the endometrium KRAS mutation is associated with endometrioid histology while in the ovary KRAS mutations are more common in mucinous and low grade serous cancers. For clear cell cancers the case is less clear. Similar gene expression profiles associated with clear cell differentiation have been identified regardless of whether the tumor originated in the endometrium or ovary [160], however the molecular pathogenesis of clear cell tumors appears quite distinct, with clear cell endometrial tumors showing defects in p53 while clear cell tumors of the ovary have PTEN mutation and p53 mutation is rare. In conclusion, the molecular pathogenesis of ovarian and endometrial cancer is best appreciated by an understanding of the epidemiology, histopathology and molecular features. In combination, these approaches may provide the best opportunity for prevention and early detection.
Acknowledgements
The guidance and comments from Dr. George Muner and Dr. Christopher Crum are gratefully acknowledged. This work was supported by NIH Grant UO1CA086381 and P50 CA105009.
References
- 1.American Career Society Cancer Facts and Figures 2008. 2008.
- 2.Parazzini F, Franceschi S, La Vecchia C, Fasoli M. The epidemiology of ovarian cancer. Gynecol Oncol. 1991;43:9–23. doi: 10.1016/0090-8258(91)90003-n. [DOI] [PubMed] [Google Scholar]
- 3.Parazzini F, La Vecchia C, Bocciolone L, Franceschi S. The epidemiology of endometrial cancer. Gynecol Oncol. 1991;41:1–16. doi: 10.1016/0090-8258(91)90246-2. [DOI] [PubMed] [Google Scholar]
- 4.Prentice RI, Thomson CA, Caan B, Hubbell FA, Anderson GL, Bcresford SA, Pettinger M, Lane DS, Lessin L, Yasmeen S, Singh B, Khandekar J, Shikany JM, Satterfield S, Chlebowski RT. Low-fat dietary pattern and cancer incidence in the Women's Health Initiative Dietary Modification Randomized Controlled Trial. J Natl Cancer Inst. 2007;99:1534–43. doi: 10.1093/jnci/djm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McPherson CP, Sellers TA, Potter JD, Bostick RM, Folsom AR. Reproductive factors and risk of endometrial cancer, The Iowa Women's Health Study. Am J Epidemiol. 1996;143:1195–202. doi: 10.1093/oxfordjournals.aje.a008707. [DOI] [PubMed] [Google Scholar]
- 6.Fathalla MF. Incessant ovulation - a factor in ovarian neoplasia? Lancet. 1971;2:163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- 7.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, Cramer DW, McKeon FD, Crum CP. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 8.Folkins AK, Jarboe EA, Saleeinuddin A, Lee Y, Callahan MJ, Drapkin R, Garber JE, Muto MG, Tworoger S, Crum CP. A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol Oncol. 2008;109:168–73. doi: 10.1016/j.ygyno.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schildkraut JM, Bastos E, Berchuck A. Relationship between lifetime ovulatory cycles and ovcrexpression of mutant p53 in epithelial ovarian cancer. J Natl Cancer Inst. 1997;89:932–938. doi: 10.1093/jnci/89.13.932. [DOI] [PubMed] [Google Scholar]
- 10.Cairns J. Mutation and cancer the antecedents to our studies of adaptive mutation. Genetics. 1998;148:1433–40. doi: 10.1093/genetics/148.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moolgavkar SH, Knudson AG., Jr. Mutation and cancer a model for human carcinogenesis. J Natl Cancer Inst. 1981;66:1037–52. doi: 10.1093/jnci/66.6.1037. [DOI] [PubMed] [Google Scholar]
- 12.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 13.Mutter GL, Ince TA, Baak JP, Kust GA, Zhou XP, Eng C. Molecular identification of latent precancers in histologically normal endometrium. Cancer Res. 2001;61:4311–4. [PubMed] [Google Scholar]
- 14.Calle EE, Kaaks R. Overweight, obesity and cancer epidemiological evidence and proposed mechanisms. Mat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 15.Leitzmann MF, Koebnick C, Danforth KN, Brinton LA, Moore SC, Hollenbeck AR, Schatzkin A, Lacey JV., Jr. Body mass index and risk of ovarian cancer. Cancer. 2009;115:812–22. doi: 10.1002/cncr.24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schouten LJ, Rivera C, Hunter DJ, Spiegclman D, Adami HO, Arslan A, Beeson WL, van den Brandt PA, Buring JE, Folsom AR, Fraser GE, Frcudenheim JL, Goldbohm RA, Hankmson SE, Lacey JV, Jr., Leitzmann M, Lukanova A, Marshall JR, Miller AB, Patel AV, Rodriguez C, Rohan TE, Ross JA, Wolk A, Zhang SM, Smith-Warner SA. Height, body mass index, and ovanan cancer: a pooled analysis of 12 cohort studies. Cancer Epidemiol Biomarkers Prev. 2008;17:902–12. doi: 10.1158/1055-9965.EPI-07-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escobedo IG, Lee NC, Peterson HB, Wingo PA. Infertility-associated endometrial cancer risk may be limited to specific subgroups of infertile women. Obstel Gynecol. 1991;77:124–8. [PubMed] [Google Scholar]
- 18.Schildkraut JM, Schwingl PJ, Bastos E, Evanoff A, Hughes C. Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstel Gynecol. 1996;88:554–9. doi: 10.1016/0029-7844(96)00226-8. [DOI] [PubMed] [Google Scholar]
- 19.Wild S, Picrpoint T, Jacobs H, McKeigue P. Long-term consequences of polycystic ovary syndrome: results of a 31 year follow-up study. Hum Fertil (Camb) 2000;3:101–105. doi: 10.1080/1464727002000198781. [DOI] [PubMed] [Google Scholar]
- 20.Sogaard M, Kjaer SK, Gaythcr S. Ovarian cancer and genetic susceptibility in relation to the BRCA1 and BRCA2 genes, Occurrence, clinical importance and intervention. Acta Obsiet Gynecol Scand. 2006;85:93–105. doi: 10.1080/00016340500324621. [DOI] [PubMed] [Google Scholar]
- 21.Beiner ME, Finch A, Rosen B, Lubmski J, Moller P, Ghadirian P, Lynch HT, Friedman E, Sun P, Narod SA. The risk of endometrial cancer in women with BRCA1 and BRCA2 mutations, A prospective study. Gynecol Oncol. 2007;104:7–10. doi: 10.1016/j.ygyno.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Malander S, Rambech E, Kristoffersson U, Halvarsson B, Ridderheim M, Borg A, Nilbert M. The contribution of the hereditary nonpolyposis colorectal cancer syndrome to the development of ovarian cancer. Gynecol Oncol. 2006;101:238–43. doi: 10.1016/j.ygyno.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 23.Lu HK, Broaddus RR. Gynecologic Cancers in Lynch Syndrome/HNPCC. Fam Cancer. 2005;4:249–54. doi: 10.1007/s10689-005-1838-3. [DOI] [PubMed] [Google Scholar]
- 24.Uppal S, Mistry D, Coatesworth AP. Cowden disease: a review. Int J Clin Pract. 2007;61:645–52. doi: 10.1111/j.1742-1241.2006.00896.x. [DOI] [PubMed] [Google Scholar]
- 25.Eng C. Will the real Cowden syndrome please stand up: revised diagnostic criteria. J Med Genet. 2003;37:828–30. doi: 10.1136/jmg.37.11.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black D, Bogomolniy F, Robson ML, Offit K, Barakat RR, Boyd J. Evaluation of germline PTEN mutations in endometrial cancer patients. Gynecol Oncol. 2005;96:21–4. doi: 10.1016/j.ygyno.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 27.Beining RM, Dennis LK, Smith EM, Dokras A. Meta-analysis of intrauterine device use and risk of endometrial cancer. Ann Epidemiol. 2008;18:492–9. doi: 10.1016/j.annepidem.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Curtis KM, Marchbanks PA, Peterson HB. Neoplasia with use of intrauterine devices. Contraception. 2007;75:S60–9. doi: 10.1016/j.contraception.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Ness RB, Grisso JA, Veigona R, Klapper J, Morgan M, Wheeler JE. Oral contraceptives, oihcr methods of contraception, and risk reduction for ovarian cancer. Epidemiology. 2001;12:307–12. doi: 10.1097/00001648-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Tworoger SS, Fairfield KM, Coldilz GA, Rosner BA, Hankinson SE. Association of Oral Contraceptive Use, Other Contraceptive Methods, and Infertility with Ovarian Cancer Risk. Am J Epidemiol. 2007 doi: 10.1093/aje/kwm157. [DOI] [PubMed] [Google Scholar]
- 31.Castellsague X, Thompson WD, Dubrow R. Tubal sterilization and the risk of endometrial cancer. Int J Cancer. 1996;65:607–12. doi: 10.1002/(SICI)1097-0215(19960301)65:5<607::AID-IJC9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Green A, Purdie D, Bain C, Siskind V, Russell P, Quinn M, Ward B. Tubal sterilisation, hysterectomy and decreased risk of ovarian cancer. Int J Cancer. 1997;71:948–951. doi: 10.1002/(sici)1097-0215(19970611)71:6<948::aid-ijc6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 33.Lacey JV, Jr., Brinton LA, Mortel R, Berman ML, Wilbanks GD, Twiggs LB, Barrett RJ. Tubal sterilization and risk of cancer of the endometrium. Gynecol Oncol. 2000;79:482–4. doi: 10.1006/gyno.2000.5970. [DOI] [PubMed] [Google Scholar]
- 34.Rosenblatt K, Thomas D. Association between tubal ligation and endometrial cancer. Int J Cancer. 1997;71:129–30. doi: 10.1002/(sici)1097-0215(19970328)71:1<129::aid-ijc22>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 35.Terry PD, Rohan TE, Franceschi S, Weiderpass E. Cigarette smoking and the risk of endometrial cancer. Lancet Oncol. 2002;3:470–80. doi: 10.1016/s1470-2045(02)00816-1. [DOI] [PubMed] [Google Scholar]
- 36.Jordan SJ, Whiteman DC, Purdie DM, Green AC, Webb PM. Does smoking increase risk of ovarian cancer? A systematic review. Gynecol Oncol. 2006;103:1122–9. doi: 10.1016/j.ygyno.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Cramer DW, Liherman RF, Titus-Ernsioff L, Welch WR, Grcenberg ER, Baron JA, Harlow BL. Genital talc exposure and risk of ovarian cancer. Int J Cancer. 1999;81:351–6. doi: 10.1002/(sici)1097-0215(19990505)81:3<351::aid-ijc7>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 38.Gross AJ, Berg PH. A meta-analytical approach examining the potential relationship between talc exposure and ovarian cancer. J Expo Anal Environ Epidemiol. 1995;5:181–95. [PubMed] [Google Scholar]
- 39.Huncharck M, Geschwind JF, Kupelnick B. Perineal application of cosmetic talc and risk of invasive epithelial ovarian cancer: a meta-analysis of 11,933 subjects from sixteen observational studies. Anticancer Res. 2003;23:1955–60. [PubMed] [Google Scholar]
- 40.Merritt MA, Green AC, Nagle CM, Webb PM. Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. Int J Cancer. 2007;122:170–6. doi: 10.1002/ijc.23017. [DOI] [PubMed] [Google Scholar]
- 41.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–7. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 42.Deligdisch L, Holinka CF. Endometrial carcinoma two diseases? Cancer Delect Prev. 1987;10:237–46. [PubMed] [Google Scholar]
- 43.Mutter GL. Endometrial carcinogenesis: an integrated molecular, histologic, and functional model of a dualistic disease. In: Giordano A, Bovicelli A, Kurman RJ, editors. Molecular Pathology of Gynecologic Cancers. Springer; 2006. [Google Scholar]
- 44.Sherman ME. Theories of endometrial carcinogenesis: a multidisciplinary approach. Mod Pathol. 2000;13:295–308. doi: 10.1038/modpathol.3880051. [DOI] [PubMed] [Google Scholar]
- 45.Sherman ME, Sturgeon S, Brinton L, Kurman RJ. Endometrial cancer chemoprevention: implications of diverse pathways of carcinogenesis. J Cell Biochem Suppl. 1995;23:160–4. doi: 10.1002/jcb.240590921. [DOI] [PubMed] [Google Scholar]
- 46.Basil JB, Goodfellow PJ, Rader JS, Mulch DG, Herzog TJ. Clinical significance of microsatellite instability in endometrial carcinoma. Cancer. 2000;89:1758–64. doi: 10.1002/1097-0142(20001015)89:8<1758::aid-cncr16>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 47.Faquin WC, Fitzgerald JT, Lin MC, Boynton KA, Muto MG, Multer GL. Sporadic microsaiellite instability is specific to neoplastic and preneoplastic endometrial tissues. Am J Clin Pathol. 2000;113:576–82. doi: 10.1309/4mgm-fmrc-6awk-yqy2. [DOI] [PubMed] [Google Scholar]
- 48.Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, Powell MA, Hcndrickson MR, Kapp DS, Chan JK. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006;94:642–6. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hendrickson M, Ross J, Eifel P, Martinez A, Kempson R. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6:93–108. doi: 10.1097/00000478-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Scully R. Tumors of the ovary and maldcvclopcd gonads. Vol Second Series. Armed Forces Institute of Pathology; Washington, DC: 1979. [Google Scholar]
- 51.Ioffe O, Simsir A, Silverberg S. Pathology. In: Berek J, Hacker N, editors. Practical Gynecologic Oncology. Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 52.Russell P. Surface epnhclial-stromal tumors of the ovary. In: Kurman RJ, editor. Blaustein's Pathology of the Female Genital Tract. Springer-Verlag; New York: 1994. pp. 705–782. [Google Scholar]
- 53.Shih L, Kurman R. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–8. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singer G, Kurman RJ, Chang HW, Cho SK, Shih Ie M. Diverse tumorigenic pathways in ovarian serous carcinoma. Am J Pathol. 2002;160:1223–8. doi: 10.1016/s0002-9440(10)62549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–9. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 56.Perucho M. Cancer of the microsatcllite mutator phcnolype. Biol Chem. 1996;377:675–84. [PubMed] [Google Scholar]
- 57.Esteller M, Levine R, Baylin SB, Ellenson LH, Herman JG. MLH1 promoter hypcrmcihvlalion is associated with the microsatcllite instability phenorype in sporadic endometrial carcinomas. Oncogene. 1998;17:2413–7. doi: 10.1038/sj.onc.1202178. [DOI] [PubMed] [Google Scholar]
- 58.Steele RJ, Thompson AM, Hall PA, Lane DP. The p53 tumour suppressor gene. Br J Surg. 1998;85:1460–7. doi: 10.1046/j.1365-2168.1998.00910.x. [DOI] [PubMed] [Google Scholar]
- 59.Vogelstein B, Lane D, Levine AJ. Sirfing the ps3 network. Nature. 2000;408:307–10. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 60.Woods DB, Vousden KH. Regulation of p53 function. Exp Cell Res. 2001;264:56–66. doi: 10.1006/excr.2000.5141. [DOI] [PubMed] [Google Scholar]
- 61.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 62.Tsatsanis C, Spandidos DA. The role of oncogenic kinases in human cancer (Review) Int J Mol Med. 2000;5:583–90. doi: 10.3892/ijmm.5.6.583. [DOI] [PubMed] [Google Scholar]
- 63.Adjei AA. Blocking oncogenic Ras signaling for cancer therapy. J Natl Cancer Inst. 2001;93:1062–74. doi: 10.1093/jnci/93.14.1062. [DOI] [PubMed] [Google Scholar]
- 64.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;221:153–83. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 65.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–90. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 66.Peyssonnaux C, Eychene A. The Raf/MEK/ERK pathway: new concepts of activation. Biol Cell. 2001;93:53–62. doi: 10.1016/s0248-4900(01)01125-x. [DOI] [PubMed] [Google Scholar]
- 67.Risinger JI, Hayes AK, Berchuck A, Barrett JC. PTEN/MMAC1 mutations in endometrial cancers. Cancer Res. 1997;57:4736–8. [PubMed] [Google Scholar]
- 68.Kong D, Suzuki A, Zou TT, Sakurada A, Kemp LW, Wakatsuki S, Yokoyama T, Yamakawa H, Furukawa T, Sato M, Ohuchi N, Sato S, Yin J, Wang S, Abraham JM, Souza RF, Smolinski KN, Meltzer SJ, Horii A. PTEN1 is frequently mutated in primary endometrial carcinomas. Nat Genet. 1997;17:143–4. doi: 10.1038/ng1097-143. [DOI] [PubMed] [Google Scholar]
- 69.Mutter GI. Pten, a protean tumor suppressor. Am J Pathol. 2001;158:1895–8. doi: 10.1016/S0002-9440(10)64656-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fukuchi T, Sakamoto M, Tsuda H, Maruyama K, Nozawa S, Hirohashi S. Beta-catenin mutation in carcinoma of the uterine endometrium. Cancer Res. 1998;58:3526–8. [PubMed] [Google Scholar]
- 71.Schlosshauer PW, Pirog EC, Levine RL, Ellenson LH. Mutational analysis of the CTNNB1 and A PC genes in uterine endometrioid carcinoma. Mod Pathol. 2000;13:1066–71. doi: 10.1038/modpathol.3880196. [DOI] [PubMed] [Google Scholar]
- 72.Moreno-Bueno G, Gamallo C, Perez-Gallego L, de Mora JC, Suarez A, Palacios J. beta-Catenin expression pattern, beta-catenin gene mutations, and microsatellite instability in endometrioid ovarian carcinomas and synchronous endometrial carcinomas. Diagn Mol Pathol. 2001;10:116–22. doi: 10.1097/00019606-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 73.Wu R, Zhai Y, Fearon ER, Cho KR. Diverse mechanisms of beta-catenin deregulation in ovarian endometrioid adenocarcinomas. Cancer Res. 2001;61:8247–55. [PubMed] [Google Scholar]
- 74.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 75.Morin PJ, Sparks AB, Konnek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catcnin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 76.Burks RT, Kessis TD, Cho KR, Hedrick L. Microsatellite instability in endometrial carcinoma. Oncogene. 1994;9:1163–6. [PubMed] [Google Scholar]
- 77.Duggan BD, Felix JC, Mudeispach LI, Tourgeman D, Zheng J, Shibata D. Microsatellite instability in sporadic endometrial carcinoma. J Natl Cancer Inst. 1994;86:1216–21. doi: 10.1093/jnci/86.16.1216. [DOI] [PubMed] [Google Scholar]
- 78.Mutter GL, Boynton KA, Faquin WC, Ruiz RE, Jovanovic AS. Allelotype mapping of unstable microsatellites establishes direct lineage continuity between endometrial precancers and cancer. Cancer Res. 1996;56:4483–6. [PubMed] [Google Scholar]
- 79.Risinger JI, Berchuck A, Kohler MF, Watson P, Lynch HT, Boyd J. Genetic instability ol microsatellites in endometrial carcinoma. Cancer Res. 1993;53:5100–3. [PubMed] [Google Scholar]
- 80.Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JP, Lees JA, Weng LP, Eng C. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 2000;92:924–30. doi: 10.1093/jnci/92.11.924. [DOI] [PubMed] [Google Scholar]
- 81.Stambolic V, Tsao MS, Maepherson D, Suzuki A, Chapman WB, Mak TW. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/− mice. Cancer Res. 2000;60:3605–11. [PubMed] [Google Scholar]
- 82.Lax SF, Kendall B, Tashiro H, Slebos RJ, Hedrick L. The frequency of p53, K-ras mutations, and microsatcllitc instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer. 2000;88:814–24. [PubMed] [Google Scholar]
- 83.Sasaki H, Nishii H, Takahashi H, Tada A, Furusato M, Terashima Y, Siegal GP, Parker SL, Kohler MF, Berchuck A, et al. Mutation of the Ki-ras protooncogene in human endometrial hyperplasia and carcinoma. Cancer Res. 1993;53:1906–10. [PubMed] [Google Scholar]
- 84.Swisher EM, Peiffer-Schneider S, Mutch DG, Herzog TJ, Rader JS, Elbendary A, Goodfellow PJ. Differences in patterns of TP53 and KKAS2 mutations in a large series of endometrial carcinomas with or without microsatellite instability. Cancer. 1999;85:119–26. doi: 10.1002/(sici)1097-0142(19990101)85:1<119::aid-cncr17>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 85.Mirabelli-Primdahl L, Gryfe R, Kim H, Millar A, Luceri C, Dale U, Holowaty E, Bapal B, Gallinger S, Redston M. Beta-catenin mutations are specific lor colorectal carcinomas with microsatellile instability but occur in endometrial carcinomas irrespective of mutator pathway. Cancer Res. 1999;59:3346–51. [PubMed] [Google Scholar]
- 86.Palacios J, Gamallo C. Mutations in the beta-catenin gene((TNNB1) in endometrioid ovarian carcinomas. Cancer Res. 1998;58:1344–7. [PubMed] [Google Scholar]
- 87.Rubinfeld B, Albert I, Porfiri F, Fiol C, Munemitsu S, Polakis P. Binding of GSK3bela to the APC-beta-catcnin complex and regulation of complex assembly. Science. 1996;272:1023–6. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 88.Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science. 1993;262:1734–7. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- 89.Pijnenborg JM, van de Broek L, Dam de Veen GC, Roemen GM, de Haan J, van Engeland M, Voncken JW, Groothuis PG. TP53 overexpression in recurrent endometrial carcinoma. Gynecol Oncol. 2006;100:397–404. doi: 10.1016/j.ygyno.2005.09.056. [DOI] [PubMed] [Google Scholar]
- 90.Schmitz MJ, Hendricks DT, Farley J, Taylor RR, Geradts J, Rose GS, Birrer MJ. p27 and cyclin D1 abnormalities in uterine papillary serous carcinoma. Gynecol Oncol. 2000;77:439–45. doi: 10.1006/gyno.2000.5814. [DOI] [PubMed] [Google Scholar]
- 91.Soslow RA, Shen PU, Chung MH, Isacson C. Distinctive p53 and mdm2 immunohislochcimcal expression profiles suggest different pathogenetic pathways in poorly differentiated endometrial carcinoma. Int J Gynecol Paihol. 1998;17:129–34. doi: 10.1097/00004347-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 92.Goodfellow PJ, Buttin BM, Herzog TJ, Rader JS, Gibb RK, Swisher E, Look K, Walls KC, Fan MY, Mutch DG. Prevalence of defective DNA mismatch repair and MSH6 mutation in an unselectcd series of endometrial cancers. Proc Natl Acad Sci USA. 2003;100:5908–13. doi: 10.1073/pnas.1030231100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sherman ME, Bur ME, Kurman RJ. p53 in endometrial cancer and its putative precursors: evidence for diverse pathways of tumongenesis. Hum Paihol. 1995;26:1268–74. doi: 10.1016/0046-8177(95)90204-x. [DOI] [PubMed] [Google Scholar]
- 94.Lax SF, Pizer ES, Ronnett BM, Kurman RJ. Clear cell carcinoma of the endometrium is characterized by a distinctive profile of p53, Ki-67, estrogen, and progesterone receptor expression. Hum Pathol. 1998;29:551–8. doi: 10.1016/s0046-8177(98)80002-6. [DOI] [PubMed] [Google Scholar]
- 95.Abramovich D, Markman M, Kennedy A, Webster K, Belinson J. Serum CA-125 as a marker of disease activity in uterine papillary serous carcinoma. J Cancer Res Clin Oncol. 1999;125:697–8. doi: 10.1007/s004320050336. [DOI] [PubMed] [Google Scholar]
- 96.Konecny GE, Santos L, Winterhoff B, Hatmal M, Keeney GL, Mariani A, Jones M, Neuper C, Thomas B, Muderspach L, Richle D, Wang HJ, Dowdy S, Podratz KC, Press MF. HER2 gene amplification and EGFR expression in a large cohort of surgically staged patients with nonendometrioid (type II)endometrial cancer. Br J Cancer. 2009;100:89–95. doi: 10.1038/sj.bjc.6604814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hetzel DJ, Wilson TO, Keeney GL, Roche PC, Cha SS, Podraiz KC. HER-2/neu expression: a major prognostie factor in endometrial cancer. Gynecol Oncol. 1992;47:179–85. doi: 10.1016/0090-8258(92)90103-p. [DOI] [PubMed] [Google Scholar]
- 98.Morrison C, Zanagnolo V, Ramirez N, Cohn DE, Kelbick N, Copeland L, Maxwell GL, Fowler JM. HER-2 is an independent prognostic factor in endometrial cancer: association with outcome in a large cohort of surgically staged patients. J Clin Oncol. 2006;24:2376–85. doi: 10.1200/JCO.2005.03.4827. [DOI] [PubMed] [Google Scholar]
- 99.Ries L, Eisner M, Kosary C, Hankey B, Miller B, Clegg L, Mariorto A, Feucr E, Edwards B. SEER Cancer Statistics Review. 2005:1975–2002. [Google Scholar]
- 100.Baak JP, Mutter GL, Robboy S, van Dicst PJ, Uyterlinde AM, Orbo A, Palazzo J, Fiane B, Lovslett K, Burger C, Voorhorst F, Verheijen RH. The molecular genetics and morphometry-based endometrial intraepithelial neoplasia classification system predicts disease progression in endometrial hyperplasia more accurately than the 1994 World Health Organization classification system. Cancer. 2005;103:2304–12. doi: 10.1002/cncr.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mutter GL, Baak JP, Crum CP, Richart RM, Ferenczy A, Faquin WC. Endometrial precancer diagnosis by histopaihology, clonal analysis, and computerized morphometry. J Pathol. 2000;190:462–9. doi: 10.1002/(SICI)1096-9896(200003)190:4<462::AID-PATH590>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 102.Esteller M, Catasus L, Matias-Guiu X, Mutter GL, Prat J, Baylin SB, Herman JG. hMLH1 promoter hypcrmethylation is an early event in human endometrial tumorigcncsis. Am J Paihol. 1999;155:1767–72. doi: 10.1016/S0002-9440(10)65492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mutter GL, Zaino RJ, Baak JP, Bentley RC, Robboy SJ. Benign endometrial hyperplasia sequence and endometrial intraepithelial neoplasia. Int J Gynecol Pathol. 2007;26:103–14. doi: 10.1097/PGP.0b013e31802e4696. [DOI] [PubMed] [Google Scholar]
- 104.Lin M, Burkholder KA, Viswarnathan AN, Neuberg D, Mutter GL. Involution of latent endometrial precancers by hormonal and non hormonal mechanisms. (In press) [DOI] [PMC free article] [PubMed]
- 105.Grimes DA, Economy KE. Primary prevention of gynecologic cancers. Am J Obsiel Gynecol. 1995;172:227–35. doi: 10.1016/0002-9378(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 106.Weiderpass E, Adami HO, Baron JA, Magnusson C, Lindgren A, Persson I. Use of oral contraceptives and endometrial cancer risk (Sweden) Cancer Causes Control. 1999;10:277–84. doi: 10.1023/a:1008945721786. [DOI] [PubMed] [Google Scholar]
- 107.Sherman ME, Bitterman P, Rosenshein NB, Delgado G, Kurman RJ. Uterine serous carcinoma. A morphologically diverse neoplasm with unifying clinicopathologic features. Am J Surg Paihol. 1992;16:600–10. doi: 10.1097/00000478-199206000-00008. [DOI] [PubMed] [Google Scholar]
- 108.Silverberg S, Mutter G, Kurman R, Kubik-Huch R, Negales F, Tavassoli F. Tumors of the uterine corpus: epithelial tumors and related lesions. In: Tavassoli F, Stratton M, editors. WHO Classification of tumors: Pathology and Genetics of Tumors of the Breast and Pemale Genital Organs. IARC Press; Lyon, France: 2003. pp. 221–232. [Google Scholar]
- 109.Zheng W, Liang SX, Yu H, Rutherford T, Chambers SK, Schwartz PE. Endometrial glandular dysplasia: a newly defined precursor lesion of uterine papillary serous carcinoma Part 1: morphologic features. Int J Surg Pathol. 2004;12:207–23. doi: 10.1177/106689690401200302. [DOI] [PubMed] [Google Scholar]
- 110.Amhros RA, Sherman MF, Zaim CM, Bmeiman P, Kurman RJ. Endometrial intraepithelial carcinoma: a distinctive lesion specifically associated with tumors displaying serous differentiation. Hum Paihol. 1995;26:1260–7. doi: 10.1016/0046-8177(95)90203-1. [DOI] [PubMed] [Google Scholar]
- 111.Zheng W, Khurana R, Farahmand S, Wang Y, Zhang ZF, Felix JC. p53 immunostaining as a significant adjunct diagnostic method for uterine surface carcinoma: precursor of uterine papillary serous carcinoma. Am J Surg Pathol. 1998;22:1463–73. doi: 10.1097/00000478-199812000-00003. [DOI] [PubMed] [Google Scholar]
- 112.Wheeler DT, Bell KA, Kurman RJ, Sherman ME. Minimal uterine serous carcinoma: diagnosis and clinicopathologic correlation. Am J Surg Paihol. 2000;24:797–806. doi: 10.1097/00000478-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 113.Liang SX, Chambers SK, Cheng L, Zhang S, Zhou Y, Zheng W. Endometrial glandular dysplasia: a putative precursor lesion of uterine papillary serous carcinoma Part II: moleculai features. Int J Surg Pathol. 2004;12:319–31. doi: 10.1177/106689690401200405. [DOI] [PubMed] [Google Scholar]
- 114.Sieben NL, Macropoulos P, Roemen CM, Kolkman-Uljee SM, Jan Fleuren G, Houmadi R, Diss T, Warren B, Al Adnani M, De Goeij AP, Krausz T, Flanagan AM. In ovarian neoplasms, BRAF, but not KRAS, mutations are restricted to low-grade serous tumours. J Pathol. 2004;202:336–40. doi: 10.1002/path.1521. [DOI] [PubMed] [Google Scholar]
- 115.Singer G, Oldt R, 3rd., Cohen Y, Wang BG, Sidransky D, Kurman RJ, Shih Ie M. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95:484–6. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- 116.Meinhold-Heerlein I, Bauerschlag D, Hilpert F, Dimitrov P, Sapinoso LM, Orlowska-Volk M, Bauknecht T, Park TW, Jonat W, Jacohsen A, Sehouli J, Luttges J, Krajewski M, Krajewski S, Reed JC, Arnold N, Hampton GM. Molecular and prognostic distinction between serous ovarian carcinomas of varying grade and malignant potential. Oncogene. 2005;24:1053–65. doi: 10.1038/sj.onc.1208298. [DOI] [PubMed] [Google Scholar]
- 117.Staebler A, Heselmeycr-Haddad K, Bell K, Riopel M, Perlman E, Ried T, Kurman RJ. Micropapillary serous carcinoma of the ovary has distinct patterns of chromosomal imbalances by comparative genomic hybridization compared with atypical proliferative serous tumors and serous carcinomas. Hum Pathol. 2002;33:47–59. doi: 10.1053/hupa.2002.30212. [DOI] [PubMed] [Google Scholar]
- 118.Allen HJ, DiCioccio RA, Hohmann P, Piver MS, Tworek H. Microsalellite instability in ovarian and other pelvic carcinomas. Cancer Genet Cytogenet. 2000;117:163–6. doi: 10.1016/s0165-4608(99)00167-3. [DOI] [PubMed] [Google Scholar]
- 119.Kmet IM, Cook IS, Magliocco AM. A review of p53 expression and mutation in human benign, low malignant potential, and invasive epithelial ovarian tumors. Cancer. 2003;97:389–404. doi: 10.1002/cncr.11064. [DOI] [PubMed] [Google Scholar]
- 120.Ross JS, Yang F, Kallakury BV, Sheehan CE, Ambros RA, Muraca PJ. HER-2/neu oncogene amplification by fluorescence in situ hybridization in epithelial tumors of the ovary. Am J Clin Pathol. 1999;111:311–6. doi: 10.1093/ajcp/111.3.311. [DOI] [PubMed] [Google Scholar]
- 121.Afify AM, Werness BA, Mark HF. HER-2/neu oncogene amplification in stage I and stage III ovarian papillary serous carcinoma. Exp Mol Pathol. 1999;66:163–9. doi: 10.1006/exmp.1999.2255. [DOI] [PubMed] [Google Scholar]
- 122.Nakayama K, Nakayama N, Kurman RJ, Cope L, Pohl G, Samuels Y, Velculescu VE, Wang TL, Shih Ie M. Sequence Mutations and Amplification of PIK3CA and AKT2 Genes in Purified Ovaiian Seious Neoplasms. Cancer Biol Ther. 2006;5:779–85. doi: 10.4161/cbt.5.7.2751. [DOI] [PubMed] [Google Scholar]
- 123.Nakayama K, Nakayama N, Jinawath N, Salani R, Kurman RJ, Shih Ie M, Wang TL. Amplicon profiles in ovarian serous carcinomas. Int J Cancer. 2007;120:2613–7. doi: 10.1002/ijc.22609. [DOI] [PubMed] [Google Scholar]
- 124.Garzetti GG, Ciavatlini A, Goteri G, De Nictolis M, Stramazzotti D, Lucarini G, Biagini G. Ki67 antigen immunostaining (MIB 1 monoclonal antibody) in serous ovarian tumors: index of proliferative activity with prognostic significance. Gynecol Oncol. 1995;56:169–74. doi: 10.1006/gyno.1995.1026. [DOI] [PubMed] [Google Scholar]
- 125.Shih L, Kurman RJ. Molecular pathogenesis of ovarian borderline tumors: new insights and old challenges. Clin Cancer Res. 2005;11:7273–9. doi: 10.1158/1078-0432.CCR-05-0755. [DOI] [PubMed] [Google Scholar]
- 126.Bell DA, Scully RE. Early de novo ovarian carcinoma. Cancer. 1994;73:1859–1864. doi: 10.1002/1097-0142(19940401)73:7<1859::aid-cncr2820730714>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 127.Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, Lee Y. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- 128.Jarboe EA, Folkins AK, Drapkin R, Ince TA, Agoston LS, Crum CP. Tubal and ovarian pathways to pelvic epithelial cancer: a pathological perspective. Histopathology. 2008;53:127–38. doi: 10.1111/j.1365-2559.2007.02938.x. [DOI] [PubMed] [Google Scholar]
- 129.Gemignani ML, Schlaerth AC, Bogomolniy F, Barakat RR, Lin O, Soslow R, Venkatraman E, Boyd J. Role of KRAS and BRAF gene mutations in mucinous ovarian carcinoma. Gynecol Oncol. 2003;90:578–81. doi: 10.1016/s0090-8258(03)00264-6. [DOI] [PubMed] [Google Scholar]
- 130.Ichikawa Y, Nishida M, Suzuki H, Yoshida S, Tsunoda H, Kubo T, Uchida K, Miwa M. Mutation of K-ras protooncogene is associated with histological subtypes in human mucinous ovarian tumors. Cancer Res. 1994;54:33–5. [PubMed] [Google Scholar]
- 131.Muk SC, Bell DA, Knapp RC, Fishbaugh PM, Welch WR, Muto MG, Berkowitz RS, Tsao SW. Mulaiion of K-ras protooncogene in human ovarian epithelial tumors of borderline malignancy. Cancer Res. 1993;53:1489–92. [PubMed] [Google Scholar]
- 132.Cuatrecasas M, Villanueva A, Matias-Guiu X, Prat J. K-ras mutations in mucinous ovarian tumors a chnieopathologic and molecular study of 95 cases. Cancer. 1997;79:1581–6. doi: 10.1002/(sici)1097-0142(19970415)79:8<1581::aid-cncr21>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 133.Mandai M, Komshi I, Kuroda H, Komatsu T, Yamamoto S, Nanbu K, Matsushita K, Fukumolo M, Yamabe H, Mori T. Heterogeneous distribution of K-ras-mutated epithelia in mucinous ovarian tumors with special reference to hislopalhology. Hum Pathol. 1998;19:34–40. doi: 10.1016/s0046-8177(98)90387-2. [DOI] [PubMed] [Google Scholar]
- 134.Litkouhi B, Litkouhi B, Fleming E, Welch WR, Berkowitz RS, Birrer MJ, Mok SC. Overexpression of CLACAM6 in borderline and invasive mucinous ovarian neoplasms. Gynecol Oncol. 2008;109:234–9. doi: 10.1016/j.ygyno.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 135.Shih YC, Kerr J, Hurst TG, Khoo SK, Ward BG, Chenevix-Treneh G. No evidence for microsatellue instability from allelotype analysis of benign and low malignant potential ovarian neoplasms. Gynecol Oncol. 1998;69:210–3. doi: 10.1006/gyno.1998.5014. [DOI] [PubMed] [Google Scholar]
- 136.Jordan S, Green A, Webb P. Benign Epithelial Ovarian Tumours-cancer Precursors or Markers for Ovarian Cancer Risk? Cancer Causes Control. 2006;17:623–32. doi: 10.1007/s10552-005-0370-y. [DOI] [PubMed] [Google Scholar]
- 137.Scully RE. Early denovo ovarian cancer and cancer developing in benign ovarian lesions. International Journal of Gynaecology and Obstetrics. 1995;49:S9–15. doi: 10.1016/0020-7292(95)02404-z. [DOI] [PubMed] [Google Scholar]
- 138.Garrett AP, Lee KR, Colitti CR, Muto MG, Bcrkowiiz RS, Mok SC. k-ras mutation may be an early eveni in mucinous ovarian tumorigencsis. Int J Gynecol Pathol. 2001;20:244–51. doi: 10.1097/00004347-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 139.Dubcau L. The cell of origin of ovarian epithelial tumors and the ovarian surface epithelium dogma: does the emperor have no Clothes? Gynecol Oncol. 1999;72:437–42. doi: 10.1006/gyno.1998.5275. [DOI] [PubMed] [Google Scholar]
- 140.Fujita M, Enomolo T, Yoshino K, Nomura T, Buzard GS, Inoue M, Okudaira Y. Microsatellite instability and alterations in the hMSH2 gene in human ovanan cancer. Int J Cancer. 1995;64:361–6. doi: 10.1002/ijc.2910640602. [DOI] [PubMed] [Google Scholar]
- 141.Gras E, Catasus I, Arguelles R, Moreno-Bueno G, Palacios J, Gamallo C, Matias-Guiu X, Prat J. Microsatellite instability, MLH-I promoter hypermethylalion, and frameshift mutations at coding mononucleotide repeat microsatcllitcs in ovarian tumors. Cancer. 2001;92:2829–36. doi: 10.1002/1097-0142(20011201)92:11<2829::aid-cncr10094>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 142.Obata K, Morland SJ, Watson RH, Hitchcock A, Chenevix-Trench G, Thomas EJ, Campbell IG. Frequent PTEN/MMAC mutations in endometrioid but not serous or mucinous epithelial ovarian tumors. Cancer Res. 1998;58:2095–7. [PubMed] [Google Scholar]
- 143.Cuatrecasas M, Erill N, Musulen E, Costa I, Matias-Guiu X, Prat J. K-ras mutations in nonmucinous ovarian epithelial tumors: a molecular analysis and clinicopathologic study of 144 patients. Cancer. 1998;82:1088–95. [PubMed] [Google Scholar]
- 144.Tan DS, Kaye S. Ovarian clear cell adenocarcinoma a continuing enigma. J Clin Paihol. 2007;60:355–60. doi: 10.1136/jcp.2006.040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sato N, Tsunoda H, Nishida M, Morishita Y, Takimoto Y, Kubo T, Noguchi M. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene P'FEN in benign endometrial cyst of the ovary possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000;60:7052–6. [PubMed] [Google Scholar]
- 146.Hashiguchi Y, Tsuda H, Inoue T, Berkowitz RS, Mok SC. PTEN expression in clear cell adenocarcinoma of the ovary. Gynecol Oncol. 2006;101:71–5. doi: 10.1016/j.ygyno.2005.09.047. [DOI] [PubMed] [Google Scholar]
- 147.Francis-Thickpenny KM, Richardson DM, van Ee CC, Love DR, Winship IM, Baguley BC, Chenevix-Trench G, Shelling AN. Analysis of the TGF beta functional pathway in epithelial ovarian carcinoma. Br J Cancer. 2001;85:687–91. doi: 10.1054/bjoc.2001.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tsuchiya A, Sakamoto M, Yasuda J, Chuma M, Ohta T, Ohki M, Yasugi T, Taketani Y, Hirohashi S. Expression profiling in ovarian clear cell carcinoma: identification of hepatoeyte nuclear factor-1 beta as a molecular marker and a possible molecular target for therapy of ovarian clear cell carcinoma. Am J Pathol. 2003;163:2503–12. doi: 10.1016/s0002-9440(10)63605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kato N, Sasou S, Moloyama T. Expression of hepatoeyte nuclear factor-1beta (HNF-1beta) in clear cell tumors and endometriosis of the ovary. Mod Pathol. 2006;19:83–9. doi: 10.1038/modpathol.3800492. [DOI] [PubMed] [Google Scholar]
- 150.Ness RB. Endometriosis and ovarian cancer thoughts on shared pathophysiology. Am J Obstet Gynecol. 2003;189:280–94. doi: 10.1067/mob.2003.408. [DOI] [PubMed] [Google Scholar]
- 151.Somigliana E, Vigano P, Parazzini F, Stoppelli S, Giambainsta E, Vercellini P. Association between endometriosis and cancer: A comprehensive review and a critical analysis of clinical and epidemiological evidence. Gynecol Oncol. 2006;101:331–41. doi: 10.1016/j.ygyno.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 152.Thomas EJ, Campbell IG. Molecular genetic defects in endometriosis. Gynecol Obstet Invest. 2000;50(Suppl 1):44–50. doi: 10.1159/000052878. [DOI] [PubMed] [Google Scholar]
- 153.Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11:63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 154.Wu Y, Halverson G, Basir Z, Strawn E, Yan P, Guo SW. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am J Obstet Gynecol. 2005;193:371–80. doi: 10.1016/j.ajog.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 155.Zheng W, Li N, Wang J, Uliikus EC, Uliikus M, Arici A, Liang SX. Initial endometriosis showing direct morphologic evidence of metaplasia in the pathogenesis of ovarian endometriosis. Int J Gynecol Pathol. 2005;24:164–72. doi: 10.1097/01.rct.0000157091.37057.b4. [DOI] [PubMed] [Google Scholar]
- 156.Callahan MJ, Crum CP, Medeiros F, Kindelbcrger DW, Elvin JA, Garber JE, Feltmate CM, Berkowitz RS, Muto MG. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol. 2007;25:3985–90. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- 157.Lee Y, Medeiros F, Kindelberger D, Callahan MJ, Muto MG, Crum CP. Advances in the recognition of tubal intraepithelial carcinoma: applications to cancer screening and the pathogenesis of ovarian cancer. Adv Anat Pathol. 2006;13:1–7. doi: 10.1097/01.pap.0000201826.46978.e5. [DOI] [PubMed] [Google Scholar]
- 158.Marquez RT, Baggerly KA, Patterson AP, Liu J, Broaddus R, Frumovitz M, Atkinson EN, Smith DI, Hartmann L, Fishman D, Berchuck A, Whitaker R, Gershenson DM, Mills GB, Bast RC, Jr., Lu KH. Patterns of gene expression in different histoiypes of epithelial ovarian cancer correlate with those in normal fillopian tube, endometrium, and colon. Clin Cancer Res. 2005;11:6116–26. doi: 10.1158/1078-0432.CCR-04-2509. [DOI] [PubMed] [Google Scholar]
- 159.Jordan SJ, Green AC, Whiteman DC, Moore SP, Bain CJ, Gertig DM, Webb PM. Serous ovarian, fallopian tube and primary peritoneal cancers: a comparative epidemiological analysis. Int J Cancer. 2008;122:1598–603. doi: 10.1002/ijc.23287. [DOI] [PubMed] [Google Scholar]
- 160.Zom KK, Bonome T, Gangi L, Chandramouii GV, Awtrey CS, Gardner GJ, Barrett JC, Boyd J, Birrer MJ. Gene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancer. Clin Cancer Res. 2005;11:6422–30. doi: 10.1158/1078-0432.CCR-05-0508. [DOI] [PubMed] [Google Scholar]
- 161.Ferlay J, Bray F, Pisani P, Parkin D. Globocan 2002. 2009, Cancer Incidence, Mortality and Prevalence Worldwide. 2004.
- 162.Brinton LA, Berman ML, Monel R, Twiggs LB, Barrett RJ, Wilbanks GD, Lannnm L, Hoover RN. Reproductive, menstrual, and medical risk factors for endometrial cancer: results from a case-control study. Am J Obstel Gynecol. 1992;167:1317–25. doi: 10.1016/s0002-9378(11)91709-8. [DOI] [PubMed] [Google Scholar]
- 163.Jordan SJ, Webb PM, Green AC. Height, age at mcnarche, and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2045–8. doi: 10.1158/1055-9965.EPI-05-0085. [DOI] [PubMed] [Google Scholar]
- 164.Titus-Emstoff L, Perez K, Cramer DW, Harlow BL, Baron JA, Greenberg ER. Menstrual and reproductive factors in relation to ovarian cancer risk. Br J Cancer. 2001;84:714–21. doi: 10.1054/bjoc.2000.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Henderson BE, Casagrande JT, Pike MC, Mack T, Rosario I, Duke A. The epidemiology of endometrial cancer in young women. Br J Cancer. 1983;47:749–56. doi: 10.1038/bjc.1983.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Henderson BF, Ross R, Bernstein L. Estrogens as a cause of human cancer: the Richard and Hmda Rosenthal Foundation award lecture. Cancer Res. 1988;48:246–53. [PubMed] [Google Scholar]
- 167.Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. II. Invasive epithelial ovarian cancers in white women. Collaborative Ovarian Cancer Group. Am J Epidemiol. 1992;136:1184–203. doi: 10.1093/oxfordjournals.aje.a116427. [DOI] [PubMed] [Google Scholar]
- 168.Kvale G, Heuch I, Eide GE. A prospective study of reproductive factors and breast cancer I. Parity. Am J Epidemiol. 1987;126:831–41. doi: 10.1093/oxfordjournals.aje.a114720. [DOI] [PubMed] [Google Scholar]
- 169.Lambe M, Wuu J, Weiderpass E, Hsieh CC. Childbearing at older age and endometrial cancer risk (Sweden) Cancer Causes Control. 1999;10:43–9. doi: 10.1023/a:1008860615584. [DOI] [PubMed] [Google Scholar]
- 170.Newcomb PA, Trentham-Dietz A. Breast feeding practices in relation to endometrial cancer risk. USA. Cancer Comes Control. 2000;11:663–7. doi: 10.1023/a:1008978624266. [DOI] [PubMed] [Google Scholar]
- 171.Okamura C, Tsubono Y, Ito K, Niikura H, Takano T, Nagasc S, Yoshinaga K, Tcrada Y, Murakami T, Sato S, Aoki D, Jobo T, Okamura K, Yaegashi N. Lactation and risk of endometrial cancer in Japan: a case-control study. Tohoku J Exp Med. 2006;208:109–15. doi: 10.1620/tjem.208.109. [DOI] [PubMed] [Google Scholar]
- 172.Rosenblatt KA, Thomas DB. Prolonged lactation and endometrial cancer. WHO Collaborative Study of Neoplasia and Steroid Contraceptives. Int J Epidemiol. 1995;24:499–503. doi: 10.1093/ije/24.3.499. [DOI] [PubMed] [Google Scholar]
- 173.Salazar-Maninez E, Lazeano-Ponce EC, Gonzalez Lira-Lira G, Escudero-De los Rios P, Salmeron-Castro J, Hernandez-Avila M. Reproductive factors of ovarian and endometrial cancer risk in a high fertility population in Mexico. Cancer Pes. 1999;59:3658–62. [PubMed] [Google Scholar]
- 174.Siskind V, Green A, Bain C, Purdie D. Breastfeeding, menopause, and epithelial ovarian cancer. Epidemiology. 1997;8:188–91. doi: 10.1097/00001648-199703000-00011. [DOI] [PubMed] [Google Scholar]
- 175.La Vecchia C. Oral contraceptives and ovarian cancer: an update. 1998-2004. Eur J Cancer Prev. 2006;15:117–24. doi: 10.1097/01.cej.0000179274.24200.9d. [DOI] [PubMed] [Google Scholar]
- 176.Maxwell GL, Schildkraul JM, Calmgaert B, Risinger JI, Dainty L, Marchbanks PA, Berchuck A, Barrett JC, Rodriguez GC. Progestin and estrogen potency of combination oral contraceptives and endometrial cancer risk. Gynecol Oncol. 2006;103:535–40. doi: 10.1016/j.ygyno.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 177.Schlesselman JJ. Risk of endometrial cancer in relation to use of combined oral contraceptives. A practitioner's guide to meta-analysis. Hum Reprod. 1997;12:1851–63. doi: 10.1093/humrep/12.9.1851. [DOI] [PubMed] [Google Scholar]
- 178.Silverberg SG, Makowski EL. Endometrial carcinoma in young women taking oral contraceptive agents. Obstet Gynecol. 1975;46:503–6. [PubMed] [Google Scholar]
- 179.Casagrande JT, Louie EW, Pike MC, Roy S, Ross RK, Henderson BE. “Incessant ovulation” and ovarian cancer. Lancer. 1979;2:170–3. doi: 10.1016/s0140-6736(79)91435-1. [DOI] [PubMed] [Google Scholar]
- 180.Purdie DM, Bain CJ, Siskind V, Webb PM, Green AC. Ovulation and risk of epithelial ovarian cancer. Int J Cancer. 2003;104:228–232. doi: 10.1002/ijc.10927. [DOI] [PubMed] [Google Scholar]
- 181.Beral V, Bull D, Reeves G. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2005;365:1543–51. doi: 10.1016/S0140-6736(05)66455-0. [DOI] [PubMed] [Google Scholar]
- 182.Garg PP, Kerlikowske K, Subak L, Grady D. Hormone replacement therapy and the risk of epithelial ovarian carcinoma: a meta-analysis. Obstet Gynecol. 1998;92:472–9. doi: 10.1016/s0029-7844(98)00139-2. [DOI] [PubMed] [Google Scholar]
- 183.Grady D, Gebreisadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol. 1995;85:304–13. doi: 10.1016/0029-7844(94)00383-O. [DOI] [PubMed] [Google Scholar]
- 184.Rodriguez C, Patel AV, Calle EM, Jacob EJ, Thun MJ. Estrogen replacement therapy and ovarian cancer mortality in a large prospective study of US women. Jama. 2001;285:1460–5. doi: 10.1001/jama.285.11.1460. [DOI] [PubMed] [Google Scholar]
- 185.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 186.Pearce CL, Chung K, Pike MC, Wu AH. Increased ovarian Cancel risk associated with menopausal estrogen therapy is leduced by adding a progestin. Cancer. 2009;115:531–9. doi: 10.1002/cncr.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kuper H, Cramer DW, Titus-Ernstoff L. Risk of ovarian cancer in the United States in relation to anthropometric measures: does the association depend on menopausal status? Cancer Causes Control. 2002;13:455–63. doi: 10.1023/a:1015751105039. [DOI] [PubMed] [Google Scholar]
- 188.Weiderpass E, Persson I, Adami HO, Magnusson C, Lindgren A, Baron JA. Body size in different periods of life, diabetes mellitus, hypertension, and risk of postmenopausal endometrial cancer (Sweden) Cancer Causes Control. 2000;11:185–92. doi: 10.1023/a:1008946825313. [DOI] [PubMed] [Google Scholar]
- 189.Nelson HD, Huffman LH, Fu R, Harris EL. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: systematic evidence review for the U.S. Preventive Services Task Eorce. Ann Intern Med. 2005;143:362–79. doi: 10.7326/0003-4819-143-5-200509060-00012. [DOI] [PubMed] [Google Scholar]
- 190.Brinton LA, Barrett RJ, Berman ML, Mortel R, Twiggs LB, Wilbanks GD. Cigarette smoking and the risk of endometrial cancer. Am J Epidemiol. 1993;137:281–91. doi: 10.1093/oxfordjournals.aje.a116675. [DOI] [PubMed] [Google Scholar]
- 191.Pan SY, Ugnat AM, Mao Y, Wen SW, Johnson KC. Association of cigarette smoking with the risk of ovarian cancer. Int J Cancer. 2004;111:124–30. doi: 10.1002/ijc.20242. [DOI] [PubMed] [Google Scholar]
- 192.Rossing MA, Cushing-Haugen KL, Wicklund KG, Weiss NS. Cigarette smoking and risk of epithelial ovarian cancer. Cancer Causes Control. 2008;19:413–20. doi: 10.1007/s10552-007-9103-8. [DOI] [PubMed] [Google Scholar]
- 193.Zhang Y, Coogan PF, Palmer JR, Strom BL, Rosenberg L. Cigarette smoking and increased risk of mucinous epithelial ovarian cancer. Am J Epidemiol. 2004;159:133–9. doi: 10.1093/aje/kwh015. [DOI] [PubMed] [Google Scholar]
- 194.Kreiger N, Sloan M, Cotterchio M, Parsons P. Surgical procedures associated with risk of ovarian cancer. Int J Epidemiol. 1997;26:710–5. doi: 10.1093/ije/26.4.710. [DOI] [PubMed] [Google Scholar]
- 195.Cramer DW, Titus-Emstoff L, McKolanis JR, Welch WR, Vitonis AF, Berkowitz RS, Finn OJ. Conditions associated with antibodies against the tumor-associated antigen MUC1 and their relationship to risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1125–31. doi: 10.1158/1055-9965.EPI-05-0035. [DOI] [PubMed] [Google Scholar]
- 196.Hubacher D, Grimes DA. Norcomraceptive health benefits of intrauterine devices a systematic review. Obstet Gynecol Surv. 2002;57:120–8. doi: 10.1097/00006254-200202000-00024. [DOI] [PubMed] [Google Scholar]
- 197.Oliva E, Clement P, Young R. Pathology of sarcomas and mixed mullerian tumors of the uterine corpus. In: Fuller A, Sciden M, Young R, editors. Uterine Cancer. BC Decker Inc.; 2004. [Google Scholar]
- 198.Young R, Clement P. Pathology of Endometrial Carcinoma. In: Fuller A, Seiden M, Young R, editors. Uterine Cancer. BC Decker Inc.; 2004. [Google Scholar]
- 199.Ries L, Melben D, Krapcho M, Stinchcomb D, Howlader N, Homer M, Mariotto A, Feucr E, Altedruse S, ewis DL, Clegg L, Eisner M, Reichman M, Edwards B. SEER Cancer Statistics Review. 2008:1975–2005. [Google Scholar]