Abstract
Many species of African nonhuman primates are naturally infected with Simian Immunodeficiency Viruses (SIVs) in the wild and in captivity. In contrast to HIV-infected humans, these natural SIV hosts typically do not develop AIDS despite chronic infection with a highly replicating virus. In this review, we will discuss the most recent advances on the mechanisms of protection from disease progression in natural SIV hosts, with emphasis on how they differ from pathogenic HIV/SIV infections of humans and rhesus macaques. These mechanisms include: i) resolution of immune activation following acute infection; ii) restricted pattern of target cell infection; and iii) protection from mother-to-infant transmission. We highlight the areas that should be pursued in future studies, focusing on potential applications for the treatment and prevention of HIV infection.
1. Introduction
Over thousands of years, species-specific strains of simian immunodeficiency virus (SIV) have endemically infected over 40 species of African nonhuman primates (1, 2) that we will refer to as "natural SIV hosts" or, simply, “natural hosts” (Box 1). Multiple cross-species transmissions of SIVcpz from chimpanzees and SIVsmm from sooty mangabeys (SMs) to humans have resulted in the current epidemics of HIV-1 and HIV-2, respectively (3). SIVsmm is also the origin of the SIVmac strains that are used to experimentally infect various species of Asian macaques, resulting in a disease called simian AIDS (4). In striking contrast to pathogenic HIV infection of humans and SIVmac infection of rhesus macaques (RMs), SIV infections of natural hosts are typically nonpathogenic despite high levels of virus replication (5–9). The only exception is the SIVcpz infection of chimpanzees that is associated with a significant increase in mortality, although not to the levels seen in HIV-1 or SIVmac infections (10). Importantly, the benign infection of natural hosts is distinct from the nonprogressive HIV/SIV infections of a rare subset of humans and RMs, called Elite Controllers (EC), in which the absence of AIDS is at least in part related to the ability of the immune system to suppress virus replication (11, 12). Understanding the mechanisms responsible for the AIDS resistance of natural SIV hosts is considered a key priority in contemporary AIDS research, with major implications for HIV prevention and therapy.
Box 1.
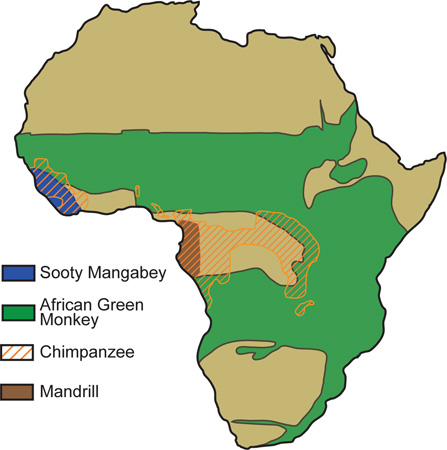
Over 40 species of African monkeys are endemically infected with a speciesspecific strain of simian immunodeficiency virus (SIV). Multiple cross-species transmissions of SIV from chimpanzees to humans during the preparation of bush-meat, aided by the rise of urbanization in early 20th century Africa, has resulted in the HIV-1 epidemic and all of its subtypes. High rates of mutation and replication as well as recombination have fueled the success of these and other zoonotic events resulting in the generation of the pathogenic lentiviruses, HIV-1, HIV-2, and SIVmac in their respective hosts. Geographic range of selected natural hosts is shown. Map adapted from B. Jacquelin et al., from Models of Protection Against HIV/SIV: Avoiding AIDS in Humans and Monkeys.
2. What is the phenotype of SIV infection in natural hosts?
Two species of natural SIV hosts that have been intensively studied as captive animals, SMs and the African green monkeys (AGMs), are housed in primate centers in the United States and Europe. Limited information is available about other species such as mandrills, drills, suntailed monkeys, and a few others. In this review we will mainly discuss data generated from SMs and AGMs because relatively little is known about the phenotype of SIV infection in other natural SIV host species. Given the many similarities of these two models we will discuss them together unless noted otherwise in specific instances. Table 1 shows the main virological and immunological aspects of pathogenic and nonpathogenic primate lentivirus infections. In summary, key similarities between SIV infection of natural hosts and pathogenic HIV/SIV infections of humans and RMs include: (i) high viremia (5–9); (ii) short in vivo lifespan of productively infected cells (13, 14); (iii) significant loss of mucosal CD4+ T cells during acute infection (15, 16); (iv) high levels of innate and adaptive immune activation during acute infection (17–21); and (v) the inability of the host cellular and humoral immune system to control virus replication (22–24). This last observation is of great theoretical and practical importance, because it shows that the AIDS-resistance of natural SIV hosts is independent of adaptive antiviral immune responses that suppress virus replication. This feature of natural SIV infection highlights the tremendous challenge of artificially inducing, with an AIDS vaccine, a type of protective immunity that has not been selected for in many thousands of years of evolutionary pressure posed by lentiviruses on the nonhuman primate immune system.
Table 1.
Main Features of SIV Infection of Natural Hosts vs. Non-Natural Hosts.
| Natural Host (SMs, AGMs) | Non-Natural Host (RMs, humans) | |
|---|---|---|
| No | AIDS | Yes |
| Healthy | Level of Peripheral CD4+ T cells | Low |
| High | Viral Load | High |
| Yes | Virus Cytopathicity | Yes |
| Ineffective | Host Immune Control | Ineffective |
| Yes, stable | Depletion of Mucosal CD4+ T cells | Yes, progressive |
| No | Mucosal Immune Dysfunction /Microbial Translocation | Yes |
| No | Chronic Immune Activation | Yes |
| Tem > Tcm | Pattern of Infected Cells | Tcm > Tem |
| Rare | Vertical Transmission | Frequent |
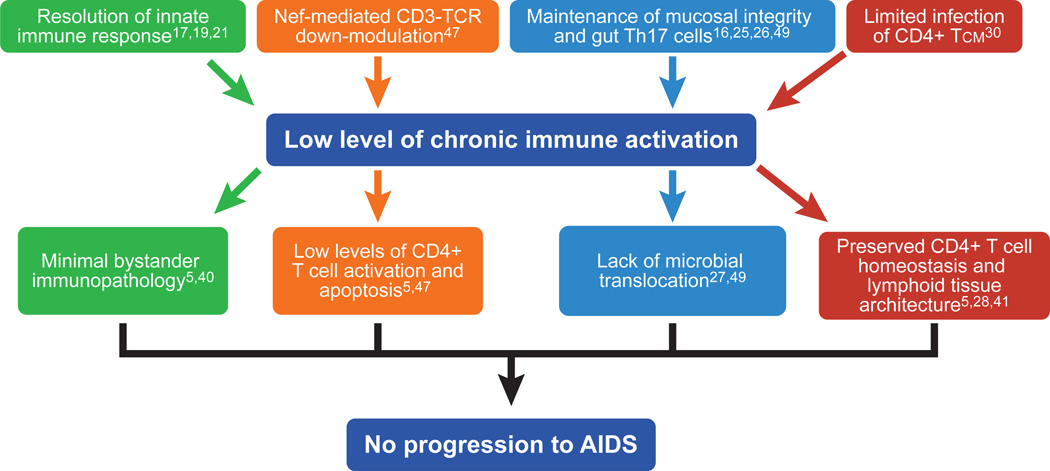
Key features of SIV infection that appear specific to natural hosts include: (i) preservation of healthy levels of peripheral CD4+ T cells (5); (ii) preservation of mucosal immunity, with normal levels of intestinal cells and absence of microbial translocation (16, 25–27); (iii) normal lymph node architecture and function (5); (iv) preservation of T cell regeneration (28); (v) preferential sparing of central memory CD4+ T cells from direct virus infection (29, 30), and (vi) lack of chronic immune activation (5, 6, 31–33). The viral and host factors that contribute to lack of chronic immune activation and their contribution to the benign phenotype of natural SIV infection are depicted in Figure 1. Another fascinating characteristic of natural SIV infections is the rarity of mother-to-infant-transmission (MTIT) as compared to pathogenic HIV/SIV infections (34–36). In this review we will discuss three key aspects of natural nonhuman primate lentiviral infections that have recently emerged as important correlates and likely determinants of the benign nature of the epidemics in these monkey species: (i) the rapid resolution of acute immune activation; (ii) the restricted pattern of target cells; and (iii) the protection from MTIT. These and other proposed mechanisms of nonpathogenic infection of natural SIV hosts are shown in Table 2.
Figure 1. Mechanisms responsible for the low immune activation and lack of disease progression in SIV-infected natural hosts.
The main immunological and virological features contributing to the lack of chronic immune activation and their key consequences resulting in protection from AIDS in natural SIV hosts are depicted.
Table 2.
Proposed Mechanisms for the Nonpathogenic Phenotype of SIV Infection of Natural Hosts
| Hypothesis | Evidence | Conclusion | |
|---|---|---|---|
| SIV strains infecting natural hosts are incapable of causing AIDS due to attentuation of cytopathicity. | Non-pathogenicity of natural host infection is not due to viral attenuation. | Hypothesis Not Supported | |
| Specific properties of SIV accessory proteins result in reduced pathogenesis in natural hosts. |
|
Coevolution of SIVs and their natural hosts has resulted in viral phenotypes that limit immune activation-mediated pathogenesis. | Hypothesis Supported |
| Long-lived central memory CD4+ T cells are relatively resistant to SIV infection resulting in preserved CD4+ T cell homeostasis and protection from SIV-induced immunopathology. | Limitation of infection to expendable T cell subsets at peripheral sites via modulation of SIV receptor/coreceptor expression reduces pathogenicity by preserving long-lived cells in primary lymphoid tissues. | Hypothesis Supported | |
| An effective adaptive immune response suppresses viral replication in natural hosts. |
|
Direct immune control of viral replication does not result in the lack of pathogenicity in natural hosts. | Hypothesis Not Supported |
| Natural hosts avoid progression to AIDS by limiting chronic immune activation and bystander immunopathology. | Avoidance of chronic immune activation in natural hosts helps to preserve T cell homeostasis and prevent bystander immunopathology and dysregulation of critical immune cell subsets and tissues. | Hypothesis Supported | |
| Natural hosts maintain the integrity of the gut mucosal barrier through preservation of the mucosal immune environment. | Maintenance of the gut mucosal immune system in natural hosts prevents the translocation of microbial products from the intestinal lumen to the systemic circulation where they can induce chronic immune activation. | Hypothesis Supported |
3. How does acute immune activation resolve in nonpathogenic SIV infection?
The early events of SIV infection in natural hosts have been studied by experimentally infecting SMs and AGMs with their species-specific virus, thus allowing detailed virological, immunological, and molecular analyses of the acute phase of SIV infection in these animals. Similar to pathogenic HIV/SIV infections, primary SIV infection of natural hosts is associated with a peak of virus replication occurring 10–15 days after virus inoculation, followed by a post-peak decline that is coincident with the emergence of cellular immune responses to SIV (37). Experimentally SIV-infected SMs and AGMs exhibit a modest but transient decline of peripheral CD4+ T cell counts, and a more severe loss of mucosal CD4+ T cells in both the gastrointestinal tract and lung (15, 16). In contrast to pathogenic infections where mucosal CD4+ T cells become progressively depleted during chronic infection, natural SIV hosts stabilize (SMs) or even recover (AGMs) their levels of mucosal CD4+ T cells (15, 16). Both SMs and AGMs mount initial strong innate and adaptive immune responses to the virus, that are characterized by production of type I interferons (IFNs) by plasmacytoid dendritic cells (pDCs), upregulation of type I IFN response genes, activation and proliferation of T cells, and production of proinflammatory cytokines (17–21). Strikingly, however, the innate immune response to the virus resolves spontaneously within 4–8 weeks post infection despite continuous virus replication (17, 19, 21). This resolution distinguishes SIV infection of natural hosts from pathogenic HIV/SIV infections, where the type I IFN response persists throughout the chronic phase of infection (38, 39).
The chronic phase of SIV infection in natural hosts is also characterized by low levels of adaptive immune activation (as measured by lymphocyte proliferation and apoptosis), preserved T cell regeneration (as measured by thymic and bone marrow function), and normal lymph node function and architecture (5, 40). The consequences of pathogenic HIV/SIV infections on lymph node function and architecture include continuous B cell activation and germinal center reaction, hypocellularity of the paracortex (where T cells reside), and the disruption of the fibroblastic reticular cell network that is associated with significant collagen deposition (41–43). These features are all conspicuously absent during the chronic phase of SIV infection in SMs and AGMs (5, 40). The presence of chronic immune activation and related immunopathology and impaired T cell renewal is clearly associated with disease progression during pathogenic HIV/SIV infections (44, 45). In fact, the level of residual immune activation in patients in which HIV replication has been successfully suppressed by antiretroviral therapy is the strongest predictor of ineffective immune recovery (46). Thus, the rapid resolution of immune activation in natural SIV infections may be a crucial factor that enables these animals to avoid AIDS.
The mechanisms underlying the resolution of immune activation in the post-acute phase of SIV infection in SMs and AGMs have been intensively investigated. Due to limited space, our discussion of these studies will focus on the mechanisms that are consistent with the bulk of the available experimental data. The first hypothesis is that natural SIV hosts actively down-regulate the innate and adaptive immune responses to the virus. This hypothesis is supported by longitudinal analysis of the transcriptome in acutely SIV-infected SMs revealing an initial immune response to the virus that is similar to that observed in pathogenic HIV/SIV infections, but that returns to a pre-infection profile within a few weeks (17–21). Molecular and histological studies have suggested that specific immune regulatory pathways, such as those involving programmed cell death-1 (PD-1) and adenosine deaminase acting on RNA-1 (ADAR-1), may be activated during the rapid resolution of immune activation in natural SIV hosts. Studies are currently being carried out in several laboratories, including ours, to better define these immune regulatory pathways and ultimately identify novel targets for immune-based interventions aimed at reducing the HIV-associated chronic immune activation.
A second hypothesized mechanism to explain the resolution of immune activation in natural hosts is that infected CD4+ T cells become resistant to activation after down-modulation of the cell surface CD3-TCR complex by the Nef protein of SIVsmm and SIVagm (47). Infected CD4+ T cells with low surface levels of CD3-TCR may be refractory to further antigenic stimulation and thus contribute to an overall less activated immunological environment. This hypothesis is supported by the finding that the Nef protein of HIV-1 and SIVcpz, strains that cause pathogenic infections with high levels of chronic immune activation, has lost the ability to down-modulate CD3-TCR (47). While the chronic immune activation and pathogenic outcome of SIVmac infection of RMs cannot be explained solely by this mechanism (SIVmac Nef can down-modulate CD3-TCR, (47)), it should be noted that the very high viral loads observed in this model of infection may favor a chronic immune activation despite the specific effects of Nef. Currently, experiments are being conducted in which AGMs are infected with SIVagm containing an "HIV-1-like" Nef to better define the in vivo role of this viral protein.
The third hypothesized mechanism for the resolution of immune activation in natural hosts is that preservation of mucosal immunity prevents translocation of microbial products from the intestinal lumen to the systemic circulation. Microbial translocation is thought to cause chronic immune activation and disease progression during pathogenic HIV/SIV infections (27), although controversy exists in the literature (48). In natural hosts, consistent data show that the significant depletion of mucosal CD4+ T cells (~50–90% from baseline levels) observed during acute SIV infection of SMs and AGMs, this depletion is not progressive (15, 16), does not result in the selective loss of Interleukin-17-producing T helper cells (Th17) (25, 26), and is not associated with focal loss of intestinal epithelial integrity (49), suggesting preservation of the mucosal barrier. Interestingly, increased immune activation was induced in SIV-infected AGMs by mimicking the effect of microbial translocation through systemic injections of bacterial lipopolysaccharide (LPS) (50), thus consistent with the hypothesis that preserved mucosal immune function protects natural SIV hosts from chronic immune activation and disease progression.
Finally, a fourth hypothesis to explain the resolution of immune activation is the low levels of virus replication in lymph nodes and other organized lymphoid tissues observed in chronic SIV infection of AGMs and SMs (37, 51). This limited viral replication in lymphoid tissues is likely related to lower levels of infection of central memory CD4+ T cells (Tcm, see next section for details, (30)). According to this hypothesis, reduced virus replication in the main anatomic sites in which immune responses are initiated allows a more effective resolution of the early virus-induced immune activation as compared to pathogenic HIV/SIV infections in which virus production in lymph nodes remains significantly higher. Although the relative contribution of each of these four mechanisms and any as-yet undescribed factors to the low immune activation observed during chronic SIV infections of natural hosts remains unknown, all available experimental data confirm that immune quiescence is a typical feature of nonpathogenic infections and is likely a key determinant of their benign nature.
4. What is the role of target cell restriction in nonpathogenic SIV infection?
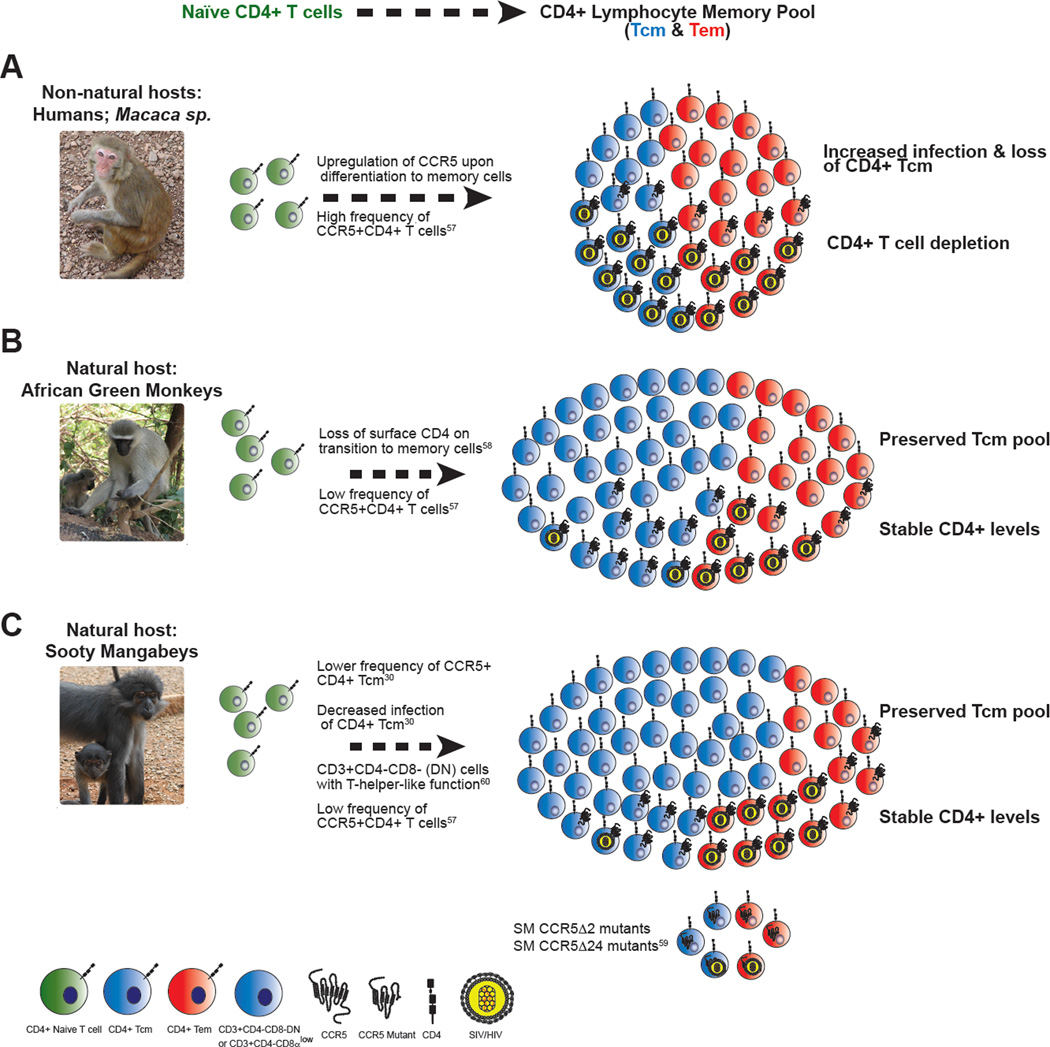
In recent years, the observation that SIV infection of SMs is characterized by a specific pattern of infected cells in vivo, in which CD4+ Tcm are relatively spared whereas CD4+ effector memory T cells (Tem) are the main viral targets (30), has led to a model of AIDS pathogenesis that proposes that the subset of cell infected is more important than the total number of infected cells or the level of plasma viremia in causing the immune deficiency (Fig. 2). CD4+ Tcm reside in lymph nodes and other inductive lymphoid tissues and show strong proliferation in response to antigenic re-stimulation with limited effector function (such as production of cytokines); in contrast, CD4+ Tem reside in non-lymphoid tissues (i.e., mucosae), and show high effector function but limited proliferation upon re-stimulation (52). Given the central role played by CD4+ Tcm in maintaining the overall homeostasis of the CD4+ T cell pool, direct infection of these cells by HIV/SIV is hypothesized to have a greater potential to damage the immune system than does infection of the likely more expendable CD4+ Tem. This idea is consistent with studies conducted in SIV-infected RMs indicating that progressive depletion of CD4+ Tcm is the key factor dictating the tempo of progression to AIDS, both in the natural history of infection and in the context of vaccination (53, 54). While the mechanisms causing the loss of CD4+ Tcm in SIV-infected RMs are likely complex and multifactorial, and include bystander cell death, proliferative senescence, and loss of anatomic niche, it is undisputed that direct virus infection is a key determinant of CD4+ Tcm depletion during pathogenic HIV/SIV infections (48). Importantly, in HIV-infected humans, the levels of in vivo infection of CD4+ Tcm are at least as high, if not higher, than those of other memory CD4+ T cell subsets, and CD4+ Tcm represent the largest reservoir of infected CD4+ T cells (56).
Figure 2. Proposed mechanisms of target cell restriction in natural host species.
(A) In pathogenic hosts such as RMs, activation of CD4+ T cells typically leads to robust surface expression of the HIV/SIV co-receptor CCR5, providing targets for infection and viral replication. Infection is distributed within both effector memory cells (Tem) and central memory cells (Tcm). Infection and disruption of the Tcm pool has been demonstrated to disrupt CD4+ T cell homeostasis in vivo (53). (B) Upon transition from naïve CD4+ T cells to memory cells, AGMs lower the surface expression of the CD4 molecule on a subset of CD3+CD4−CD8αlow lymphocytes that exhibit CD4+ T cell function and are infected by SIV at a lower frequency than naïve or memory CD4+ cells (58). AGMs also exhibit lower levels of CCR5 expression on CD4+ T cells than non-natural hosts (57). (C) Multiple, non-mutually exclusive mechanisms modulating SIV tropism have been described in SMs: (i) Upon activation, SM CD4+ Tcm cells maintain low levels of CCR5 relative to CD4+ Tem cells, resulting in significantly decreased levels of SIV infection (30); (ii) SMs have high levels of CD3+CD4−CD8− “double-negative” lymphocytes that exhibit T helper function, providing a potential surrogate for CD4+ T cells that is resistant to infection (60); (iii) Approximately 7% of captive SMs are homozygous for CCR5 mutations that abrogate surface expression, although these animals can be infected with SIV (59). Photo credit: S.E.B. and K.D. Mir.
In SMs, the protection of CD4+ Tcm from SIV infection has been observed both in vivo and in vitro. Ex vivo sorted CD4+ Tcm of naturally SIV-infected SMs have significantly fewer copies of cell-associated SIV-DNA as compared to CD4+ Tcm of SIV-infected RMs, and sorted CD4+ Tcm of SIV-uninfected SMs show significantly lower levels of virus production upon experimental in vitro SIV infection when compared to sorted CD4+ Tcm of healthy, uninfected RMs (30). The relative resistance to SIV infection by SM CD4+ Tcm as compared to SM CD4+ Tem or CD4+ Tcm of RMs suggests that SMs may have adapted to protect their CD4+ Tcm from virus infection, and that this preferential sparing of CD4+ Tcm is a key determinant of the benign nature of SIV infection in these animals. Interestingly, CD4+ Tcm of SMs express lower levels of the main virus coreceptor, the chemokine receptor CCR5, both ex vivo and, even more dramatically, following in vitro activation with various stimuli (30, 57), thus leading to the hypothesis that lower levels of CCR5 represent a mechanism of intrinsic resistance of SM CD4+ Tcm to virus infection that acts at the entry level. Somewhat analogously, CD4+ T cells of AGMs have been shown to down-modulate the expression of the CD4 molecule, the virus receptor, from their surface during the transition from naive to memory cells, thus acquiring resistance to SIV infection (58). Interestingly, a recent genetic study revealed that ~7% of SMs are homozygous for a mutant CCR5 allele (consisting of a two base-pair deletion in the region corresponding to the fourth transmembrane domain of CCR5) that abrogates surface expression of CCR5 (59). When infected with SIV, these CCR5Δ2 SMs also remain healthy despite robust virus replication (albeit ~0.5 log lower than CCR5 wild-type animals), indicating that selective CCR5 down-modulation is not the only mechanism protecting CD4+ Tcm from direct virus infection. The discovery of CCR5Δ2 SMs also suggests that the expression of alternative virus coreceptors (i.e., CXCR6, GPR1, etc.) is an additional determinant of the different patterns of target cells observed in SIV-infected natural hosts and RMs.
The role of virus co-receptor tropism in the pathophysiology of SIV infection in SMs was emphasized in a series of elegant studies conducted by Sodora and colleagues, in which development of CXCR4-tropic variants of SIVsmm was found to be associated with a rare phenotype characterized by generalized depletion of CD4+ T cells (60, 61). Intriguingly, these "CD4-low" SIV-infected SMs maintain a population of CD3+CD4−CD8− (i.e., double negative, DN) T cells that exhibit phenotypic and functional features of Tcm cells and mediate a strong helper T cell effect in vivo when animals are exposed to neo-antigens (60). Another key feature of these CD4-low SIV-infected SMs is the reduced viremia (~2 logs lower than the "CD4-high" SMs), which may help explain why immune activation remains low in these animals.
This association between low CD4+ T cell counts and low virus replication in SMs was recreated experimentally by monoclonal antibody-mediated in vivo CD4+ lymphocyte depletion in SIV-infected SMs (29). When a similar experimental CD4+ lymphocyte depletion was conducted prior to SIVmac infection of RMs, the subsequent levels of virus replication were remarkably high (~108–109 copies/ml of plasma), with the majority of virus-producing cells in both lymph nodes and mucosal tissues belonging to the macrophage lineage (62). The reason(s) why CD4+ lymphocyte depletion is associated with massive virus replication in macrophages in RMs but not in SMs are currently under investigation, and may involve both entry and post-entry mechanisms that restrict virus infection and/or replication in these cells in natural hosts. The SIVsmm and SIVmac (and SIVagm and HIV-2, but not SIVmnd-1, SIVcpz, or HIV-1) viruses express Vpx that antagonizes the newly described SAMHD1 host restriction factor in myeloid cells (63, 64) and further exploration of the impact of this virus-host interaction, as well as other such interactions --in particular that of BST-2 tetherin with several viral gene products (65)-- is the subject of ongoing research in the natural host field. Collectively, these studies delineate a model in which, in the majority of SIV-infected SMs, CD4+ Tcm are protected from direct virus infection thus favoring the preservation of CD4+ T cell homeostasis. In the relatively rare case of infection with a CXCR4-tropic, CD4+ T cell-depleting virus, a combination of "off-shoring" of T helper function to DN T cells and a reduction of virus replication due to the inability to infect macrophages appears sufficient to avoid AIDS.
The resolution of immune activation and target cell restriction that we have thus far described in this review may protect natural SIV hosts from disease progression, and it is likely that these two phenomena favor each other in a virtuous cycle whose establishment is crucial to avoid the immune deficiency that follows HIV/SIV infections of humans and RMs. On the one hand, low levels of infected CD4+ Tcm may limit immune activation by decreasing virus replication and thus antigenic burden in the tissues where innate and adaptive immune responses are primed (lymph nodes, spleen, Peyer’s patches). On the other hand, low chronic immune activation may promote CD4+ Tcm preservation by reducing direct virus infection (that is enhanced by cell activation), minimizing bystander cell death of uninfected CD4+ Tcm, and maintaining the anatomic niche of these cells, which is replaced by tissue fibrosis during pathogenic SIV infection of RMs (41). We propose that low immune activation and reduced infection of CD4+ Tcm are the evolutionary result of a mutually beneficial co-adaptation between primate lentiviruses and their natural hosts that took place over the many thousands of years in which these viruses have infected African monkey species.
5. How are natural hosts protected from mother-to-infant transmission?
A striking feature of SIV infection in natural hosts is the rarity of mother-to-infant-transmission (MTIT) (34–36). In HIV-1-infected humans, mother-to-child transmission can occur in utero, during labor and delivery, and as a consequence of breastfeeding, with a total HIV-1 transmission rate of 35–40% in absence of antiretroviral therapy or other preventative measures. In stark contrast, MTIT of SIV in SMs occurs at a rate of <7% and appears to be even less frequent in AGMs and mandrills (34–36). Interestingly, when infant SMs or AGMs do become infected, either as a consequence of MTIT or through experimental inoculation, the levels of virus replication are significantly lower than those observed in adult animals (34, 66). The mechanisms responsible for the low rate of MTIT in natural SIV hosts remain poorly understood; however, strong preliminary evidence implicates low levels of target cells (and particularly activated CD4+CCR5+ T cells (36)) as a factor involved in both restricted transmission and low viral loads in infants of natural SIV host species. As such, an emerging theory is that there exists a convergence of the immunological mechanisms protecting from disease progression in adult animals and MTIT in offspring. We are not surprised by this relationship. The evolutionary pressure selecting for the benign nature of current SIV infections in natural host species would almost certainly have encompassed more than a disease course similar to that seen in HIV-infected individuals (approximately 10 years from infection to death) in animals infected as adults and with an average lifespan of only 15–20 years. Most likely, the original epidemics of SIV infection with ancestral, putatively pathogenic viruses were characterized by high levels of transmission from mother to offspring, particularly if one considers the obvious deleterious effect of moving the "clock" of infection back 4–5 years to infancy, along with the observation that pathogenic HIV/SIV infections appear to be more severe in children compared to adults. In this view, the genetic features of natural SIV hosts that underlie two key mechanisms of AIDS resistance (i.e., low immune activation and target cell restriction) may at least in part reflect evolutionary selection to protect from MTIT. Further studies are needed to better elucidate the mechanisms restricting MTIT in natural hosts, and to understand the relationship between these mechanisms and those that protect SIV-infected animals from disease progression.
6. Future studies and implications for human health
Although significant progress has been made in understanding the pathophysiology of natural SIV infections, several important questions remain unanswered, and further studies are clearly warranted to fully elucidate the mechanisms by which natural SIV hosts avoid AIDS. Key areas of investigation that should be prioritized include: (i) the mechanisms responsible for the resolution of immune activation; (ii) the factors restricting virus replication in CD4+ Tcm and macrophages; (iii) the ways in which specific viral gene products (Nef, Vpu, Vpx) interact with the species-specific host immune system to reduce pathogenicity; (iv) the immunological events involved in the preservation of gut mucosal immunity and integrity; and (v) the mechanisms responsible for protection from MTIT. More detailed studies in SMs and AGMs - the two species of natural SIV hosts available for research in the United States and Europe - would certainly benefit from a more aggressive use of high throughput genetic, genomic, and proteomic techniques. These studies should be conducted in parallel in humans and RMs, and could include the establishment of a basic transcriptome and proteome resource for the key immunological cell types in these different species. In addition, it would be very useful if more species of natural SIV hosts become accessible to researchers in the field. In particular, an increased understanding of lentiviral pathogenesis may be obtained by collecting basic information on the outcome of infection in certain nonhuman primate species (i.e., greater spot nosed monkey, mustached guenons, and monas) that are hosts of SIVs that include vpu and whose Nef protein is unable to down-modulate the CD3-TCR complex (47). Examination of a broader range of natural hosts will likely reveal additional mechanisms that prevent SIV infection from progressing to AIDS in these animals.
It is clear that rigorous investigations of the mechanisms by which African nonhuman primate natural hosts and their lentiviruses have coevolved to reach a pacific coexistence in nature will continue to provide critical insights into human HIV/AIDS pathogenesis. The direct relevance of studies of natural SIV infection to human health has been emphasized by the recent observation that rare HIV-infected humans exhibit a “natural host-like” phenotype: stable CD4+ T cell counts despite high viremia, with a transcriptional profile similar to that of SIV-infected SMs (67). Ultimately, these insights will translate into successful interventions to prevent and treat HIV infection in humans. In a previous article we discussed how studies of natural SIV infections inform the development of an AIDS vaccine (68), and here we will focus more on the potential impact on HIV therapy. Elucidating the mechanisms responsible for the resolution of immune activation in natural hosts will likely guide the development of novel agents to treat the chronic immune activation associated with HIV infection that is a key determinant of disease progression. In particular, these studies could identify molecular pathways of immune regulation that are active in natural SIV hosts and that could be targeted to reduce or eliminate the residual immune activation and inflammation that is associated with increased morbidity and end-organ disease during long-term antiretroviral treatment of HIV-infected individuals. In addition, uncovering the mechanisms of Th17 preservation during natural SIV infection could identify therapeutic targets to improve Th17 cell homeostasis in HIV-infected individuals, thereby promoting the immunologic restoration of the intestinal mucosal barrier. Furthermore, the observation that the pattern of infected cells may be critical in dictating the progression to AIDS in HIV/SIV infections should prompt more investigations aimed at defining the determinants of CD4+ Tcm infection in HIV-infected humans and whether the level of CD4+ Tcm infection correlates with disease progression and/or residual morbidity in treated individuals. Another area to pursue is the role of CD4+ Tcm infection in establishing the reservoirs of latently infected cells, and, given the long lifespan of these cells, in causing HIV persistence during antiretroviral therapy. It is hoped that therapeutic interventions will be developed that may more aggressively protect CD4+ Tcm from infection. Finally, a more complete understanding of the block to vertical transmission in natural hosts could majorly impact current treatment of HIV-infected pregnant women and their infants and possibly reduce the number of perinatal HIV infections worldwide.
In the title of this review we expressed the hope that natural SIV hosts will tell us how to "show AIDS the door.” Indeed, if we are to reach this aim, it would be foolish to ignore the lessons of this experiment conducted by nature over thousands of years. After all, natural SIV hosts are the door through which HIV came to humans, and we believe they will give us the tools to finally close the door on AIDS, just as they did long ago.
Acknowledgements
The authors would like to gratefully acknowledge the following individuals, whose unparalleled scientific insight and dedication have contributed significantly to our understanding of the natural SIV hosts: J. Allan, A. Ansari, C. Apetrei, J. Brenchley, L. Chakrabarti, R. Collman, M. Davenport, D. Douek, J. Else, J. Estes, M. Feinberg, R. Grant, A. Haase, B. Hahn, V. Hirsch, A. Kaur, F. Kirchhoff, M. Lederman, P. Marx, M. Muller-Trutwin, I. Pandrea, D. Sodora, J. Schmitz, S. Staprans, R. Veazey, and F. Villinger. We apologize that, due to space constraints, we were only able to cite selected works from these and other authors. The Authors wish to thank Drs. C. Dieffenbach, S. Plaeger, J. Young, A. Embry, and D. Finzi at the Division of AIDS of the NIAID, NIH, for their continuous intellectual and logistical support of this work. This work was supported by NIH grants R01-AI066998 to G.S. and P51-RR00165 to YNPRC.
References
- 1.Worobey M, et al. Island biogeography reveals the deep history of SIV. Science. 2010 Sep 17;329:1487. doi: 10.1126/science.1193550. [DOI] [PubMed] [Google Scholar]
- 2.VandeWoude S, Apetrei C. Going wild: lessons from naturally occurring Tlymphotropic lentiviruses. Clinical Microbiology Reviews. 2006 Oct;19:728. doi: 10.1128/CMR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hahn BH, Shaw GM, De Cock KM, Sharp PM. AIDS as a zoonosis: scientific and public health implications. Science. 2000 Jan;287:607. doi: 10.1126/science.287.5453.607. [DOI] [PubMed] [Google Scholar]
- 4.Letvin NL, et al. Induction of AIDS-like disease in macaque monkeys with T-celltropic retrovirus STLV-III. Science. 1985 Oct 4;230:71. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- 5.Silvestri G. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003 Mar;18:441. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 6.Apetrei C, et al. Immunovirological Analyses of Chronically Simian Immunodeficiency Virus SIVmnd-1- and SIVmnd-2-Infected Mandrills (Mandrillus sphinx) J Virol. 2011 Dec;85:13077. doi: 10.1128/JVI.05693-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein S, et al. Comparison of simian immunodeficiency virus SIVagmVer replication and CD4+ T-cell dynamics in vervet and sabaeus African green monkeys. J Virol. 2006 May;80:4868. doi: 10.1128/JVI.80.10.4868-4877.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandrea I, et al. Simian immunodeficiency viruses replication dynamics in African non-human primate hosts: common patterns and species-specific differences. J Med Primatol. 2006 Aug;35:194. doi: 10.1111/j.1600-0684.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- 9.Rey-Cuille MA, et al. Simian immunodeficiency virus replicates to high levels in sooty mangabeys without inducing disease. J Virol. 1998 May;72:3872. doi: 10.1128/jvi.72.5.3872-3886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keele BF, et al. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature. 2009 Jul 23;460:515. doi: 10.1038/nature08200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007 Sep;27:406. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Loffredo JT, et al. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J Virol. 2007 Aug;81:8827. doi: 10.1128/JVI.00895-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon SN, et al. Short-lived infected cells support virus replication in sooty mangabeys naturally infected with simian immunodeficiency virus: implications for AIDS pathogenesis. J Virol. 2008 Apr;82:3725. doi: 10.1128/JVI.02408-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandrea I, et al. Simian immunodeficiency virus SIVagm dynamics in African green monkeys. J Virol. 2008 Apr;82:3713. doi: 10.1128/JVI.02402-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon SN, et al. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J Immunol. 2007 Sep 1;179:3026. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandrea IV, et al. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol. 2007 Sep 1;179:3035. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosinger SE, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009 Dec;119:3556. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estes JD, et al. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. J Immunol. 2008 May 15;180:6798. doi: 10.4049/jimmunol.180.10.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacquelin B, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009 Dec;119:3544. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris LD, et al. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J Virol. 2010 Aug;84:7886. doi: 10.1128/JVI.02612-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lederer S, et al. Transcriptional profiling in pathogenic and non-pathogenic SIV infections revealed significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog. 2009 Feb;5:e1000296. doi: 10.1371/journal.ppat.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunham R, et al. The AIDS resistance of naturally SIV-infected sooty mangabeys is independent of cellular immunity to the virus. Blood. 2006 Jul 1;108:209. doi: 10.1182/blood-2005-12-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B, et al. Nonpathogenic simian immunodeficiency virus infection of sooty mangabeys is not associated with high levels of autologous neutralizing antibodies. J Virol. 2010 Jun;84:6248. doi: 10.1128/JVI.00295-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Metcalf B, Ribeiro RM, McClure H, Kaur A. Th-1-type cytotoxic CD8+ T-lymphocyte responses to simian immunodeficiency virus (SIV) are a consistent feature of natural SIV infection in sooty mangabeys. J Virol. 2006 Mar;80:2771. doi: 10.1128/JVI.80.6.2771-2783.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenchley JM, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008 Oct 1;112:2826. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Favre D, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009 Feb;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006 Dec;12:1365. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 28.Paiardini M, et al. Bone marrow-based homeostatic proliferation of mature T cells in nonhuman primates: implications for AIDS pathogenesis. Blood. 2009 Jan 15;113:612. doi: 10.1182/blood-2008-06-159442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klatt NR, et al. Availability of activated CD4+ T cells dictates the level of viremia in naturally SIV-infected sooty mangabeys. J Clin Invest. 2008 Jun;118:2039. doi: 10.1172/JCI33814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paiardini M, et al. Low levels of SIV infection in sooty mangabey central memory CD T cells are associated with limited CCR5 expression. Nat Med. 2011 Jul;17:830. doi: 10.1038/nm.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakrabarti LA, et al. Normal T-cell turnover in sooty mangabeys harboring active simian immunodeficiency virus infection. J Virol. 2000 Feb;74:1209. doi: 10.1128/jvi.74.3.1209-1223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandrea I, et al. Simian immunodeficiency virus SIVagm.sab infection of Caribbean African green monkeys: a new model for the study of SIV pathogenesis in natural hosts. J Virol. 2006 May;80:4858. doi: 10.1128/JVI.80.10.4858-4867.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sumpter B, et al. Correlates of preserved CD4(+) T cell homeostasis during natural, nonpathogenic simian immunodeficiency virus infection of sooty mangabeys: implications for AIDS pathogenesis. J Immunol. 2007 Feb 1;178:1680. doi: 10.4049/jimmunol.178.3.1680. [DOI] [PubMed] [Google Scholar]
- 34.Chahroudi A, et al. Mother-to-infant transmission of simian immunodeficiency virus is rare in sooty mangabeys and is associated with low viremia. J Virol. 2011 Jun;85:5757. doi: 10.1128/JVI.02690-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otsyula MG, et al. Apparent lack of vertical transmission of simian immunodeficiency virus (SIV) in naturally infected African green monkeys, Cercopithecus aethiops. Annals of tropical medicine and parasitology. 1995 Oct;89:573. doi: 10.1080/00034983.1995.11812990. [DOI] [PubMed] [Google Scholar]
- 36.Pandrea I, et al. Paucity of CD4+ CCR5+ T cells may prevent transmission of simian immunodeficiency virus in natural nonhuman primate hosts by breast-feeding. J Virol. 2008 Jun;82:5501. doi: 10.1128/JVI.02555-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meythaler M, et al. Early induction of polyfunctional simian immunodeficiency virus (SIV)-specific T lymphocytes and rapid disappearance of SIV from lymph nodes of sooty mangabeys during primary infection. J Immunol. 2011 May 1;186:5151. doi: 10.4049/jimmunol.1004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sedaghat AR, et al. Chronic CD4+ T-cell activation and depletion in human immunodeficiency virus type 1 infection: Type I interferon-mediated disruption of T-cell dynamics. J. Virol. 2008;82:1870. doi: 10.1128/JVI.02228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hyrcza MD, et al. Distinct transcriptional profiles in ex vivo CD4+ and CD8+ T cells are established early in human immunodeficiency virus type 1 infection and are characterized by a chronic interferon response as well as extensive transcriptional changes in CD8+ T cells. J. Virol. 2007;81:3477. doi: 10.1128/JVI.01552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Broussard SR, et al. Simian immunodeficiency virus replicates to high levels in naturally infected African green monkeys without inducing immunologic or neurologic disease. J. Virol. 2001;75:2262. doi: 10.1128/JVI.75.5.2262-2275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng M, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J. Clin. Invest. 2011;121:998. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pantaleo G, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 43.Schacker TW, et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J. Clin. Invest. 2002;110:1133. doi: 10.1172/JCI16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heeney JL. AIDS: A disease of impaired Th-cell renewal? Immunol. Today. 1995;16:515. doi: 10.1016/0167-5699(95)80043-3. [DOI] [PubMed] [Google Scholar]
- 45.Sodora DL, Silvestri G. Immune activation and AIDS pathogenesis. AIDS. 2008;22:439. doi: 10.1097/QAD.0b013e3282f2dbe7. [DOI] [PubMed] [Google Scholar]
- 46.Hunt PW, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J. Infect. Dis. 2003;187:1534. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 47.Schindler M, et al. Nef-mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell. 2006;125:1055. doi: 10.1016/j.cell.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 48.Redd AD, et al. Microbial translocation, the innate cytokine response, and HIV-1 disease progression in Africa. Proc. Natl. Acad. Sci. U.S.A. 2009;106:6718. doi: 10.1073/pnas.0901983106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Estes JD, et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 2010;6:e1001052. doi: 10.1371/journal.ppat.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pandrea I, et al. Cutting edge: Experimentally induced immune activation in natural hosts of simian immunodeficiency virus induces significant increases in viral replication and CD4+ T cell depletion. J. Immunol. 2008;181:6687. doi: 10.4049/jimmunol.181.10.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diop OM, et al. High levels of viral replication during primary simian immunodeficiency virus SIVagm infection are rapidly and strongly controlled in African green monkeys. J. Virol. 2000;74:7538. doi: 10.1128/jvi.74.16.7538-7547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pepper M, Jenkins MK. Origins of CD4+ effector and central memory T cells. Nat. Immunol. 2011;12:467. doi: 10.1038/ni.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okoye A, et al. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J. Exp. Med. 2007;204:2171. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Letvin NL, et al. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science. 2006;312:1530. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brenchley JM, Silvestri G, Douek DC. Nonprogressive and progressive primate immunodeficiency lentivirus infections. Immunity. 2010;32:737. doi: 10.1016/j.immuni.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brenchley JM, et al. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: Implications for HIV pathogenesis. J. Virol. 2004;78:1160. doi: 10.1128/JVI.78.3.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pandrea I, et al. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood. 2007;109:1069. doi: 10.1182/blood-2006-05-024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beaumier CM, et al. CD4 downregulation by memory CD4+ T cells in vivo renders African green monkeys resistant to progressive SIVagm infection. Nat. Med. 2009;15:879. doi: 10.1038/nm.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riddick NE, et al. A novel CCR5 mutation common in sooty mangabeys reveals SIVsmm infection of CCR5-null natural hosts and efficient alternative coreceptor use in vivo. PLoS Pathog. 2010;6:e1001064. doi: 10.1371/journal.ppat.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Milush JM, et al. Lack of clinical AIDS in SIV-infected sooty mangabeys with significant CD4+ T cell loss is associated with double-negative T cells. J. Clin. Invest. 2011;121:1102. doi: 10.1172/JCI44876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Milush JM, et al. Virally induced CD4+ T cell depletion is not sufficient to induce AIDS in a natural host. J. Immunol. 2007;179:3047. doi: 10.4049/jimmunol.179.5.3047. [DOI] [PubMed] [Google Scholar]
- 62.Ortiz AM, et al. Depletion of CD4+ T cells abrogates post-peak decline of viremia in SIV-infected rhesus macaques. J. Clin. Invest. 2011;121:4433. doi: 10.1172/JCI46023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laguette N, et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474:654. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hrecka K, et al. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011;474:658. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sauter D, et al. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe. 2009;6:409. doi: 10.1016/j.chom.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]