Abstract
Recently the new term ‘telocytes’ has been proposed for cells formerly known as interstitial Cajal-like cells. In fact, telocytes are not really Cajal-like cells, they being different from all other interstitial cells by the presence of telopodes, which are cell-body prolongations, very thin, extremely long with a moniliform aspect. The identification of these cells is based on ultrastructural criteria. The presence of telocytes in others organs was previously documented. We reported for the first time, an ultrastructural study of telocytes in the lamina propria of rat duodenum. Our findings show that typical telocytes are present in the rat duodenum. Telocytes are located in the lamina propria, immediately below mucosal crypts. Telopodes frequently establish close spatial relationships with immune cells, blood vessels and nerve endings. On the basis of their distribution and morphology, we suggest that these cells may be involved in immune response and in our opinion, it may be possible that different locations of telocytes could be associated with different roles.
Keywords: telocytes, telopodes, lamina propria, transmission electron microscopy, duodenum, ultrastructure
Introduction
In 1893, Santiago Ramon and Cajal used the Golgi technique and methylene blue staining to describe, in amphibians [1] and several mammals [2], spindle-shaped or stellate cells associated with autonomic nerve endings of the intestine; he termed them ‘primitive neurons’. At present, the term ‘interstitial cells of Cajal’ (ICC) refers to several types of cells located in the musculature of the gastrointestinal tract and, morphologically and functionally, intercalated between the segments of the enteric nervous system and smooth muscle cells.
For the past 10 years many groups were interested in whether or not ICC are present outside the gastrointestinal tract, and indeed, peculiar interstitial cells were found in: upper and lower urinary tracts [3–7], blood vessels [8–12], pancreas [13–14], male and female reproductive tracts [15], mammary gland [16], placenta [17], heart [18] and gut [19]. Such cells, now mostly known as interstitial Cajal-like cells (ICLC), were given different and confusing names.
Recently the new term ‘telocytes’ (telos, i.e. provided with long-distance cell projections) has been proposed for cells formerly known as ICLC, and the term ‘telopodes’ has been proposed for their very long and very thin prolongations [20]. We share these arguments and for the sake of standardization; these terms will be used from this point of view.
Here we show the presence of telocytes and telopodes in the lamina propria, immediately below mucosal crypts, of rat duodenum by transmission electron microscopy (TEM). Their functional significance in the lamina propria must be studied more extensively.
Materials and methods
Animal use
Six adult Wistar rats, 3 months old (The Jackson Laboratory, Bar Harbor, ME, USA)), were used in accordance with institutional guidelines (Ethics Advisory Commission for Animal Experimentation, PI 22/08). Each animal had ad libitum access to food and water and was fed on a complete and balanced standard laboratory diet (Teklad 4% rat diet 7001; Harlan Teklad, Madison, WI, USA). They were housed in temperature controlled rooms (20 ± 1°C) and natural light.
All rats were anesthetized, killed and perfused intracardially with the specific fixative of the technique described below.
Transmission electron microscopy
Duodenum samples (about 1–1.5 mm3) were washed in phosphate buffer and fixed with 2.5% glutaraldehyde and 2% paraformaldehyde. The pieces were fixed overnight at room temperature in the same fixative, washed in 0.1 M phosphate buffer for 5 min., post-fixed with 2% osmium, rinsed, dehydrated in graded acetones (30%, 50%, 70% with 2% uranyl-acetate, 90%, 100%), cleared in propylene oxide and embedded in araldite (Durcupan, Fluka AG, Buchs SG, Switzerland). Semi-thin sections (1.5 μm) were cut with a diamond knife and stained lightly with 1% toluidine blue. Later, ultrathin (0.08 μm) sections were cut with a diamond knife, collected on Formvar coated single-slot grids, counterstained with 1% uranyl acetate and with Reynold’s lead citrate for 10 min. and examined under a FEI Tecnai G2 Spirit TEM. The images were achieved with Advanced Microscopy Techniques, Corp.’s Charge-Coupled Device (CCD) (Danvers, MA, USA) imaging system.
Semi-thin sections (<1 μm thick) were stained with toluidine blue and examined by light microscopy (Olympus BX51 microscope, Olympus Imaging Corporation, Tokyo, Japan).
Results
Light microscopy on semi-thin sections
Telocytes were easily identifiable as cells with long cellular processes that extend into the connective tissue beneath mucosal crypts in toluidine blue semi-thin sections (Fig. 1).
Fig 1.
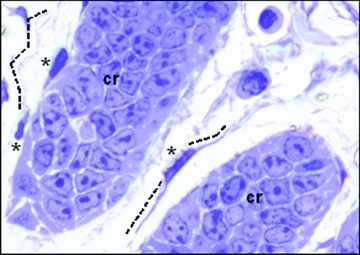
Rat duodenum. Non-conventional light microscopy; objective 100×. Tissue fixed with glutaraldehyde–paraformaldehyde and post-fixed in OsO4. Thin section of Aradilte-embedded material was stained with toluidine blue. At least three telocytes (asterisks) are present in the interstitium among crypt (cr).
Transmission electron microscopy
TEM examination is fundamental in identifying the telocytes.
Figure 2(A) shows general aspect of the telocyte: small cellular body, containing a nucleus, surrounded by a small amount of cytoplasm. The nucleus contains a thin band of marginal heterochromatin attached to the nuclear envelope.
Fig 2.
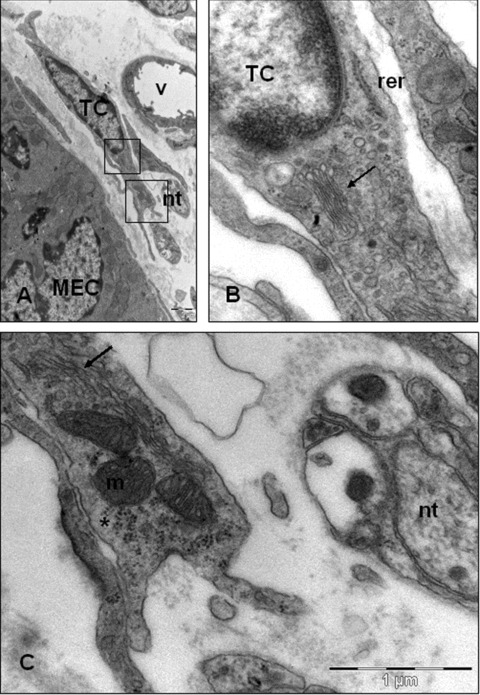
Telocyte typical ultrastructural features. (A) Telocytes are found in close opposition to nervous trunks (nt) and blood vessels (v). Square marked area in (A) are enlarged in (B) and (C). (B, C) The perinuclear cytoplasm contains a small Golgi complex (arrows), elements of rough endoplasmic reticulum (rer) and distally, presents abundant mitochondria (m) and polyribosomes (asterisks). In this case, the surface cell membranes not covered with a basal lamina. TC: telocyte; MEC: mucosal epithelial cell.
The perinuclear cytoplasm contains a small Golgi complex, cytoskeletal elements as well as, elements of rough and smooth endoplasmic reticulum. Distally, the telocyte presents abundant mitochondria and polyribosomes (Fig. 2B and C).
The shape of the telocytes is according to the number of their telopodes: piriform for one prolongation (Fig. 3A), spindle for two telopodes, triangular for three, stellate, etc. (Fig. 3B). Their spatial appearance would be that of a polyhedron with a different number of vertices, depending on their telopode number. Of course, the number of prolongations as well as the ramifications of telopodes depend on the site and angle of section, because TEM is essentially a 2D examination of an extremely thin section (∼60 nm).
Fig 3.
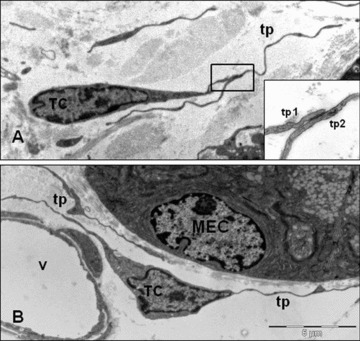
The shape of the telocytes is according to the number of their telopodes (tp). (A) Piriform for one prolongation. Inset: gap junction connecting the processes of two telopodes (tp1, tp2). (B) Triangular shape for two telopodes in the vicinity of a blood vessel (v). TC: telocytes; MEC: mucosal epithelial cell.
Figure 4(A) presents several examples of complex systems of telopodes which connect, for example, telocytes with immune cells via cell-to-cell contacts. Telopodes present a moniliform aspect which represent cellular prolongations of the telocytes.
Fig 4.
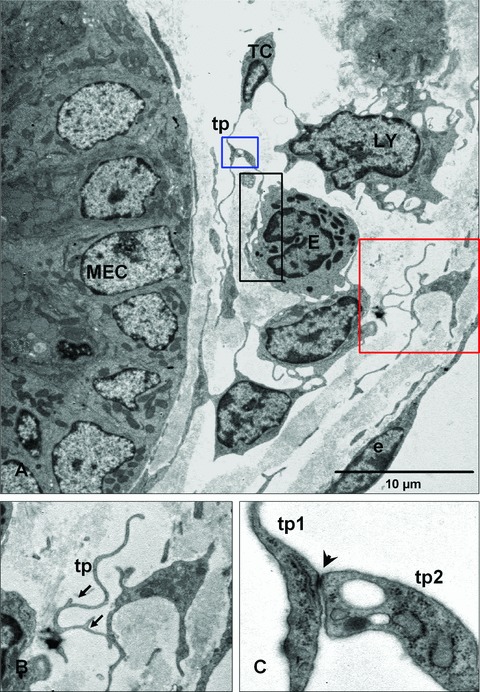
Relation of telocytes with other cells in the lamina propria. (A) The processes of a telocyte establish numerous interactions with adjacent cells in the lamina propria. A multicontact synapsis between an eosinophil and a telopode indicated by black rectangular area. Blue and red square illustrating higher magnification of telopodes (tp) from (A) in (B) and (C), respectively. (B) Distinctive dichotomous pattern of branching (arrows). (C) Direct cellular contact (arrowhead) between two telopodes (tp1, tp2) showing junctional complexes. MEC: mucosal epithelial cell; LY: lymphocyte; E: eosinophile cells; e: endothelial cell.
Another distinctive ultrastructural feature of telopodes is the formation of labyrinthine apparatus with very convoluted profile and a dichotomous patterns of branching (Fig. 4B), but, we have observed another having a surface without flexions or irregularities. Also, it is common that telopodes present dilations, where accommodate mitochondria and endoplasmic reticulum, and establish junctional complexes between two telopodes (Fig. 4C).
Figure 5 illustrates the exchange of molecules between telocytes and the extracellular matrix or other cells in lamina propria; the shape of the telopodes seems to help this telocyte to deliver multivesicular bodies as exosomes.
Fig 5.
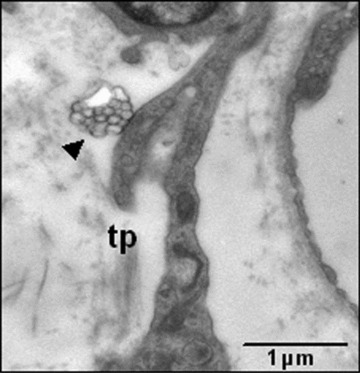
Electron micrographs show details of telopodes (tp) from lamina propria of rat duodenum. Exosomes (arrowhead) emerge from telopodes in the interstitial space.
For the last one the close vicinity with nerve fibres and blood vessel deserves to be mentioned. Telocytes have ‘strategic’ positioning in a tissue, in between blood capillaries and their specific target cells (mucosa epithelial cells) and in close contact with nerve endings (Fig. 2A). Some telocytes were observed very close to enterochromaffin cells, where they form close contacts with them. Enterochromaffin cells (neuroendocrine cells) present secreting granules generally spherical or spindle shaped, which contain electron-dense core (Fig. 6).
Fig 6.
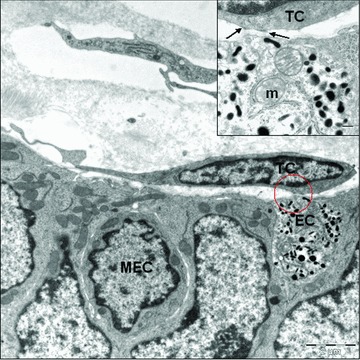
Close contact between telocyte (TC) and enterochromaffin cells (EC). Inset: electron-dense reinforcements in telocyte membrane (arrows). Note the enterochromaffin cell with large amounts of pleomorphic secretory granules. MEC: mucosal epithelial cell; m: mitochondria.
Discussion
Electron microscopy study unequivocally demonstrated the presence of the telocytes in the duodenum lamina propria, having their distinctive ultrastructural features according to the TEM diagnostic criteria for telocytes [20]. However, the ultrastructural features of telocytes comprise distinct cellular compartments: cell body, cellular prolongations (telopodes) and the labyrinthine system made of telopodes.
(1) Location in the connective interstitium, in the extraepithelial space, among functional elements: blood vessels, nerve endings, immunoreactive cells.
(2) Telopodes: (a) typical long, thin, moniliform prolongations, with (b) dichotomic branching pattern; (c) two to three telopodes are observed on single section; (d) specialized cell-to-cell junctions (gap junctions, junctional complexes).
Although the term telocytes is relatively new, the screening of the literature revealed several studies evaluating the presence the telocytes themselves in other organs: epicardium [21], myocardium [22], endocardium [23] and placenta [24].
The role of telocyte is unknown to us. However, based on several publications of telocytes, some relevant and potential roles were proposed. Popescu and Faussone-Pellegrini [20] suggested that telocytes are involved in intercellular signalling, taking into account the 3D network of telopodes and their strategic position in between target cells, blood capillary and nerve ending. They considered at least two mechanisms could be involved: (1) a paracrine and/or juxtacrine secretion of small signal molecules and (2) shedding microvesicles, which play unique roles in horizontal transfer of important macromolecules among neighbouring cells (e.g. proteins or RNAs, microRNA included).
Kostin (2010) suggested possible functions of telocytes in the myocardium:
(1) Telocytes are involved in intercellular signalling at distance because they are situated very close to blood capillaries and nerve endings.
(2) Telocytes, owing to their extremely long telopodes and their ability to form attachment plaques connecting them to the extracellular matrix, are predicted to function as mechanoreceptors/transducers.
(3) Telocytes are key players in cardiac renewal and, likewise, in cardiac repair.
Telocytes relations with the other elements of the lamina propria
Telocytes were often in close proximity to nerve bundles and blood vessels as shown also in other localization.
We observed the relationship between telocyte and neuroendocrine cells. Gut neuroendocrine cells are highly specialized mucosal epithelial cells that produce a wide range of hormones. Studies on the distribution and frequency of endocrine cells have been performed in the gastrointestinal tract of the rat [25] and human beings [26] and others, with a view to understanding their role in the gut. The neuroendocrine cells heterogeneity and concentration in the duodenum may be related to regulation of the secretion of pancreatic juice and bile as well as to functional control of the small intestine [27–29]. Instead, the telocyte–enterochromaffin cells proximity may have a special significance here.
Popescu described the close contacts (synapses) that establish ICC and ICC-like cells (now termed ‘telocytes’) with several types of immunoreactive cells: lymphocytes, plasma cells, eosinophils, basophils, macrophages and mast cells in human mammary gland and myometrium, rat stomach, gut, bladder and uterus. We also observed these contacts with eosinophils or lymphocytes present in the rat duodenum lamina propria. This author proposed that ICC-like processes might also act as ‘cellular’ guides for immune cells that arrive via blood stream. ICC-like might display gradients of membrane-bound or soluble chemoattractants and these cells might contribute to the process of leucocytes homing in connective tissues [30].
In conclusion, this study shows that telocytes exist in duodenum lamia propria. On the basis of their distribution and morphology, we suggest that these cells may be involved in immune response and in our opinion, it could be possible that different locations of telocytes could be associated with different roles. Currently, however, the presumed role(s) for lamina propria telocytes is the subject of speculation and further studies are required.
Acknowledgments
This research has received financial support from Aragon Institute of Health Sciences (I+CS) (PIPAMER 0903) and the European Social Fund (ESF), DGA (B83).
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Ramón y, Cajal S. Notes on the Auerbach’s plexus of the frog. Histological laboratory works of the Medicine Faculty of Barcelona. 1892:23–8. [Google Scholar]
- 2.Ramón y, Cajal S. Ganglia and nerve plexus of the mammal gut and some contributions to our studies on the spine and sympathetic nervous system. Madrid: Imprenta y Librerí de Nicolás Moya. 1893:5–37. [Google Scholar]
- 3.Hashitani H, Suzuki H. Identification of interstitial cells of Cajal in corporal tissues of the guinea-pig penis. Br J Pharmacol. 2004;141:199–204. doi: 10.1038/sj.bjp.0705622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidson RA, McCloskey KD. Morphology and localization of interstitial cells in the guinea pig bladder: structural relationships with smooth muscle and neurons. J Urol. 2005;173:1385–90. doi: 10.1097/01.ju.0000146272.80848.37. [DOI] [PubMed] [Google Scholar]
- 5.McHale NG, Hollywood MA, Sergeant GP, et al. Organization and function of ICC in the urinary tract. J Physiol. 2006;576:689–94. doi: 10.1113/jphysiol.2006.116657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sergeant GP, Thornbury KD, McHale NG, et al. Interstitial cells of Cajal in the urethra. J Cell Mol Med. 2006;10:280–91. doi: 10.1111/j.1582-4934.2006.tb00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen H, Rumessen JJ, Hansen A, et al. Ultrastructure of Cajal-like interstitial cells in the human detrusor. Cell Tissue Res. 2009;335:517–27. doi: 10.1007/s00441-008-0736-z. [DOI] [PubMed] [Google Scholar]
- 8.Bobryshev YV. Subset of cells immunopositive for neurokinin-1 receptor identified as arterial interstitial cells of Cajal in human large arteries. Cell Tissue Res. 2005;321:45–55. doi: 10.1007/s00441-004-1061-9. [DOI] [PubMed] [Google Scholar]
- 9.Harhun MI, Pucovsky V, Povstyan OV, et al. Interstitial cells in the vasculature. J Cell Mol Med. 2005;9:232–43. doi: 10.1111/j.1582-4934.2005.tb00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harhun M, Gordienko D, Kryshtal D, et al. Role of intracellular stores in the regulation of rhythmical [Ca2]i changes in interstitial cells of Cajal from rabbit portal vein. Cell Calcium. 2006;40:287–98. doi: 10.1016/j.ceca.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Pucovsky V, Harhun MI, Povstyan OV, et al. Close relation of arterial ICC-like cells to the contractile phenotype of vascular smooth muscle cell. J Cell Mol Med. 2007;11:764–75. doi: 10.1111/j.1582-4934.2007.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morel E, Meyronet D, Thivolet-Bejuy F, et al. Identification and distribution of interstitial Cajal cells in human pulmonary veins. Heart Rhythm. 2008;5:1063–7. doi: 10.1016/j.hrthm.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 13.Popescu L, Hinescu M, Radu E, et al. CD117/c-kit positive interstitial (Cajal-like) cells in human pancreas. J Cell Mol Med. 2005;9:738–9. doi: 10.1111/j.1582-4934.2005.tb00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popescu LM, Hinescu ME, Ionescu N, et al. Interstitial cells of Cajal in pancreas. J Cell Mol Med. 2005;9:169–90. doi: 10.1111/j.1582-4934.2005.tb00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutchings G, Williams O, Cretoiu D, et al. Myometrial interstitial cells and the coordination of myometrial contractility. J Cell Mol Med. 2009;13:4268–82. doi: 10.1111/j.1582-4934.2009.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gherghiceanu M, Popescu LM. Interstitial Cajal-like cells (ICLC) in human resting mammary gland stroma. Transmission electron microscope (TEM) identification. J Cell Mol Med. 2005;9:893–910. doi: 10.1111/j.1582-4934.2005.tb00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suciu L, Popescu LM, Gherghiceanu M. Human placenta: de visu demonstration of interstitial Cajal-like cells. J Cell Mol Med. 2007;11:590–7. doi: 10.1111/j.1582-4934.2007.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kostin S, Popescu LM. A distinct type of cell in myocardium: interstitial Cajal-like cells (ICLCs) J Cell Mol Med. 2009;13:295–308. doi: 10.1111/j.1582-4934.2008.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pieri L, Vannucchi MG, Faussone-Pellegrini MS. Histochemical and ultrastructural characteristics of an interstitial cell type different from ICC and resident in the muscle coat of human gut. J Cell Mol Med. 2008;12:1944–55. doi: 10.1111/j.1582-4934.2008.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popescu LM, Faussone-Pellegrini MS. TELOCYTES – a case of serendipity: the winding way from interstitial cells of Cajal (ICC), via interstitial Cajal-like cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010;14:729–40. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popescu LM, Manole CG, Gherghiceanu M, et al. Telocytes in human epicardium. J Cell Mol Med. 2010;14:2085–93. doi: 10.1111/j.1582-4934.2010.01129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kostin S. Myocardial telocytes: a specific new cellular entity. J Cell Mol Med. 2010;14:1917–21. doi: 10.1111/j.1582-4934.2010.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gherghiceanu M, Manole CG, Popescu LM. Telocytes in endocardium: electron microscope evidence. J Cell Mol Med. 2010;14:2330–34. doi: 10.1111/j.1582-4934.2010.01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suciu L, Popescu LM, Gherghiceanu M, et al. Telocytes in human term placenta: morphology and phenotype. Cells Tissues Organs. 2010;192:325–39. doi: 10.1159/000319467. [DOI] [PubMed] [Google Scholar]
- 25.Lundqvist M, Arnberg H, Candell J, et al. Silver stains for identification of neuroendocrine cells. A study of the chemical background. Histochem J. 1990;22:615–23. doi: 10.1007/BF01072943. [DOI] [PubMed] [Google Scholar]
- 26.Sjolund K, Sanden G, Hakanson R, et al. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1993;85:1120–30. [PubMed] [Google Scholar]
- 27.Kitamura N, Yamada J, Yamashita T, et al. Endocrine cells in the gastrointestinal tract of cat as revealed by various staining methods. Jap J Vet Sci. 1982;44:427–31. doi: 10.1292/jvms1939.44.427. [DOI] [PubMed] [Google Scholar]
- 28.Kitamura N, Yamada J, Calingasan NY, et al. Histologic and immunocytochemical study of endocrine cells in the gastrointestinal tract of the cow and calf. Am J Vet Res. 1985;46:1381–86. [PubMed] [Google Scholar]
- 29.Krause WJ, Yamada J, Cutts JH. Quantitative distribution of endocrine cells in the gastrointestinal tract of the adult opossum, Didelphis virginiana. J Anat. 1985;140:591–605. [PMC free article] [PubMed] [Google Scholar]
- 30.Popescu LM, Gherghiceanu M, Cretoiu D, et al. The connective connection: interstitial cells of Cajal (ICC) and ICC-like cells establish synapses with immunoreactive cells. Electron microscope study in situ. J Cell Mol Med. 2005;9:714–30. doi: 10.1111/j.1582-4934.2005.tb00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


