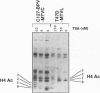
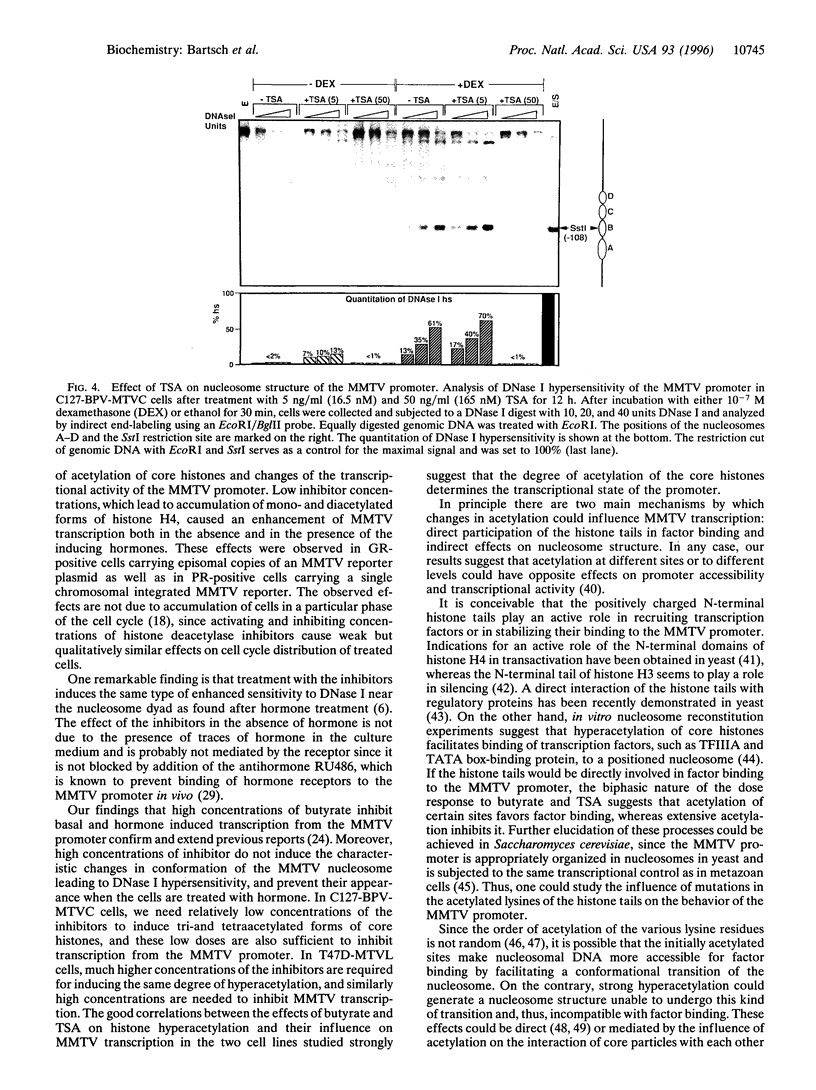
Abstract
The mouse mammary tumor virus (MMTV) promoter is regulated by steroid hormones through a hormone-responsive region that is organized in a positioned nucleosome. Hormone induction leads to a structural change of this nucleosome which makes its DNA more sensitive to cleavage by DNase I and enables simultaneous binding of all relevant transcription factors. In cells carrying either episomal or chromosomally integrated MMTV promoters, moderate acetylation of core histones, generated by treatment with low concentrations of the histone deacetylase inhibitors sodium butyrate or trichostatin A, enhances transcription from the MMTV promoter in the absence of hormone and potentiates transactivation by either glucocorticoids or progestins. At higher concentrations, histone deacetylase inhibitors reduce basal and hormone induced MMTV transcription. Inducing inhibitor concentrations lead to the same type of nucleosomal DNase I hypersensitivity as hormone treatment, suggesting that moderate acetylation of core histone activates the MMTV promoter by mechanisms involving chromatin remodeling similar to that generated by the inducing hormones.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfageme C. R., Zweidler A., Mahowald A., Cohen L. H. Histones of Drosophila embryos. Electrophoretic isolation and structural studies. J Biol Chem. 1974 Jun 25;249(12):3729–3736. [PubMed] [Google Scholar]
- Ausio J., van Holde K. E. Histone hyperacetylation: its effects on nucleosome conformation and stability. Biochemistry. 1986 Mar 25;25(6):1421–1428. doi: 10.1021/bi00354a035. [DOI] [PubMed] [Google Scholar]
- Bode J., Gómez-Lira M. M., Schröter H. Nucleosomal particles open as the histone core becomes hyperacetylated. Eur J Biochem. 1983 Feb 15;130(3):437–445. doi: 10.1111/j.1432-1033.1983.tb07170.x. [DOI] [PubMed] [Google Scholar]
- Boffa L. C., Vidali G., Mann R. S., Allfrey V. G. Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. J Biol Chem. 1978 May 25;253(10):3364–3366. [PubMed] [Google Scholar]
- Bresnick E. H., John S., Berard D. S., LeFebvre P., Hager G. L. Glucocorticoid receptor-dependent disruption of a specific nucleosome on the mouse mammary tumor virus promoter is prevented by sodium butyrate. Proc Natl Acad Sci U S A. 1990 May;87(10):3977–3981. doi: 10.1073/pnas.87.10.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candau R., Moore P. A., Wang L., Barlev N., Ying C. Y., Rosen C. A., Berger S. L. Identification of human proteins functionally conserved with the yeast putative adaptors ADA2 and GCN5. Mol Cell Biol. 1996 Feb;16(2):593–602. doi: 10.1128/mcb.16.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candido E. P., Reeves R., Davie J. R. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978 May;14(1):105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- Cartwright I. L., Hertzberg R. P., Dervan P. B., Elgin S. C. Cleavage of chromatin with methidiumpropyl-EDTA . iron(II). Proc Natl Acad Sci U S A. 1983 Jun;80(11):3213–3217. doi: 10.1073/pnas.80.11.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez S., Candau R., Truss M., Beato M. Constitutive repression and nuclear factor I-dependent hormone activation of the mouse mammary tumor virus promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1995 Dec;15(12):6987–6998. doi: 10.1128/mcb.15.12.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Anna J. A., Tobey R. A., Gurley L. R. Concentration-dependent effects of sodium butyrate in Chinese hamster cells: cell-cycle progression, inner-histone acetylation, histone H1 dephosphorylation, and induction of an H1-like protein. Biochemistry. 1980 Jun 10;19(12):2656–2671. doi: 10.1021/bi00553a019. [DOI] [PubMed] [Google Scholar]
- Durrin L. K., Mann R. K., Kayne P. S., Grunstein M. Yeast histone H4 N-terminal sequence is required for promoter activation in vivo. Cell. 1991 Jun 14;65(6):1023–1031. doi: 10.1016/0092-8674(91)90554-c. [DOI] [PubMed] [Google Scholar]
- Elgin S. C. The formation and function of DNase I hypersensitive sites in the process of gene activation. J Biol Chem. 1988 Dec 25;263(36):19259–19262. [PubMed] [Google Scholar]
- Goodrich J. A., Cutler G., Tjian R. Contacts in context: promoter specificity and macromolecular interactions in transcription. Cell. 1996 Mar 22;84(6):825–830. doi: 10.1016/s0092-8674(00)81061-2. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Grove G. W., Zweidler A. Regulation of nucleosomal core histone variant levels in differentiating murine erythroleukemia cells. Biochemistry. 1984 Sep 11;23(19):4436–4443. doi: 10.1021/bi00314a030. [DOI] [PubMed] [Google Scholar]
- Hebbes T. R., Clayton A. L., Thorne A. W., Crane-Robinson C. Core histone hyperacetylation co-maps with generalized DNase I sensitivity in the chicken beta-globin chromosomal domain. EMBO J. 1994 Apr 15;13(8):1823–1830. doi: 10.1002/j.1460-2075.1994.tb06451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbes T. R., Thorne A. W., Clayton A. L., Crane-Robinson C. Histone acetylation and globin gene switching. Nucleic Acids Res. 1992 Mar 11;20(5):1017–1022. doi: 10.1093/nar/20.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbes T. R., Thorne A. W., Crane-Robinson C. A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 1988 May;7(5):1395–1402. doi: 10.1002/j.1460-2075.1988.tb02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht A., Laroche T., Strahl-Bolsinger S., Gasser S. M., Grunstein M. Histone H3 and H4 N-termini interact with SIR3 and SIR4 proteins: a molecular model for the formation of heterochromatin in yeast. Cell. 1995 Feb 24;80(4):583–592. doi: 10.1016/0092-8674(95)90512-x. [DOI] [PubMed] [Google Scholar]
- Juan L. J., Utley R. T., Adams C. C., Vettese-Dadey M., Workman J. L. Differential repression of transcription factor binding by histone H1 is regulated by the core histone amino termini. EMBO J. 1994 Dec 15;13(24):6031–6040. doi: 10.1002/j.1460-2075.1994.tb06949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. Y., Hayes J. J., Pruss D., Wolffe A. P. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell. 1993 Jan 15;72(1):73–84. doi: 10.1016/0092-8674(93)90051-q. [DOI] [PubMed] [Google Scholar]
- Littlefield B. A., Cidlowski J. A. Increased steroid responsiveness during sodium butyrate-induced "differentiation" of HeLa S3 cells. Endocrinology. 1984 Feb;114(2):566–575. doi: 10.1210/endo-114-2-566. [DOI] [PubMed] [Google Scholar]
- Lucibello F. C., Truss M., Zwicker J., Ehlert F., Beato M., Müller R. Periodic cdc25C transcription is mediated by a novel cell cycle-regulated repressor element (CDE). EMBO J. 1995 Jan 3;14(1):132–142. doi: 10.1002/j.1460-2075.1995.tb06983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Hager L., Palmiter R. D. Butyrate and related inhibitors of histone deacetylation block the induction of egg white genes by steroid hormones. Cell. 1980 Nov;22(2 Pt 2):469–477. doi: 10.1016/0092-8674(80)90357-8. [DOI] [PubMed] [Google Scholar]
- Müller H., Beato M. RNA synthesis in rabbit endometrial nuclei. Hormonal regulation of transcription of the uteroglobin gene. Eur J Biochem. 1980 Nov;112(2):235–241. doi: 10.1111/j.1432-1033.1980.tb07199.x. [DOI] [PubMed] [Google Scholar]
- Norton V. G., Imai B. S., Yau P., Bradbury E. M. Histone acetylation reduces nucleosome core particle linking number change. Cell. 1989 May 5;57(3):449–457. doi: 10.1016/0092-8674(89)90920-3. [DOI] [PubMed] [Google Scholar]
- Norton V. G., Marvin K. W., Yau P., Bradbury E. M. Nucleosome linking number change controlled by acetylation of histones H3 and H4. J Biol Chem. 1990 Nov 15;265(32):19848–19852. [PubMed] [Google Scholar]
- Oliva R., Bazett-Jones D. P., Locklear L., Dixon G. H. Histone hyperacetylation can induce unfolding of the nucleosome core particle. Nucleic Acids Res. 1990 May 11;18(9):2739–2747. doi: 10.1093/nar/18.9.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry C. A., Annunziato A. T. Histone acetylation reduces H1-mediated nucleosome interactions during chromatin assembly. Exp Cell Res. 1991 Oct;196(2):337–345. doi: 10.1016/0014-4827(91)90269-z. [DOI] [PubMed] [Google Scholar]
- Piña B., Brüggemeier U., Beato M. Nucleosome positioning modulates accessibility of regulatory proteins to the mouse mammary tumor virus promoter. Cell. 1990 Mar 9;60(5):719–731. doi: 10.1016/0092-8674(90)90087-u. [DOI] [PubMed] [Google Scholar]
- Richard-Foy H., Hager G. L. Sequence-specific positioning of nucleosomes over the steroid-inducible MMTV promoter. EMBO J. 1987 Aug;6(8):2321–2328. doi: 10.1002/j.1460-2075.1987.tb02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridsdale J. A., Hendzel M. J., Delcuve G. P., Davie J. R. Histone acetylation alters the capacity of the H1 histones to condense transcriptionally active/competent chromatin. J Biol Chem. 1990 Mar 25;265(9):5150–5156. [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Schlake T., Klehr-Wirth D., Yoshida M., Beppu T., Bode J. Gene expression within a chromatin domain: the role of core histone hyperacetylation. Biochemistry. 1994 Apr 12;33(14):4197–4206. doi: 10.1021/bi00180a012. [DOI] [PubMed] [Google Scholar]
- Sealy L., Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978 May;14(1):115–121. doi: 10.1016/0092-8674(78)90306-9. [DOI] [PubMed] [Google Scholar]
- Sommerville J., Baird J., Turner B. M. Histone H4 acetylation and transcription in amphibian chromatin. J Cell Biol. 1993 Jan;120(2):277–290. doi: 10.1083/jcb.120.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. H., Elgin S. C. Protein/DNA architecture of the DNase I hypersensitive region of the Drosophila hsp26 promoter. EMBO J. 1988 Jul;7(7):2191–2201. doi: 10.1002/j.1460-2075.1988.tb03058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. S., Hecht A., Grunstein M. Histones and the regulation of heterochromatin in yeast. Cold Spring Harb Symp Quant Biol. 1993;58:247–256. doi: 10.1101/sqb.1993.058.01.029. [DOI] [PubMed] [Google Scholar]
- Thompson J. S., Ling X., Grunstein M. Histone H3 amino terminus is required for telomeric and silent mating locus repression in yeast. Nature. 1994 May 19;369(6477):245–247. doi: 10.1038/369245a0. [DOI] [PubMed] [Google Scholar]
- Truss M., Bartsch J., Beato M. Antiprogestins prevent progesterone receptor binding to hormone responsive elements in vivo. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11333–11337. doi: 10.1073/pnas.91.24.11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truss M., Bartsch J., Schelbert A., Haché R. J., Beato M. Hormone induces binding of receptors and transcription factors to a rearranged nucleosome on the MMTV promoter in vivo. EMBO J. 1995 Apr 18;14(8):1737–1751. doi: 10.1002/j.1460-2075.1995.tb07163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truss M., Chalepakis G., Slater E. P., Mader S., Beato M. Functional interaction of hybrid response elements with wild-type and mutant steroid hormone receptors. Mol Cell Biol. 1991 Jun;11(6):3247–3258. doi: 10.1128/mcb.11.6.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B. M. Decoding the nucleosome. Cell. 1993 Oct 8;75(1):5–8. [PubMed] [Google Scholar]
- Turner B. M., Fellows G. Specific antibodies reveal ordered and cell-cycle-related use of histone-H4 acetylation sites in mammalian cells. Eur J Biochem. 1989 Jan 15;179(1):131–139. doi: 10.1111/j.1432-1033.1989.tb14530.x. [DOI] [PubMed] [Google Scholar]
- Turner B. M., O'Neill L. P. Histone acetylation in chromatin and chromosomes. Semin Cell Biol. 1995 Aug;6(4):229–236. doi: 10.1006/scel.1995.0031. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P., Almouzni G., Ura K., Pruss D., Hayes J. J. Transcription factor access to DNA in the nucleosome. Cold Spring Harb Symp Quant Biol. 1993;58:225–235. doi: 10.1101/sqb.1993.058.01.027. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Kijima M., Akita M., Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990 Oct 5;265(28):17174–17179. [PubMed] [Google Scholar]
- Zaret K. S., Yamamoto K. R. Reversible and persistent changes in chromatin structure accompany activation of a glucocorticoid-dependent enhancer element. Cell. 1984 Aug;38(1):29–38. doi: 10.1016/0092-8674(84)90523-3. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]
- van Holde K. Transcription. The omnipotent nucleosome. Nature. 1993 Mar 11;362(6416):111–112. doi: 10.1038/362111a0. [DOI] [PubMed] [Google Scholar]