Abstract
Heart disease and atherosclerosis are the leading causes of morbidity and mortality worldwide. The lack of suitable autologous grafts has produced a need for artificial grafts; however, current artificial grafts carry significant limitations, including thrombosis, infection, limited durability and the inability to grow. Tissue engineering of blood vessels, cardiovascular structures and whole organs is a promising approach for creating replacement tissues to repair congenital defects and/or diseased tissues. In an attempt to surmount the shortcomings of artificial grafts, tissue-engineered cardiovascular graft (TECVG), constructs obtained using cultured autologous vascular cells seeded onto a synthetic biodegradable polymer scaffold, have been developed. Autologous TECVGs have the potential advantages of growth, durability, resistance to infection, and freedom from problems of rejection, thrombogenicity and donor scarcity. Moreover polymers engrafted with growth factors, cytokines, drugs have been developed allowing drug-releasing systems capable of focused and localized delivery of molecules depending on the environmental requirements and the milieu in which the scaffold is placed. A broad range of applications for compound-releasing, tissue-engineered grafts have been suggested ranging from drug delivery to gene therapy. This review will describe advances in the development of drug-delivery systems for cardiovascular applications focusing on the manufacturing techniques and on the compounds delivered by these systems to date.
Keywords: tissue engineering, cardiovascular graft, drug delivery, growth factors
Introduction
Heart disease and atherosclerosis still represent the leading causes of morbidity and mortality worldwide. The lack of suitable autologous grafts has produced a need for artificial grafts but the patency of these grafts is limited in comparison to natural materials. Tissue engineering, of blood vessels, cardiovascular structures and whole organs holds promises as a new approach for creating replacement tissues to repair congenital defects and/or diseased tissues. Current artificial grafts carry significant limitations, including thrombosis, infection, limited durability and inability to grow. In an attempt to surmount these shortcomings, creation of tissue-engineered cardiovascular vascular grafts (TECVG), constructs by using cultured autologous vascular wall cells seeded onto a synthetic biodegradable polymer scaffold, have been developed [1, 2]. Autologous TECVGs have the potential advantages of growth, durability, resistance to infection, and freedom from problems of rejection, thrombogenicity and donor scarcity. Both pre-clinical and clinical studies have demonstrated the feasibility of constructing functional large-diameter (6 mm) vascular grafts from autologous vascular cells seeded onto a biodegradable tubular matrix [3–5].
Until now several types of polymers have been proposed to obtain vascular grafts and poly(lactic-co-glycolic acid) polymers (PLGA) and poly-L-lactic acid (PLLA) represent the most attractive avenue for this purpose as their advantageous mechanical properties, plasticity and biocompatibility [6]. These materials are biodegradable, with the aim of in vivo vascular cell remodelling to enable the final functional conduit to perform the structural role as well. To this extent, the degradation process with further possibility of remodelling is to be pursued especially to avoid aneurysmal degeneration and rupture, as it does confer adequate strength to the conduit. There is a good piece of evidence underlining biodegradable porous polymer scaffolds as an attractive alternative to develop temporal templates for tissue regeneration, prosthetic devices and stents.
Many different techniques have been developed for the construction of vascular scaffolds including solvent casting/particulate leaching, phase separation, emulsion freeze drying, fibre extrusion and fabric forming processing, gas foaming and lately the electro-spinning technique [7].
Moreover, polymers engrafted with growth factors, cytokines and drugs have been developed [8–10], thus obtaining drug-releasing systems capable of focused and localized delivery of molecules depending on the environment requirements and the actual milieu in which the scaffold is placed. A broad range of applications for electrospun fibres have been suggested [11, 12] ranging from drug delivery [13–17] to gene therapy [18].
However, in many cases of tissue-engineering applications, cell differentiation towards a specific phenotype is highly desirable. In situ local delivery of various drugs and protein growth factors within tissue-forming cell/scaffold constructs has been investigated [19, 20]. Local sustained delivery of various growth factors such as epidermal growth factor [21], transforming growth factor (TGF) [22], vascular endothelial growth factor (VEGF) [23, 24] and bone morphogenic proteins (BMPs) [25] within the biodegradable scaffolds has been used to stimulate the differentiation of seeded cells to desirable specific organized tissues. To this extent, because of their biochemical properties, it is possible to produce growth factor microspheres to encapsulate within polymeric biomaterials or direct incorporate into the void space of the scaffolds to achieve sustained release [20].
In tissue engineering of blood vessels and cardiovascular structures, several autologous cell types have been used to seed the constructs in particular endothelial cells obtained from great saphenous vein even if this raise a good number of issues concerning cells amount and availability [26]. Recently, bone marrow stem cells were seeded into macroporous biodegradable polymer scaffolds and implanted to regenerate or heal injured tissues [27]. Previously, a 3D collagen type I matrix has been seeded with human umbilical cord blood mononuclear cells and grafted onto infarcted ventricles in an ischemic heart murine model with notable improvement in the left ventricular function and myocardial remodelling [28]. Recently, a similar approach has been successfully used in a clinical trial (MAGNUM Trial), with a bone marrow cells seeded collagen sponge applied on top of the scarred area aiming at regenerating myocardial cells and restoring the extracellular matrix (ECM) function which is deeply altered following myocardial infarction [29, 30].
This review will describe the actual advances in development of drug-delivery systems (DDSs) for cardiovascular applications focusing on the manufacturing techniques and on the molecules delivered by these systems until now.
Drug delivery systems in cardiovascular applications – what are the aims/targets?
In the cardiovascular research field, biosynthetic and biodegradable materials have been largely investigated. A wide range of natural materials ranging from collagen [31–33], fibrin [34], hyaluronic acid [35] to approaches involving tissue decellularization [36, 37] have been evaluated (Table 1). With the aim to improve the long- and short-term management of the tissue-engineering vascular graft following its implantation, a variety of local DDSs have been developed.
1.
Biomaterial used for cardiovascular applications
| Source | Biomaterial | Application | ||
|---|---|---|---|---|
| Natural | Matrigel [31, 209, 210] | Cardiac | ||
| Matrigel [32] | Cell differentiation | |||
| Collagen [28, 31, 33] | Cardiac | |||
| Collagen [211] | Vascular | |||
| Hyaluronic acid [35] | Vascular | |||
| Alginate [61] | Cell differentiation | |||
| Fibrin [34] | Cardiac | |||
| Fibrin [35] | Vascular | |||
| Decellularized vessel [37] | Vascular | |||
| Decellularized small intestine [36] | Vascular | |||
| submucosa | ||||
| Synthetic | Poly-L-lactic acid (PLLA) [212] | Cell differentiation | ||
| PLLA [213] | Vascular | |||
| Poly(lactic-co-glycolic acid) (PLGA) [212] | Cell differentiation | |||
| PLGA [40] | Vascular | |||
| Polyglycolic acid [214] | Vascular | |||
| poly(epsilon-caprolactone) (PCL) [215] | Vascular | |||
| PCL- Poly Urethan [216] | Vascular | |||
| Peptide nanofibres [64, 217] | Cardiac | |||
| Peptide nanofibres [64] | Vascular | |||
| Poly(diol citrates) [218, 219] | Vascular | |||
| Poly(glycerol-sebacate) [220] | Cardiac and vascular | |||
| Bioglass [221] | Vascular | |||
In the early post-implantation phase, the local environment undergoes a number of dramatic changes that are considered to be at the root of the loss of in vivo performance of biomedical devices. Implanted devices have been shown to typically trigger a cascade of immunogenic reactions in response to the implantation-related trauma that results in acute inflammation with dense infiltration of inflammation-mediating cells at the device–tissue interface [38]. The acute inflammation associated to the presence of a foreign object can eventually lead to complications that can adversely impact or compromise the performance of implantable devices [39]. The maintaining of the inflammatory stimulus provoked by chronically implanted devices turns in the accumulation of dense fibrotic tissue which encapsulates the device with the regression of blood vessel growth, remarking a shift to a chronic state of the inflammation process [40]. In myocardial tissue engineering this process results further in issues concerning mechanical resistance and endothelialization of the inner graft's surface [41].
Cells survival, proliferation and differentiation require the development of an adequate vascularization especially at the early stage of implantation. Even if the foreign body response to the implanted material is known to induce a certain degree of capillary ingrowth over time, this native angiogenic process is likely to be either too slow or quantitatively inadequate in terms of capillary density, especially considering dimensions and thickness of implanted grafts. Mass transfer limitations and fibrosis associated with the foreign body response can result in non-sufficient nutrient transport for most cells in constructs approximately 1-mm thick and greater [42–44] leading to implant necrosis [45]. Carrier et al.[46, 47] and Radisic et al.[48–50] demonstrated the importance of the oxygen supply in producing thick and resistant new tissue on tissue-engineered cardiac grafts (TECGs). They found that only increasing the oxygen concentration supply to an in vitro TECG, improved the production of cardiac muscle [47, 51]. Increasing oxygen availability by increasing endothelialization is especially important as most TECGs and myocardial patches are directly applied to an infracted area in in vivo experimental models to provide ventricular mechanical support.
Thus, stimulation of rapid formation of high-density local vasculature is crucial for tissue regeneration. A highly open porous and sponge-like structure is desirable in order to provide a sufficient cell seeding density and to ease mass transfer rates of nutrients and oxygen within the cell-seeded scaffolds. In this environment, microvessels may sprout from host vasculatures and penetrate into the scaffolds by a natural angiogenesis process. However, this type of neovascularization phenomenon is rare and insufficient to support the whole area of implanted constructs leading to insufficient tissue formation with massive cell death often observed in the early-phase of implantation [52, 53].
Therefore, inducing angiogenesis into the inner pore space of scaffolds is essential. Incorporation of angiogenic growth factors such as basic fibroblast growth factor (bFGF) and VEGF, among others, into scaffolds for controlled release has been shown to promote local angiogenesis [42, 54] (Fig. 1). This effect could be crucial in pathological states as critical limb ischemia. Here the sustained pro-angiogenic stimulus driven by the inappropriate tissue oxygen level could be fostered by the release of specific growth factors leading to a rapid vessel sprouting and collateralization. Moreover, addition of growth factors and other types of molecular signals to the scaffolds could result in the generation of a molecular device providing a leading framework for guided cell differentiation and tissue regeneration (Table 2). In the vascular field, trophic cutaneous lesions related to microvessel diseases as diabetic or venous insufficiency-related ulcers, could benefit from these approaches for skin reconstruction following tissue loss. But this concept becomes even more relevant in cardiac tissue engineering where use of artificial myocardial patches is desirable to both limit geometrical and shape remodelling of the infarcted myocardium and to promote tissue restoration. At the cellular level the sudden suppression of oxygen supply triggers molecular cascades and a series of other dramatic biological events all modulated by intricate molecular networks. The inflammatory state associated with the early-phases of wound healing is characterized by a high regenerative and proliferative capacity of both cardiac and mobilized stem cells. These initial adaptive mechanisms, especially sustained by neurohormonal activation, further lead to a deleterious counterpart represented by fibroblast proliferation and ECM deposition with the aim to compensate tissue loss and prevent ventricular dilation. At this point the proliferative capacity and the actual viability of myocardiocytes are dramatically reduced with the generation of a non-functional scar. To this extent, engineered functionalized myocardium might represent an amenable alternative as it could provide both a mean for mechanical restraint and prevention of ventricular dilation. At the same time it could generate an environment to maintain the proliferative capacity of the cells surrounding the infarcted area providing a molecular pathway to promote cell differentiation (Fig. 2).
1.
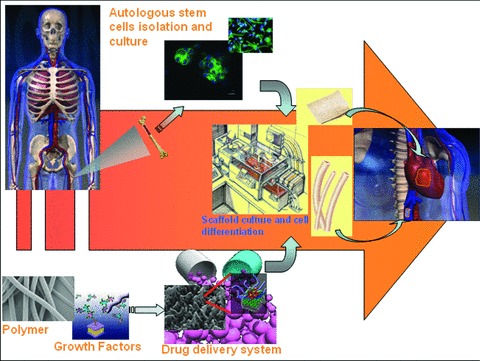
Basic concept of application of drug-delivery systems (DDS) to tissue engineering. Use of stem cells and DDS could constitute a bridge between cell biology, tissue engineering and pharmacology eventually leading to a final optimized product for clinical applications.
2.
Drug and growth factor used in tissue engineering for cardiovascular purposes
| Drug/growth factor | Biomaterial |
|---|---|
| Dexamethasone | Poly(D,L-lactic-co-glycolic acid) (PLGA) [89] |
| PLGA microspheres/poly(vinyl alcohol) hydrogel composite [40] | |
| VEGF | Poly(lactide-co-glycolide) (PLG) [109] |
| Fibrin [222] | |
| Collagen [223] | |
| Gelatine [224] | |
| Alginate [114] | |
| PDGF-BB b-FGF | Poly(D,L-lactic-co-glycolic acid) (PLGA) [71] Alginate [69] |
| poly(D,L-lactic-co-glycolic acid) (PLGA) [132] | |
| Poly(ester urethane)urea [73] | |
| Fibrin [225] | |
| Gelatine [226] | |
| TGF-β | Gelatine [227] |
| Insulin-like growth factor 1 | Self-assembling nanofibres [134] |
| HGF | Gelatine [228] |
| Heparin | Alginate [81] |
| Chitosan [82] | |
| Hyaluronic acid [83] | |
| Poly(D,L-lactic-co-glycolic acid) (PLGA) [80] | |
| Heparin | Poly(epsilon-caprolactone)(PCL) [9] |
| Polymer-coated metallic wires [139] | |
| Diazeniumdiolates nitric oxide donor | Polyurethane [143] |
| Polyethylene glycol (PEG) [146] | |
| Plasmid DNA encoding platelet-derived growth factor (PDGF) gene | Poly(D,L-lactic-co-glycolic acid) (PLGA) [180] |
2.
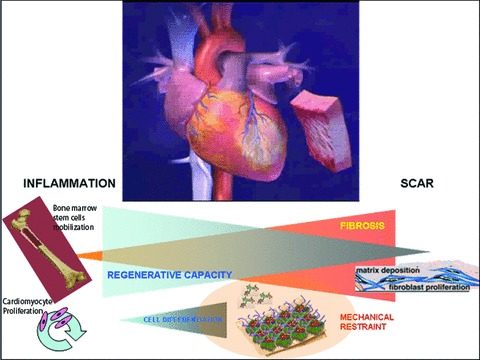
Potential mechanisms of action of engineered functionalized myocardium. Following a myocardial infarction a progressive decrease in the regenerative capacity of cardiac and mobilized stem cells together with a parallel shifting towards fibrosis is seen. Artificial functionalized myocardial patches could provide both a mean for mechanical restraint and prevention of ventricular dilation. At the same time they could generate an environment to maintain the proliferative capacity of the cells surrounding the infarcted area providing a molecular pathway to promote cell differentiation.
Approaches, methodologies and manufacturing techniques
Since a lack of blood supply to transplanted cells at the early stage of implantation retards the survival, proliferation and differentiation of cells, therapeutic angiogenesis via sustained delivery of angiogenic growth factors at a localized region is essential for tissue regeneration [23, 55] Several different techniques have been used for the fabrication of scaffolds for tissue engineering. These include solvent casting/particulate leaching, phase separation, emulsion freeze drying, fibre extrusion and fabric forming processing and gas foaming [7]. Of late, the electrospinning technique is becoming one of the most effective and functional approaches [56] with the possibility to be combined to the newest methodologies of cell seeding [57, 58]. The electrospinning process allows for control over the morphology of the fibres and utilization of a wide variety of polymers. The small fibre diameters produced by electrospinning have the advantage of a large surface-to volume ratio, as well as a high permeability and interconnecting pore structure, both of which are desirable in a biological setting (Fig. 3). Further, this material can be functionalized with compounds encouraging cell survival, proliferation and differentiation obtaining a DDS.
3.
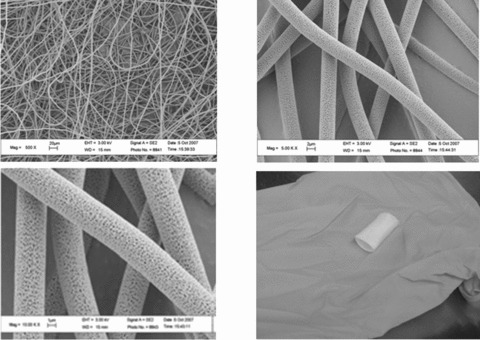
S.E.M. microphotographs of electrospun poly-L-lactic acid polymer for vascular tissue engineering. Electrospinning technique provides a useful mean to generate a native extracellular matrix like structure to mimic the native histoarchitecture thereby facilitating cell attachment, proliferation and differentiation. Bottom right: vascular prosthesis realized by electrospinning. Data from Chemistry and Biomaterials Laboratory, University ‘Campus Bio-Medico’ of Rome, Italy.
An appealing class of delivery vehicles is represented by hydrogels, as they can be introduced into the body with minimally invasive procedures [59] and are often highly biocompatible, owing to their high water content. Alginate is a naturally occurring (linear unbranched) polysaccharide composed of an a-L-guluronic and b-D-mannuronic acid sugar residues. Sodium salts of alginate are water soluble, but in the presence of divalent cations (such as Ca2+), alginate chains form ionic cross-links, leading to gelation. Owing to the gentle nature of this gelation process, Ca + cross-linked alginate hydrogels have been widely used in several medical applications (dental impressions, drug-delivery devices and as immobilization matrices for cells) [60, 61]. The main issue with these materials concerns the fact that, despite their biocompatibility, these gels undergo a slow and unpredictable degradation in vivo harnessing their utility. Several studies have reported attempts to control alginate gel degradation by partial oxidization of the polymer chains with sodium periodate [62], with the lower levels of oxidation maintaining the gel biocompatibility [63]. An alternative strategy relies on controlling the polymer molecular weight distribution, with the use of a binary molecular weight formulation that allows the incorporation of low molecular weight chains, which more readily disassociate from the gel and can be subsequently excreted from the body [62]. To this extent, growth factor release could be tuned by controlling both diffusion and degradation. Another interesting class of compound amenable for tissue engineering and drug-releasing systems is represented by self-assembling peptides, a class of synthetic, ionic, oligopeptides that spontaneously assemble into gels with an ECM-like microarchitecture when exposed to salt [64].
These peptide scaffolds can be further functionalized through direct solid-phase synthesis extension of short-sequence motifs from two major proteins of the basement membrane, laminin 1 (YIGSR, RYVVLPR) and collagen IV (TAGSCLRKFSTM). It has been shown that these tailor-made scaffolds increased the formation of confluent cell monolayers with cobblestone phenotype of human aortic endothelial cells in culture. These functionalized scaffolds can maintain the typical phenotype of endothelial cells which not only preserve low-density lipoprotein uptake activity but also enhance their nitric oxide release capacity, and can promote basement membrane protein deposition as laminin 1 and collagen IV, which are physiological prerequisites for successful culture and expansion of endothelial cells [64]. Moreover, these biomimetic ECM-like materials support capillary morphogenesis and thus may be useful for supporting vascularization [65].
However, for tissue regeneration of complex structures such as cardiovascular components, scaffolds aiming to recapitulate the native histoarchitecture are needed. Again, the necessity to create a specific microenvironment with factors able to promote and orientate cell proliferation requires a careful evaluation and widely spans in the drug-delivery spectrum of interest. Until now several methods to incorporate growth factors into synthetic scaffolds have been developed [42, 55, 66–69]. Growth factors can be adsorbed to the scaffold [70]. Adsorption efficiency is material related and concerns not only the chemical influence but also physical properties of the scaffold itself as the porosity and the nature of the pores. Moreover, it depends on the structure of the growth factors too, which can in turn affect release kinetic. As proteins are generally adsorbed through electrostatic attractions between anionic group and the scaffold surface, growth factors presenting only a few of these reactive groups can display lower adsorption capacities on biomaterials as elegantly showed by Ziegler and colleagues who combined ceramic materials with bFGF and VEGF [70].
It is also possible to blend growth factor containing microspheres into the scaffold [71] without interfering with its macro and microstructure. Microsphere incorporation allows for a fine modulation of the release kinetics by adjusting the molecular weight and composition of the copolymer, eventually avoiding undesirable initial releases bursts.
Recently, Patil and colleagues developed a novel PLGA micros-phere/polyvinyl alcohol (PVA) hydrogel composite DDS that may be suitable for coating implantable devices for localized delivery of medicinal agents. These composites combine the structural mechanical properties and relative tissue compatibility of physically cross-linked PVA hydrogels with the slow release properties of PLGA microspheres to provide a drug-delivery coating device able to provide a zero-order release profile [40].
Alternatively, a direct mixing growth factor containing protein powder into the scaffold during processing can be performed [24, 53, 72].
Adsorbing growth factor onto the scaffold has the drawback of low loading efficiencies and rapid release together with the degradation of growth factors such as rhVEGF, bone morphogenetic protein 4 (BMP-4) and bFGF during a very short release time [70]. Loading growth factor into microspheres, even if elicits a regulated sustained release, could be harnessed by a loss in bioactivity due to harsh solvents such as methylene. Incorporating growth factors directly into the scaffold can potentially surmount these shortcomings. In particular, the use of a thermoplastic scaffold would be amenable to the mixing of growth factor during polymer processing providing an appropriately mild solvent or low processing temperature. Guan and colleagues have developed a biodegradable, elastomeric poly(ester urethane)urea (PEUU) scaffold capable of releasing bioactive bFGF over a period of several weeks [73].
Nevertheless, more controllable growth factor delivery systems are definitely needed for angiogenesis. Another approach to the controlled delivery issues regards the modification of the scaffold itself.
Surface-modified PLGA scaffolds have been developed in order to enhance adhesion and function of rat hepatocytes, bovine chondrocytes and bone marrow stem cells by surface immobilization of bioactive molecules such as galactose, cell adhesive pep-tides and hyaluronic acid [74–77].
The idea at the basis of this strategy concerns the mimicry of the natural ECM with the final aim to improve cell adhesion, proliferation and differentiation. Among the ECM components, heparin has been known to specifically bind various angiogenic growth factors such VEGF, bFGF and TGF-β[23, 78, 79]. Through specific interactions occurring between both 2-O-sulphate group and N-sulphate group of heparin and lysine and arginine residues of the growth factors, heparin could be reliably considered as playing a depot role in the ECM for the growth factors, enabling them to diffuse out in a sustained manner. Thus addition of heparin on a supporting scaffold could represent a functionalization strategy enabling the scaffold itself to increase its drug-delivery capabilities. Furthermore, it has been shown that the binding of bFGF to heparin augments its stability against thermal denaturation and enzymatic digestion at physiological conditions [80] by inducing a conformational change in the molecule. In relation to both this protective effect and the heparin-specific affinity towards angiogenic growth factors, a good piece of literature has been focused on controlled release of growth factors using heparin-conjugated natural polysaccharides such as alginate, chitosan and hyaluronic acid [81–83]. In these studies, heparin-modified hydrogel matrices loaded with growth factors were intended to be injected into a desired tissue defect site to induce local angiogenesis.
However, it is important to consider that the in vivo release of proteins may result in a more severe inflammatory response as a consequence of the body's response to the foreign protein, in comparison to that resulting from the implant alone. It can further exacerbate the condition and thus aggravate the adverse effects.
Another approach for scaffold surface modification concerns the addition to the polymer of other bioactive materials that present angiogenic capabilities. To this extent, glasses represent an interesting class of compounds. Since the observation by
Hench and colleagues in 1971 that glasses in the system SiO2–CaO–NaO–P2O5 possess bioactivity, these materials have found use in a wide range of biomedical applications including permanent implants, dentistry, bone reconstruction and tissue-engineering scaffolds [84]. More recently, it has been discovered that certain compositions of bioactive glass stimulate a significant increase in the secretion of angiogenic growth factors from fibroblasts and can induce infiltration of a significantly increased number of blood vessels into synthetic scaffolds [85, 86]. This effect can be incorporated into biodegradable tissue-engineering scaffolds in order to obtain bioactive glass-coated polymer composites able to induce angiogenesis. The ability of bioactive glass-coated polymer scaffolds to stimulate the secretion of angiogenic growth factors and subsequent in-growth of blood vessel into the scaffolds could offer a novel way of ensuring that soft-tissue-engineered constructs become sufficiently vascularized. These composites can be obtained by phase separation, solvent casting/salt leaching and gel foaming adding Bioglass particles to the polymer solution and have been shown to retain the surrounding granulation tissue and a higher number of blood vessels in an in vivo model [87]. Moreover, it has been recently suggested that the pro-angiogenic capacity of this material is related to the soluble dissolution products of Bioglass and the subsequent production of cell-secreted angiogenic factors by stimulated cells [88].
Drug and molecules released
Steroids
With the aim to reduce the foreign body response following graft implantation and interrupt the vicious circle eventually leading to the failure of the biomedical device, corticosteroids have been used during the scaffolds fabrication. Considering this rationale, Yoon and colleagues developed a sustained releasing dexamethasone biodegradable scaffold showing the possibility to achieve an inhibiting effect on smooth muscle cell proliferation [89]. From this point of view, dexamethasone releasing porous and biodegradable scaffolds could be potentially used either as an anti-inflammatory porous prosthetic device or as a temporal anti-inflammatory biodegradable stent for reducing intimal hyper-plasia in restenosis. Patil and colleagues developed a PLGA microsphere/PVA hydrogel composite DDS with zero-order release profile that provides a reservoir with a constant amount drug delivery. Dexamethasone loading of this device could be more adequate in the control of inflammatory responses at the tissue/implant interface.
Moreover, due to its capability to induce cell differentiation, corticosteroid drug-releasing scaffolds could be usefully employed in tissue engineering of stem cells [90]. Dexamethasone, along with other cytokines, has been used as a stimulating agent for the differentiation of stem cells into bone cells [91, 92]. With this in mind drug loaded scaffolds could be employed to create a specific differentiating microenvironment useful in tissue regeneration. For instance, a scaffold implanted in a bone defect site in vivo could be colonized by bone marrow stem cells recruited and migrated from the local tissue, that might more readily differentiate into bony osteoblast cells due to the locally sustained released dexamethasone.
However, although administration of anti-inflammatory agents such as dexamethasone can minimize implantation-associated inflammation [40, 93, 94], their delivery can inhibit endogenous blood vessel growth. A large number of studies proved that dexamethasone administration can inhibit or down-regulate some of the key factors responsible for the formation of blood vessels such as endogenous VEGF and other angiogenic growth factors [95–98]. A decrease in the available blood circulation surrounding the implant could potentially limit graft functionality and retard healing of the trauma related to the implantation itself. Thus an amenable strategy could be the co-administration of both anti-inflammatory corticosteroids as well as growth factors in a localized environment at low doses so that both agents can function symbiotically without interference [93].
Growth factors
Localized delivery of growth factors using implantable DDSs has been successfully used to achieve site-specific pharmacological effects such as neo-angiogenesis using VEGF [99–102] and bone growth using BMPs [103, 104]. As far as pro-angiogenic growth factor treatment is concerned, considering the limited success in clinical investigations of direct bolus injections of pro-angiogenic growth factors [52, 105], a good piece of literature has been focused on the controlled (i.e. sustained and localized) VEGF delivery from polymeric vehicles. This method has been proving to drive vascularization at a desired tissue site, as indicated by increased microvessel density [106]. Moreover, considering the short half-lives of these molecules in vivo[107], a confined delivery of such potent initiators of angiogenesis to the specific site where intervention is desired would be an attractive alternative in order to compensate the short time tissue exposure, minimizing undesired systemic effects.
Delivery of VEGF has been shown to not only stimulate new blood vessels but also improve circulation in the intended target area [100, 102, 104, 108, 109]. Moreover, combinations of anti-inflammatory agents and growth factors to simultaneously control inflammation and ensure neo-angiogenesis have been reported [93]. This could represent an adequate strategy to circumvent the anti-angiogenic effects of the corticosteroids.
A sustained delivery of VEGF by means of a polymer [85:15 poly(lactide-co-glycolide) (PLG)] has been shown to increase angiogenesis and improve perfusion in an ischemic murine hind limb model [109]. VEGF delivery resulted in a greater capillary density and a more mature vasculature in both the muscle surrounding the implanted area and within the scaffold, suggesting that angiogenic process was regulated by the polymer-derived VEGF. Interestingly, VEGF was not sufficient to induce a detectable significant vessel enlargement suggesting the need for a synergic action of additional factors such as platelet-derived growth factor (PDGF) [20].
PDGF has been demonstrated to promote wound healing through enhancing the formation of granulation tissues, and recombinant human PDGF-BB has been approved by US Food and Drug Administration for use in diabetic foot ulcers [110].
Despite its potential role in tissue regeneration enhancement, clinical application of PDGF encounters some hurdles basically represented by requirement for frequent administrations to maintain sufficient therapeutic concentration. In order to surmount these shortcomings, engineering of biomaterials developed a microsphere-based scaffold release system in which PDGF-BB encapsulated PLGA microspheres were incorporated into a biodegradable PLLA scaffold with well interconnected macroporous and nano-fibrous structures [71]. Moreover, given its protective action on cardiomyocyte [111], local myocardial delivery of PDGF by self-assembling peptide nanofibres at the time of myocardial infarction has been attempted, leading to a sustained improvement in cardiac function without induction of cardiac fibrosis or pulmonary hypertension [112].
Polymeric delivery systems allowing localized and sustained release of therapeutic agents may bypass the current limitations of growth factors delivery [113]. Alginate hydrogels may represent an attractive alternative for localized VEGF delivery [114, 115] and recently an injectable biodegradable alginate hydrogel allowing sustained and localized release of VEGF at a desirable concentration has been developed [116]. This system provided a spatiotemporal factor bioavailability leading to a significant angiogenic response in ischemic hind limbs. The importance of a regulated VEGF-induced angiogenesis is demonstrated by the lack of efficient perfusion when an uncontrolled induction of angiogenesis leads to leaky vessels with tortuous profiles that are readily remodelled and eliminated through regression [117, 118].
It is important to focus on the fact that the biocompatibility of the materials currently used does not necessary imply in its significance that they are inert and will not exert any collateral effect once in in vivo setting, specially when drugs and growth factor are involved in the experimental arena. Indeed, several natural mechanisms involved in circulatory homeostasis maintenance may also affect engineered vascular networks and, on the other side, the addition of exogenous signals would surely affect this delicate equilibrium. One of these mechanisms concerns blood flow to capillary beds. Vasodilating (e.g. nitric oxide, carbon dioxide) and vasoconstricting (e.g. vasopressin, endothelin) factors originated from the vessel endothelium and the surrounding tissue modulate blood flow in a tissue region according to changes in tissue metabolism and blood pressure [119].
The immune system may also affect the functionality of engineered vessels, as invading immune cells secrete angiogenic growth factors and also release these factors from the ECM [120, 121]. Chen and colleagues demonstrated differences in the angiogenic local response in severe combined immunodeficiency (SCID) and immunocompetent mice (C56BL/6) to a porous PLG construct engineered to provide a sustained delivery of VEGF [122]. The VEGF delivered from this system maintained at least 90% bioactivity and induced sprouting angiogenesis from surrounding host vasculature, which infiltrates the highly porous scaffold, resulting in the rapid formation of neovasculature in vivo[20, 123] both within and around the PLG system. The defined area of the scaffold allowed to differentiate between new tissue within the scaffold (i.e. local effects), and the surrounding host tissue (i.e. regional effects), and thus to distinguish between perfusion changes resulting from growth factor-induced angiogenesis (within the scaffold), and the combined effects of angiogenesis and arteriogenesis in the tissue surrounding the scaffold. This study proved that local angiogenesis may be augmented by the combined effects of angiogenesis initiated by the immune response and delivered growth factors, clearly stressing the idea of the need for a careful evaluation of the ‘host factor’ in tissue engineering of cardiovascular structures. Moreover, dosage of the delivered growth factors has to be considered in order to avoid some potential adverse effects as tumourigenesis.
Along with VEGF, b-FGF has been one of the most studied cytokine as for its angiogenic properties. This heparin-binding growth factor is known to induce the proliferation of endothelial cells and has been studied as a potent mitogen for tissue regeneration, wound healing and angiogenesis [124]. In addition, heparin-bound bFGF improved recognition by cellular receptors and enhanced mitogenic activity. b-FGF has been widely used for vascular applications. Intracoronary delivery with increased collateralization has been shown [125]. Treatment of expanded polytetra-fluoroethylene (ePTFE) graft with bFGF in order to increase endothelialization [126, 127] and coating with the peptide sequence Arg-Gly-Asp to improve endothelial cell adhesion through the integrins system, have been performed [128]. However, those attempts encounter hurdles related to the synthetic graft materials which still present issues including high mechanical stiffness, thrombogenecity and the elicitation of foreign-body reactions. Additionally, endothelialization has been shown not to inhibit intimal thickening in vascular prostheses [129]. The use of a naturally occurring ECM materials in the form of a decellularized artery as a substrate for an endothelial cell seeded vascular graft, has been experimented with the aim to maintain similar mechanical properties and structure to a native artery [130]. This system has been further biologically improved by means of covalent link of heparin to the graft to reduce throm-bogenicity and to act as a substrate for heparin-binding growth factors. Further coating of the decellularized functionalized artery with b-FGF resulted in an increase in cell retention and proliferation rate of both human microvascular endothelial cells and canine endothelial progenitor cells in vitro and may therefore help expedite the endothelialization [131].
Furthermore, b-FGF has been used in an alginate hydrogel sustained delivery system showing, after a subcutaneous implantation, an increase in the number of blood vessels in the granulation tissue even higher than in a VEGF-based similar model [69]. This system could enable maintenance of a growth factor concentration ranging in the therapeutic dosage for a prolonged time window within a distance from the vehicle that is regulated by the effective amount of factor incorporated in the scaffold [69].
More recently, Guan and colleagues developed a thermoplastic elastomer system to provide a controlled release of bFGF. Biodegradable, PEUU scaffolds in tubular and cylinder forms with the ability to release bioactive bFGF over a 3-week period in vitro have been seeded with murine smooth muscle cells. These scaffolds showed mechanical properties attractive for future application in soft tissue engineering as demonstrated by tensile strength, elongation at break and suture retention studies [73]. The release profile of bFGF from heparin containing PEUU scaffolds was found to be similar to that from PLGA scaffolds and methylidene malonate polymer. Previously, a bFGF-loaded PLGA scaffold fabricated using supercritical CO2 exhibited 45% initial burst release in 1 day [72, 132]. The release of bFGF from methyli-dene malonate polymer films had over 30% initial burst release in the first day [133].
These studies clearly demonstrate the possibility to develop a cell-seeded construct that can stimulate local angiogenesis useful for blood vessel and cardiovascular structures tissue engineering.
Additionally, the drug-delivery technology can address issues hampering the effectiveness of stem cell therapy regarding cell engraftment, survival and differentiation engineering the local cellular microenvironment. Davies and colleagues designed self-assembling peptide nanofibres for prolonged delivery of insulin-like growth factor 1, a cardiomyocyte growth and differentiation factor, to the myocardium, using a ‘biotin sandwich’ approach [134]. The ability to control the intramyocardial environment by delivering growth factors to injured myocardium maybe crucial to preventing heart failure as it might sustain organ function and improve the endogenous regenerative response providing a chemoattractant signal to promote stem cell migration.
Heparin
Heparin is widely used for a variety of important cardiovascular conditions including deep venous thrombosis, myocardial infarction and to circumvent thrombotic phenomena arising from bare metal stent implantation in coronary arteries or other vascular procedures. However, heparin has profound effects on many of the other events that accompany vascular repair. Heparin markedly and rapidly inhibits DNA and RNA synthesis in cultured vascular smooth muscle cells, limits leucocyte adhesion to injured endothelium, restores endothelial integrity, enhances complement biology and interacts with vascular growth factors being involved in their signalling cascade. To this extent, heparin is considered the best alternative to reduce intimal hyperplasia after vascular interventions [135–138]. Based on the specific affinity between heparin and angiogenic growth factors, and its capability to increase stability against thermal denaturation and enzymatic digestion at physiological conditions, there have been numerous reports about controlled release of growth factors using heparin-conjugated natural polysaccharides such as alginate, chitosan and hyaluronic acid [81–83]. Recently, heparin has been used in association with biodegradable scaffolds by means of immobilization onto the surface of PLGA constructs to obtain local and sustained delivery of bFGF [80]. In this study, heparin was immobilized onto the surface of PLGA scaffolds by covalent conjugation between carboxylic groups of heparin and surface-exposed amine groups on the PLGA scaffolds. The heparin-immobilized scaffolds loaded with bFGF showed a proliferative effect on human umbilical vein endothelial cells and when implanted subcutaneously in vivo, they effectively induced the formation of blood vessels in the vicinity of the implant site. This study demonstrated that local and sustained delivery of angiogenic growth factor for tissue regeneration could be achieved by surface modification of porous scaffolds with heparin.
In consideration of the antithrombogenic properties, coiled tubular structures, constructed from polymer-coated metallic wires with the addition of heparin to the surface, have been also developed and demonstrated to support growth of an endothelial cell layer in vitro. In this study the constructs have been treated with human plasma proteins in order to mimic the in vivo conditions, and human microvascular endothelial cells grew adequately with a further a moderate enhancing effect in the heparin-coated group [139].
Moreover, heparin exerts an important inhibitory effect on neointimal hyperplasia. Edelman and colleagues described the design and development of a novel biodegradable system for the perivascular delivery of heparin to the blood vessel wall with well-defined release kinetics. Heparin-encapsulated PLGA microspheres were sequestered in an alginate gel and evaluated for their ability to inhibit growth of bovine vascular smooth muscle cells in tissue culture. Moreover functionalized alginate gel sheets were used to wrap arterial vessel in a murine model of vascular injury induced by endothelial denudation and in a vein vascular graft interposition intervention. This system showed ability to both inhibit smooth muscle cell proliferation in vitro and to reduce neointimal hyperplasia in in vivo models of vascular disease [140].
Nitric oxide
Nitric oxide plays a pivotal role in vascular homeostasis modulating the activation and adhesion of leucocytes, limiting platelet aggregation and thrombus formation, and maintaining the vascular smooth muscle in a non-proliferative state, thereby preserving blood vessels from adverse vascular remodelling [141]. Nitric oxide biological characteristics could therefore constitute an attractive alternative to ameliorate properties of biomaterials for cardiovascular applications. Polyethylene terephthalate (Dacron, Invista, KS, USA) and ePTFE (Teflon, DuPont, VA, USA) grafts currently used for large-diameter (more than 6 mm) vascular substitutes, encounter several limitations especially concerning thrombosis, intimal hyperplasia and thus, failure in small-diameter applications. It is noteworthy that one of the reasons thought to be at the root of the early failure of saphenous vein grafts is the relative paucity of nitric oxide production by these conduits as compared with arterial ones.
To this extent, Jun and colleagues developed a diazeniumdiolate-modified polyurethane for vascular applications. Diazeniumdiolates are interesting compounds containing the [N(O)NO]- functional group [142, 143]. The anionic portions of these compounds spontaneously decompose in solution to release nitric oxide, leaving the amine group as a byproduct [144] with rates of dissociation depending on several factors, such as structure, temperature and pH of solution [145].
Using this bioactive material authors described a two phases releasing kinetic with a rapid burst during the first 48 hrs, followed by a slower, sustained release over a 2-month period. Along with these findings enhanced rates of proliferation of endothelial cells and increased rates of migration and coverage of the synthetic surface during both phases have been demonstrated. Moreover, nitric oxide releasing polyurethane, while greatly inhibiting smooth muscle cell growth, reduced platelets adhesion as revealed by a mepacrine-based assay. More recently, incorporation of a diazeniumdiolate nitric oxide donor into the backbone of a polyurethane containing both Polyethylene glycol (PEG) and the cell adhesive YIGSR peptide sequence has been performed in order to provide a nitric oxide local release during the period of graft endothelialization [146]. PEG has been shown to resist to protein adsorption, platelet adhesion and bacterial adhesion, and numerous methods have been developed to integrate PEG into biomaterials, including polyurethanes, through co-polymerization and surface modification [147–152]. The laminin-derived cell adhesive peptide sequence tyrosine-isoleucine-glycine-serine-arginine (YIGSR) increases endothelial cell adhesion and migration [153], while the nitric oxide release improves endothelial cell proliferation during the period of graft endothelialization. Nitric oxide generating, cell adhesive polyurethane-PEG copolymer showed the ability to reduce platelet adhesion and smooth muscle proliferation enhancing endothelial cells attachment and proliferation. If the addition of the YIGSR peptide sequence, known to improve cell attachment, spreading and resistance to shear stress, could give an answer to potential issues concerning the influence of physical forces of the circulation in vivo settings, other questions regarding the response of bone marrow-derived endothelial progenitor cells need to be evaluated [154].
However, the therapeutic potential of nitric oxide generating polyurethane remains high and it may be suitable as a candidate material for small-diameter vascular grafts.
Gene delivery
Until now several attempts have been done to promote regeneration of blood vessels using tissue-engineering approaches with genes. The idea at the basis of this strategy regards the possibility of inducing cell proliferation and differentiation or the secretion of ECM components for tissue regeneration by the transfection of genes encoding growth factors [155]. For these purposes, it is crucial that the development of controlled DDSs which allow a therapeutic gene to be delivered specifically to the target cell in an appropriate sequence and timing [156–158]. In gene therapy two kinds of vectors are basically available, viral and non-viral vectors. Viral vectors, such as adenovirus, retrovirus and adeno-associated virus, have been mainly used because of the high efficiency of gene transfection. Nevertheless, limited size of DNA molecules inserted into viral vectors, possible mutagenesis of cells transfected, high immunogenicity and toxicity represent some of the shortcomings related to this approach. On the other hand, despite their low transfection efficiency, non-viral vectors, such as naked plasmid DNA and plasmid DNA complexed with cationic groups may represent a more feasible alternative as for their safety and the greater molecular size of DNA. Therefore, a lot of literature has been focused on the development of synthetic materials, including cationic liposomes [159–161] and cationic polymers like polylysine [162–165] and polyethylenimine, molecularly designed to improve delivery of plasmid DNA to the target site at high expression levels [166–174].
On account of the similar negative charge of both cell membranes and plasmid DNA used in trasfections, complexation of the genomic material with cationic elements is well recognized to be useful to reduce the plasmid DNA molecular size and to promote electrostatic interactions with the cell membrane resulting in the internalization.
However, the plasmid DNA, when given to cells or injected into the body in the solution form, is readily degraded and inactivated by enzymes or cells and it does not have any inherent ability to accumulate in a certain tissue or organ. Moreover, considering gene pharmacokinetics, a successful gene therapy requires a target-specific sustained expression of the construct [175–179]. The time period of gene expression can be prolonged by the controlled release technology of plasmid DNA. To this extent, DDS technology and methodology are promising to enhance the biological effects of genes for therapeutic purposes. Several studies have been done about the controlled release of plasmid DNA with different biodegradable biomaterials [155, 180–203].
This release technology could enhance the level of gene transfection and promote a prolonged transfection time, which are both important to effectively induce tissue regeneration. Use of biodegradable hydrogels has been widely studied. Modifications of their chemical properties by alkaline or acidic processing have been investigated in order to obtain materials with different iso-electric points to improve interaction with growth factor, plasmid DNA, decoy DNA and small interference RNA for release purposes. The time profile of growth factor and DNA release could be controlled by adjusting hydrogel degradation, which is dependent on the extent of the hydrogel cross-linking [204, 205].
Derivatization with cationic compounds gives gelatine cationic moieties which can polyionically interact with plasmid DNA of anionic nature. The cationized gelatine could be processed with glutaraldehyde to obtain different degrees of cross-linking. Cross-linking extent is important as it directly affects the hydrogel degradation rate. The release of the DNA molecules ionically complexed with the cationized gelatine is thought to occur during the hydrogel degradation process to generate water-soluble cationized gelatine fragments. The combined release of the plasmid DNA molecules as a complex with degraded gelatine fragments of positive charge is conceived to be at the basis of the ionic interaction with the cell surface of negative charge promoting internalization. Moreover, this complex is thought to prevent DNA enzymatic degradation by Dnase [206–208]. In this approach DNA is biologically stabilized by the incorporation into the hydrogel and the controlled release enhances the local concentration of plasmid DNA around cells to be transfected, consequently increasing the efficiency of gene transfection.
Biomaterial based release of genomic material has been experimented in several fields of medical interest including muscle and bone regeneration. For cardiovascular applications, Mooney et al. have reported that the in vivo sustained release of a plasmid DNA encoding PDGF gene with the carrier matrix of PLGA, enhanced matrix deposition and blood vessel formation [180, 181]. The possibility to create polymer scaffolds capable of controlled release of intact and functional plasmid DNA could represent a mean to enhance and regulate gene transfer within a developing tissue, which will increase their utility in tissue engineering avoiding use of viral vectors.
Conclusion
DDSs represent an attractive option in cardiovascular tissue-engineering applications. Controlled delivery may provide an appropriate alternative capable of releasing localized doses of bioactive molecules over sustained periods of time, while minimizing undesired systemic effects. However, until now the current limitation of TECVG lies in the fact that artificial vessels and cardiovascular structures need to have growth potential, and the inherent ability to remodel and to warranty coagulation homeostasis while maintaining biocompatibility. Scaffold-related limitations and the issues of off-the-shelf availability, and ease of harvest and propagation of autologous cells, are the greatest obstacles to the widespread acceptance of tissue-engineering technology in clinical practice. This gap is where stem cell application can be fully utilized. The use of stem cells could be proposed as an adequate alternative to other autologous primary cell lines because of their easy recruitment in clinical settings (i.e. bone marrow aspiration) and their notable plasticity. The exciting bridge that the stem cell represents could be a breakthrough in tissue engineering especially if combined with biomaterials that actively guide and provide the correct sequence of signals to allow a harmonic ongoing lineage-specific differentiation of these pluripotent precursor cells.
Acknowledgments
This work has been supported by the Heart, Lung and Esophageal Surgery Institute at University of Pittsburgh Medical Center.
References
- 1.Shinoka T, Shum-Tim D, Ma PX, Tanel RE, Isogai N, Langer R, Vacanti JP, Mayer JE., Jr Creation of viable pulmonary artery autografts through tissue engineering. J Thorac Cardiovasc Surg. 1998;115:536–45. doi: 10.1016/S0022-5223(98)70315-0. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe M, Shin’oka T, Tohyama S, Hibino N, Konuma T, Matsumura G, Kosaka Y, Ishida T, Imai Y, Yamakawa M, Ikada Y, Morita S. Tissue-engineered vascular autograft: inferior vena cava replacement in a dog model. Tissue Eng. 2001;7:429–39. doi: 10.1089/10763270152436481. [DOI] [PubMed] [Google Scholar]
- 3.Shum-Tim D, Stock U, Hrkach J, Shinoka T, Lien J, Moses MA, Stamp A, Taylor G, Moran AM, Landis W, Langer R, Vacanti JP, Mayer JE., Jr Tissue engineering of autologous aorta using a new biodegradable polymer. Ann Thorac Surg. 1999;68:2298–304. doi: 10.1016/s0003-4975(99)01055-3. [DOI] [PubMed] [Google Scholar]
- 4.Shin’oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, Sakamoto T, Nagatsu M, Kurosawa H. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330–8. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 5.Hibino N, Shin’oka T, Matsumura G, Ikada Y, Kurosawa H. The tissue-engineered vascular graft using bone marrow without culture. J Thorac Cardiovasc Surg. 2005;129:1064–70. doi: 10.1016/j.jtcvs.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 6.Sarkar S, Lee GY, Wong JY, Desai TA. Development and characterization of a porous micro-patterned scaffold for vascular tissue engineering applications. Biomaterials. 2006;27:4775–82. doi: 10.1016/j.biomaterials.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 7.Seunarine K, Gadegaard N, Tormen M, Meredith DO, Riehle MO, Wilkinson CD. 3D polymer scaffolds for tissue engineering. Nanomed. 2006;1:281–96. doi: 10.2217/17435889.1.3.281. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Cheng X, Wang J, Wang Y, Shi B, Huang C, Yang X, Liu T. Novel chitosan/collagen scaffold containing transforming growth factor-beta1 DNA for periodontal tissue engineering. Biochem Biophys Res Commun. 2006;344:362–9. doi: 10.1016/j.bbrc.2006.03.106. [DOI] [PubMed] [Google Scholar]
- 9.Luong-Van E, Grondahl L, Chua KN, Leong KW, Nurcombe V, Cool SM. Controlled release of heparin from poly(epsilon-caprolactone) electrospun fibers. Biomaterials. 2006;27:2042–50. doi: 10.1016/j.biomaterials.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Zhang G, Suggs LJ. Matrices and scaffolds for drug delivery in vascular tissue engineering. Adv Drug Deliv Rev. 2007;59:360–73. doi: 10.1016/j.addr.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Huang ZMZY, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electro-spinning and their applications in nanocomposites. Compos Sci Technol. 2003;63:2223–53. [Google Scholar]
- 12.Zhang YZ, Venugopal J, Huang ZM, Lim CT, Ramakrishna S. Characterization of the surface biocompatibility of the electro-spun PCL-collagen nanofibers using fibroblasts. Biomacromolecules. 2005;6:2583–9. doi: 10.1021/bm050314k. [DOI] [PubMed] [Google Scholar]
- 13.Katti DS, Robinson KW, Ko FK, Laurencin CT. Bioresorbable nanofiber-based systems for wound healing and drug delivery: optimization of fabrication parameters. J Biomed Mater Res B Appl Biomater. 2004;70:286–96. doi: 10.1002/jbm.b.30041. [DOI] [PubMed] [Google Scholar]
- 14.Kim K, Luu YK, Chang C, Fang D, Hsiao BS, Chu B, Hadjiargyrou M. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J Control Release. 2004;98:47–56. doi: 10.1016/j.jconrel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Zeng J, Xu X, Chen X, Liang Q, Bian X, Yang L, Jing X. Biodegradable electrospun fibers for drug delivery. J Control Release. 2003;92:227–31. doi: 10.1016/s0168-3659(03)00372-9. [DOI] [PubMed] [Google Scholar]
- 16.Kenawy ElR, Bowlin GL, Mansfield K, Layman J, Simpson DG, Sanders EH, Wnek GE. Release of tetracycline hydrochloride from electrospun poly (ethyl-ene-co-vinylacetate), poly(lactic acid), and a blend. J Control Release. 2002;81:57–64. doi: 10.1016/s0168-3659(02)00041-x. [DOI] [PubMed] [Google Scholar]
- 17.Verreck G, Chun I, Rosenblatt J, Peeters J, Dijck AV, Mensch J, Noppe M, Brewster ME. Incorporation of drugs in an amorphous state into electrospun nanofibers composed of a water-insoluble, non-biodegradable polymer. J Control Release. 2003;92:349–60. doi: 10.1016/s0168-3659(03)00342-0. [DOI] [PubMed] [Google Scholar]
- 18.Luu YK, Kim K, Hsiao BS, Chu B, Hadjiargyrou M. Development of a nanos-tructured DNA delivery scaffold viaelectro-spinning of PLGA and PLA-PEG block copolymers. J Control Release. 2003;89:341–53. doi: 10.1016/s0168-3659(03)00097-x. [DOI] [PubMed] [Google Scholar]
- 19.Tabata Y. Significance of release technology in tissue engineering. Drug Discov Today. 2005;10:1639–46. doi: 10.1016/S1359-6446(05)03639-1. [DOI] [PubMed] [Google Scholar]
- 20.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 21.Zeng F, Lee H, Allen C. Epidermal growth factor-conjugated poly(ethylene glycol)-block-poly(delta-valerolactone) copolymer micelles for targeted delivery of chemotherapeutics. Bioconjug Chem. 2006;17:399–409. doi: 10.1021/bc050350g. [DOI] [PubMed] [Google Scholar]
- 22.Vehof JW, Fisher JP, Dean D, Van Der Waerden JP, Spauwen PH, Mikos AG, Jansen JA. Bone formation in transforming growth factor beta-1-coated porous poly(propylene fumarate) scaffolds. J Biomed Mater Res. 2002;60:241–51. doi: 10.1002/jbm.10073. [DOI] [PubMed] [Google Scholar]
- 23.Wissink MJ, Beernink R, Poot AA, Engbers GH, Beugeling T, Van Aken WG, Feijen J. Improved endothelialization of vascular grafts by local release of growth factor from heparinized collagen matrices. J Control Release. 2000;64:103–14. doi: 10.1016/s0168-3659(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 24.Murphy WL, Peters MC, Kohn DH, Mooney DJ. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21:2521–7. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 25.Whang K, Goldstick TK, Healy KE. A biodegradable polymer scaffold for delivery of osteotropic factors. Biomaterials. 2000;21:2545–51. doi: 10.1016/s0142-9612(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 26.Zilla P, Deutsch M, Meinhart J, Puschmann R, Eberl T, Minar E, Dudczak R, Lugmaier H, Schmidt P, Noszian I. Clinical in vitro endothelialization of femoropopliteal bypass grafts: an actuarial follow-up over three years. J Vasc Surg. 1994;19:540–8. doi: 10.1016/s0741-5214(94)70083-4. [DOI] [PubMed] [Google Scholar]
- 27.Hashi CK, Zhu Y, Yang GY, Young WL, Hsiao BS, Wang K, Chu B, Li S. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci USA. 2007;104:11915–20. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortes-Morichetti M, Frati G, Schussler O, Van Huyen JP, Lauret E, Genovese JA, Carpentier AF, Chachques JC. Association between a cell-seeded collagen matrix and cellular cardiomyoplasty for myocardial support and regeneration. Tissue Eng. 2007;13:2681–7. doi: 10.1089/ten.2006.0447. [DOI] [PubMed] [Google Scholar]
- 29.Chachques JC, Trainini JC, Lago N, Masoli OH, Barisani JL, Cortes-Morichetti M, Schussler O, Carpentier A. Myocardial assistance by grafting a new bioartificial upgraded myocardium (MAGNUM clinical trial): one year follow-up. Cell Transplant. 2007;16:927–34. doi: 10.3727/096368907783338217. [DOI] [PubMed] [Google Scholar]
- 30.Chachques JC, Trainini JC, Lago N, Cortes- Morichetti M, Schussler O, Carpentier A. Myocardial assistance by grafting a new bioartificial upgraded myocardium (MAGNUM trial): clinical feasibility study. Ann Thorac Surg. 2008;85:901–8. doi: 10.1016/j.athoracsur.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 31.Huang NF, Yu J, Sievers R, Li S, Lee RJ. Injectable biopolymers enhance angiogenesis after myocardial infarction. Tissue Eng. 2005;11:1860–6. doi: 10.1089/ten.2005.11.1860. [DOI] [PubMed] [Google Scholar]
- 32.Philp D, Chen SS, Fitzgerald W, Orenstein J, Margolis L, Kleinman HK. Complex extracellular matrices promote tissue-specific stem cell differentiation. Stem Cells. 2005;23:288–96. doi: 10.1634/stemcells.2002-0109. [DOI] [PubMed] [Google Scholar]
- 33.Dai W, Wold LE, Dow JS, Kloner RA. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 2005;46:714–9. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 34.Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10:403–9. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 35.Remuzzi A, Mantero S, Colombo M, Morigi M, Binda E, Camozzi D, Imberti B. Tissue Eng. 2004. Vascular smooth muscle cells on hyaluronic acid: culture and mechanical characterization of an engineered vascular construct; pp. 699–710. [DOI] [PubMed] [Google Scholar]
- 36.Badylak S, Liang A, Record R, Tullius R, Hodde J. Endothelial cell adherence to small intestinal submucosa: an acellular bioscaffold. Biomaterials. 1999;20:2257–63. doi: 10.1016/s0142-9612(99)00156-8. [DOI] [PubMed] [Google Scholar]
- 37.Schaner PJ, Martin ND, Tulenko TN, Shapiro IM, Tarola NA, Leichter RF, Carabasi RA, Dimuzio PJ. Decellularized vein as a potential scaffold for vascular tissue engineering. J Vasc Surg. 2004;40:146–53. doi: 10.1016/j.jvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 38.Gaspardone A, Versaci F. Coronary stenting and inflammation. Am J Cardiol. 2005;96:65L–70L. doi: 10.1016/j.amjcard.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 39.Versaci F, Gaspardone A. Prevention of restenosis after stenting: the emerging role of inflammation. Coron Artery Dis. 2004;15:307–11. doi: 10.1097/00019501-200409000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Patil SD, Papadimitrakopoulos F, Burgess DJ. Dexamethasone-loaded poly (lactic-co-glycolic) acid microspheres/ poly(vinyl alcohol) hydrogel composite coatings for inflammation control. Diabetes Technol Ther. 2004;6:887–97. doi: 10.1089/dia.2004.6.887. [DOI] [PubMed] [Google Scholar]
- 41.Stankus JJ, Guan J, Wagner WR. Fabrication of biodegradable elastomeric scaffolds with sub-micron morphologies. J Biomed Mater Res A. 2004;70:603–14. doi: 10.1002/jbm.a.30122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perets A, Baruch Y, Weisbuch F, Shoshany G, Neufeld G, Cohen S. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J Biomed Mater Res A. 2003;65:489–97. doi: 10.1002/jbm.a.10542. [DOI] [PubMed] [Google Scholar]
- 43.Radisic M, Euloth M, Yang L, Langer R, Freed LE, Vunjak-Novakovic G. High-density seeding of myocyte cells for cardiac tissue engineering. Biotechnol Bioeng. 2003;82:403–14. doi: 10.1002/bit.10594. [DOI] [PubMed] [Google Scholar]
- 44.Mikos AG, G G, Ingber DE, Vacanti JP, Langer R. Prevascularization of porous biodegradable polymers. Biotech Bioeng. 1993;42:716–23. doi: 10.1002/bit.260420606. [DOI] [PubMed] [Google Scholar]
- 45.Dee KC, D AP, Bizios R. An introduction to tissue – biomaterial interactions. Hoboken: John Wiley & Sons; 2002. pp. 127–47. [Google Scholar]
- 46.Carrier RL, Rupnick M, Langer R, Schoen FJ, Freed LE, Vunjak-Novakovic G. Perfusion improves tissue architecture of engineered cardiac muscle. Tissue Eng. 2002;8:175–88. doi: 10.1089/107632702753724950. [DOI] [PubMed] [Google Scholar]
- 47.Carrier RL, Rupnick M, Langer R, Schoen FJ, Freed LE, Vunjak-Novakovic G. Effects of oxygen on engineered cardiac muscle. Biotechnol Bioeng. 2002;78:617–25. doi: 10.1002/bit.10245. [DOI] [PubMed] [Google Scholar]
- 48.Radisic M, Malda J, Epping E, Geng W, Langer R, Vunjak-Novakovic G. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol Bioeng. 2006;93:332–43. doi: 10.1002/bit.20722. [DOI] [PubMed] [Google Scholar]
- 49.Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G. Bio-mimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12:2077–91. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 50.Radisic M, Park H, Gerecht S, Cannizzaro C, Langer R, Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering. Philos Trans R Soc B Biol Sci. 2007;362:1357–68. doi: 10.1098/rstb.2007.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zimmermann WH, Fink C, Kralisch D, Remmers U, Weil J, Eschenhagen T. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol Bioeng. 2000;68:106–14. [PubMed] [Google Scholar]
- 52.Ennett AB, Mooney DJ. Tissue engineering strategies for in vivo neovascularization. Expert Opin Biol Ther. 2002;2:805–18. doi: 10.1517/14712598.2.8.805. [DOI] [PubMed] [Google Scholar]
- 53.Lee H, Cusick RA, Browne F, Ho Kim T, Ma PX, Utsunomiya H, Langer R, Vacanti JP. Local delivery of basic fibroblast growth factor increases both angiogenesis and engraftment of hepatocytes in tissue-engineered polymer devices. Trans -plantation. 2002;73:1589–93. doi: 10.1097/00007890-200205270-00011. [DOI] [PubMed] [Google Scholar]
- 54.Mooney DJ, Kaufmann PM, Sano K, McNamara KM, Vacanti JP, Langer R. Transplantation of hepatocytes using porous, biodegradable sponges. Transplant Proc. 1994;26:3425–6. [PubMed] [Google Scholar]
- 55.Zisch AH, Lutolf MP, Hubbell JA. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc Pathol. 2003;12:295–310. doi: 10.1016/s1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 56.Xu C, Inai R, Kotaki M, Ramakrishna S. Electrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineering. Tissue Eng. 2004;10:1160–8. doi: 10.1089/ten.2004.10.1160. [DOI] [PubMed] [Google Scholar]
- 57.Stankus JJ, Soletti L, Fujimoto K, Hong Y, Vorp DA, Wagner WR. Fabrication of cell microintegrated blood vessel constructs through electrohydrodynamic atomization. Biomaterials. 2007;28:2738–46. doi: 10.1016/j.biomaterials.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soletti L, Nieponice A, Guan J, Stankus JJ, Wagner WR, Vorp DA. A seeding device for tissue engineered tubular structures. Biomaterials. 2006;27:4863–70. doi: 10.1016/j.biomaterials.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 59.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–79. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 60.Matthew IR, Browne RM, Frame JW, Millar BG. Subperiosteal behaviour of alginate and cellulose wound dressing materials. Biomaterials. 1995;16:275–8. doi: 10.1016/0142-9612(95)93254-b. [DOI] [PubMed] [Google Scholar]
- 61.Gerecht-Nir S, Cohen S, Ziskind A, Itskovitz-Eldor J. Three-dimensional porous alginate scaffolds provide a conducive environment for generation of well-vascularized embryoid bodies from human embryonic stem cells. Biotechnol Bioeng. 2004;88:313–20. doi: 10.1002/bit.20248. [DOI] [PubMed] [Google Scholar]
- 62.Bouhadir KH, Lee KY, Alsberg E, Damm KL, Anderson KW, Mooney DJ. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol Prog. 2001;17:945–50. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
- 63.Boontheekul T, Kong HJ, Mooney DJ. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26:2455–65. doi: 10.1016/j.biomaterials.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 64.Genove E, Shen C, Zhang S, Semino CE. The effect of functionalized self-assembling peptide scaffolds on human aortic endothelial cell function. Biomaterials. 2005;26:3341–51. doi: 10.1016/j.biomaterials.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 65.Sieminski AL, Semino CE, Gong H, Kamm RD. Primary sequence of ionic self-assembling peptide gels affects endothelial cell adhesion and capillary morphogenesis. J Biomed Mater Res A. 2008;87:494–504. doi: 10.1002/jbm.a.31785. [DOI] [PubMed] [Google Scholar]
- 66.Kedem A, Perets A, Gamlieli-Bonshtein I, Dvir-Ginzberg M, Mizrahi S, Cohen S. Vascular endothelial growth factor-releasing scaffolds enhance vascularization and engraftment of hepatocytes transplanted on liver lobes. Tissue Eng. 2005;11:715–22. doi: 10.1089/ten.2005.11.715. [DOI] [PubMed] [Google Scholar]
- 67.Cleland JL, Duenas ET, Park A, Daugherty A, Kahn J, Kowalski J, Cuthbertson A. Development of poly-(D,L-lactide–coglycolide) microsphere formulations containing recombinant human vascular endothelial growth factor to promote local angiogenesis. J Control Release. 2001;72:13–24. doi: 10.1016/s0168-3659(01)00258-9. [DOI] [PubMed] [Google Scholar]
- 68.Tabata Y. Tissue regeneration based on tissue engineering technology. Congenit Anom. 2004;44:111–24. doi: 10.1111/j.1741-4520.2004.00024.x. [DOI] [PubMed] [Google Scholar]
- 69.Lee KY, Peters MC, Mooney DJ. Comparison of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in SCID mice. J Control Release. 2003;87:49–56. doi: 10.1016/s0168-3659(02)00349-8. [DOI] [PubMed] [Google Scholar]
- 70.Ziegler J, Mayr-Wohlfart U, Kessler S, Breitig D, Gunther KP. Adsorption and release properties of growth factors from biodegradable implants. J Biomed Mater Res. 2002;59:422–8. doi: 10.1002/jbm.1258. [DOI] [PubMed] [Google Scholar]
- 71.Wei G, Jin Q, Giannobile WV, Ma PX. Nano-fibrous scaffold for controlled delivery of recombinant human PDGF-BB. J Control Release. 2006;112:103–10. doi: 10.1016/j.jconrel.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hile DD, Pishko MV. Solvent-free protein encapsulation within biodegradable polymer foams. Drug Deliv. 2004;11:287–93. doi: 10.1080/10717540490493961. [DOI] [PubMed] [Google Scholar]
- 73.Guan J, Stankus JJ, Wagner WR. Biodegradable elastomeric scaffolds with basic fibroblast growth factor release. J Control Release. 2007;120:70–8. doi: 10.1016/j.jconrel.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoo HS, Lee EA, Yoon JJ, Park TG. Hyaluronic acid modified biodegradable scaffolds for cartilage tissue engineering. Biomaterials. 2005;26:1925–33. doi: 10.1016/j.biomaterials.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 75.Yoon JJ, Song SH, Lee DS, Park TG. Immobilization of cell adhesive RGD peptide onto the surface of highly porous biodegradable polymer scaffolds fabricated by a gas foaming/salt leaching method. Biomaterials. 2004;25:5613–20. doi: 10.1016/j.biomaterials.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 76.Yoon JJ, Nam YS, Kim JH, Park TG. Surface immobilization of galactose onto aliphatic biodegradable polymers for hepatocyte culture. Biotechnol Bioeng. 2002;78:1–10. doi: 10.1002/bit.10239. [DOI] [PubMed] [Google Scholar]
- 77.Park TG. Perfusion culture of hepatocytes within galactose-derivatized biodegradable poly(lactide-co-glycolide) scaffolds prepared by gas foaming of effervescent salts. J Biomed Mater Res. 2002;59:127–35. doi: 10.1002/jbm.1224. [DOI] [PubMed] [Google Scholar]
- 78.Ishihara M, Sato M, Hattori H, Saito Y, Yura H, Ono K, Masuoka K, Kikuchi M, Fujikawa K, Kurita A. Heparin-carrying polystyrene (HCPS)-bound collagen substratum to immobilize heparin-binding growth factors and to enhance cellular growth. J Biomed Mater Res. 2001;56:536–44. doi: 10.1002/1097-4636(20010915)56:4<536::aid-jbm1125>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 79.Gospodarowicz D, Cheng J. Heparin protects basic and acidic FGF from inactivation. J Cell Physiol. 1986;128:475–84. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- 80.Yoon JJ, Chung HJ, Lee HJ, Park TG. Heparin-immobilized biodegradable scaffolds for local and sustained release of angiogenic growth factor. J Biomed Mater Res A. 2006;79:934–42. doi: 10.1002/jbm.a.30843. [DOI] [PubMed] [Google Scholar]
- 81.Andreopoulos FM, Persaud I. Delivery of basic fibroblast growth factor (bFGF) from photoresponsive hydrogel scaffolds. Biomaterials. 2006;27:2468–76. doi: 10.1016/j.biomaterials.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 82.Cai S, Liu Y, Zheng Shu X, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26:6054–67. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 83.Liu LS, Ng CK, Thompson AY, Poser JW, Spiro RC. Hyaluronate-heparin conjugate gels for the delivery of basic fibroblast growth factor (FGF-2) J Biomed Mater Res. 2002;62:128–35. doi: 10.1002/jbm.10238. [DOI] [PubMed] [Google Scholar]
- 84.Boccaccini ARMV. Bioresorbable and bioactive polymer/Bioglass composites with tailored pore structure for tissue engineering applications. Comp Sci Technol. 2003:2417–29. [Google Scholar]
- 85.Keshaw HFA, Day R. Release of angio-genic growth factors from cells encapsulated in alginate beads with bioactive glass. Biomaterials. 2005;19:4171–9. doi: 10.1016/j.biomaterials.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 86.Day RMBA, Shurey S, Roether JA, Forbes A, Hench LL, Gabe SM. Assessment of polyglycolic acid mesh and bioactive glass for soft-tissue engineering scaffolds. Biomaterials. 2004:5857–66. doi: 10.1016/j.biomaterials.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 87.Day R, Maquet V, Boccaccini AR, Robert Jerome R, Alastair Forbes A. In vitro and in vivo analysis of macroporous biodegradable poly(D,L-lactide-co-glycolide) scaffolds containing bioactive glass. J Biomed Mater Res A. 2005;75:778–87. doi: 10.1002/jbm.a.30433. [DOI] [PubMed] [Google Scholar]
- 88.Leu A, Leach JK. Proangiogenic potential of a collagen/bioactive glass substrate. Pharm Res. 2008;25:1222–9. doi: 10.1007/s11095-007-9508-9. [DOI] [PubMed] [Google Scholar]
- 89.Yoon JJ, Kim JH, Park TG. Dexamethasone-releasing biodegradable polymer scaffolds fabricated by a gas-foaming/salt-leaching method. Biomaterials. 2003;24:2323–9. doi: 10.1016/s0142-9612(03)00024-3. [DOI] [PubMed] [Google Scholar]
- 90.Tabata Y. Current status of regenerative medical therapy based on drug delivery technology. Reprod Biomed Online. 2008;16:70–80. doi: 10.1016/s1472-6483(10)60558-5. [DOI] [PubMed] [Google Scholar]
- 91.Martin I, Shastri VP, Padera RF, Yang J, Mackay AJ, Langer R, Vunjak-Novakovic G, Freed LE. Selective differentiation of mammalian bone marrow stromal cells cultured on three-dimensional polymer foams. J Biomed Mater Res. 2001;55:229–35. doi: 10.1002/1097-4636(200105)55:2<229::aid-jbm1009>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 92.Peter SJ, Liang CR, Kim DJ, Widmer MS, Mikos AG. Osteoblastic phenotype of rat marrow stromal cells cultured in the presence of dexamethasone, beta-glycerolphosphate, and L-ascorbic acid. J Cell Biochem. 1998;71:55–62. doi: 10.1002/(sici)1097-4644(19981001)71:1<55::aid-jcb6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 93.Patil SD, Papadmitrakopoulos F, Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. J Control Release. 2007;117:68–79. doi: 10.1016/j.jconrel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 94.Galeska I, Kim TK, Patil SD, Bhardwaj U, Chatttopadhyay D, Papadimitrakopoulos F, Burgess DJ. Controlled release of dexamethasone from PLGA microspheres embedded within polyacid-containing PVA hydrogels. Aaps J. 2005;7:E231–40. doi: 10.1208/aapsj070122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koedam JA, Smink JJ, Van Buul-Offers SC. Glucocorticoids inhibit vascular endothelial growth factor expression in growth plate chondrocytes. Mol Cell Endocrinol. 2002;197:35–44. doi: 10.1016/s0303-7207(02)00276-9. [DOI] [PubMed] [Google Scholar]
- 96.Luo JC, Chi CW, Lin HY, Chang FY, Lu CL, Chen CY, Lee SD. Dexamethasone inhibits epidermal growth factor-stimulated gastric epithelial cell proliferation. J Pharmacol Exp Ther. 2007;320:687–94. doi: 10.1124/jpet.106.113035. [DOI] [PubMed] [Google Scholar]
- 97.Luo JC, Shin VY, Liu ES, Ye YN, Wu WK, So WH, Chang FY, Cho CH. Dexamethasone delays ulcer healing by inhibition of angiogenesis in rat stomachs. Eur J Pharmacol. 2004;485:275–81. doi: 10.1016/j.ejphar.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 98.Iwai A, Fujii Y, Kawakami S, Takazawa R, Kageyama Y, Yoshida MA, Kihara K. Down-regulation of vascular endothelial growth factor in renal cell carcinoma cells by glucocorticoids. Mol Cell Endocrinol. 2004;226:11–7. doi: 10.1016/j.mce.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 99.Elcin AE, Elcin YM. Localized angiogene- sis induced by human vascular endothelial growth factor-activated PLGA sponge. Tissue Eng. 2006;12:959–68. doi: 10.1089/ten.2006.12.959. [DOI] [PubMed] [Google Scholar]
- 100.Elcin YM, Dixit V, Gitnick G. Extensive in vivo angiogenesis following controlled release of human vascular endothelial cell growth factor: implications for tissue engineering and wound healing. Artif Organs. 2001;25:558–65. doi: 10.1046/j.1525-1594.2001.025007558.x. [DOI] [PubMed] [Google Scholar]
- 101.Linn T, Erb D, Schneider D, Kidszun A, Elcin AE, Bretzel RG, Elcin YM. Polymers for induction of revascularization in the rat fascial flap: application of vascular endothelial growth factor and pancreatic islet cells. Cell Transplant. 2003;12:769–78. doi: 10.3727/000000003108747244. [DOI] [PubMed] [Google Scholar]
- 102.Gu F, Amsden B, Neufeld R. Sustained delivery of vascular endothelial growth factor with alginate beads. J Control Release. 2004;96:463–72. doi: 10.1016/j.jconrel.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 103.Jung RE, Zwahlen R, Weber FE, Molenberg A, Van Lenthe GH, Hammerle CH. Evaluation of an in situ formed synthetic hydrogel as a biodegradable membrane for guided bone regeneration. Clin Oral Implants Res. 2006;17:426–33. doi: 10.1111/j.1600-0501.2005.01228.x. [DOI] [PubMed] [Google Scholar]
- 104.Weber FE, Eyrich G, Gratz KW, Maly FE, Sailer HF. Slow and continuous application of human recombinant bone morphogenetic protein via biodegradable poly(lactide-co-glycolide) foamspheres. Int J Oral Maxillofac Surg. 2002;31:60–5. doi: 10.1054/ijom.2001.0154. [DOI] [PubMed] [Google Scholar]
- 105.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER. The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107:1359–65. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 106.Smith MK, Peters MC, Richardson TP, Garbern JC, Mooney DJ. Locally enhanced angiogenesis promotes transplanted cell survival. Tissue Eng. 2004;10:63–71. doi: 10.1089/107632704322791709. [DOI] [PubMed] [Google Scholar]
- 107.Lazarous DF, Shou M, Scheinowitz M, Hodge E, Thirumurti V, Kitsiou AN, Stiber JA, Lobo AD, Hunsberger S, Guetta E, Epstein SE, Unger EF. Comparative effects of basic fibroblast growth factor and vascular endothelial growth factor on coronary collateral development and the arterial response to injury. Circulation. 1996;94:1074–82. doi: 10.1161/01.cir.94.5.1074. [DOI] [PubMed] [Google Scholar]
- 108.Gu F, Neufeld R, Amsden B. Maintenance of vascular endothelial growth factor and potentially other therapeutic proteins bioactivity during a photo-initiated free radical cross-linking reaction forming biodegradable elastomers. Eur J Pharm Biopharm. 2007;66:21–7. doi: 10.1016/j.ejpb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 109.Sun Q, Chen RR, Shen Y, Mooney DJ, Rajagopalan S, Grossman PM. Sustained vascular endothelial growth factor delivery enhances angiogenesis and perfusion in ischemic hind limb. Pharm Res. 2005;22:1110–6. doi: 10.1007/s11095-005-5644-2. [DOI] [PubMed] [Google Scholar]
- 110.Robson MC, Mustoe TA, Hunt TK. The future of recombinant growth factors in wound healing. Am J Surg. 1998;176:80S–2S. doi: 10.1016/s0002-9610(98)00186-x. [DOI] [PubMed] [Google Scholar]
- 111.Hsieh PC, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–48. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hsieh PC, MacGillivray C, Gannon J, Cruz FU, Lee RT. Local controlled intramyocardial delivery of platelet-derived growth factor improves postinfarction ventricular function without pulmonary toxicity. Circulation. 2006;114:637–44. doi: 10.1161/CIRCULATIONAHA.106.639831. [DOI] [PubMed] [Google Scholar]
- 113.Silva EA, Mooney DJ. Synthetic extracellular matrices for tissue engineering and regeneration. Curr Top Dev Biol. 2004;64:181–205. doi: 10.1016/S0070-2153(04)64008-7. [DOI] [PubMed] [Google Scholar]
- 114.Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408:998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 115.Peters MC, Isenberg BC, Rowley JA, Mooney DJ. Release from alginate enhances the biological activity of vascular endothelial growth factor. J Biomater Sci Polym Ed. 1998;9:1267–78. doi: 10.1163/156856298x00389. [DOI] [PubMed] [Google Scholar]
- 116.Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5:590–8. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 117.Dor Y, Djonov V, Keshet E. Making vascular networks in the adult: branching morphogenesis without a roadmap. Trends Cell Biol. 2003;13:131–6. doi: 10.1016/s0962-8924(03)00022-9. [DOI] [PubMed] [Google Scholar]
- 118.Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y. Angiogenic synergism, vascular stability and improvement of hindlimb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–13. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 119.Klabunde R. Cardiovascular physiology concepts. Philadelphia: Lippincott, Williams & Wilkins; 2004. pp. 91–140. [Google Scholar]
- 120.Fowlkes JL, Winkler MK. Exploring the interface between metallo-proteinase activity and growth factor and cytokine bioavailability. Cytokine Growth Factor Rev. 2002;13:277–87. doi: 10.1016/s1359-6101(02)00005-9. [DOI] [PubMed] [Google Scholar]
- 121.Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65:550–63. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 122.Chen RR, Snow JK, Palmer JP, Lin AS, Duvall CL, Guldberg RE, Mooney DJ. Host immune competence and local ischemia affects the functionality of engineered vasculature. Microcirculation. 2007;14:77–88. doi: 10.1080/10739680601131101. [DOI] [PubMed] [Google Scholar]
- 123.Peters MC, Polverini PJ, Mooney DJ. Engineering vascular networks in porous polymer matrices. J Biomed Mater Res. 2002;60:668–78. doi: 10.1002/jbm.10134. [DOI] [PubMed] [Google Scholar]
- 124.Raman R, Venkataraman G, Ernst S, Sasisekharan V, Sasisekharan R. Structural specificity of heparin binding in the fibroblast growth factor family of proteins. Proc Natl Acad Sci USA. 2003;100:2357–62. doi: 10.1073/pnas.0437842100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rajanayagam MA, Shou M, Thirumurti V, Lazarous DF, Quyyumi AA, Goncalves L, Stiber J, Epstein SE, Unger EF. Intracoronary basic fibroblast growth factor enhances myocardial collateral perfusion in dogs. J Am Coll Cardiol. 2000;35:519–26. doi: 10.1016/s0735-1097(99)00550-1. [DOI] [PubMed] [Google Scholar]
- 126.Greisler HP, Gosselin C, Ren D, Kang SS, Kim DU. Biointeractive polymers and tissue engineered blood vessels. Biomaterials. 1996;17:329–36. doi: 10.1016/0142-9612(96)85571-2. [DOI] [PubMed] [Google Scholar]
- 127.Gray JL, Kang SS, Zenni GC, Kim DU, Kim PI, Burgess WH, Drohan W, Winkles JA, Haudenschild CC, Greisler HP. FGF-1 affixation stimulates ePTFE endothelialization without intimal hyperplasia. J Surg Res. 1994;57:596–612. doi: 10.1006/jsre.1994.1189. [DOI] [PubMed] [Google Scholar]
- 128.Massia SP, Hubbell JA. Human endothelial cell interactions with surface-coupled adhesion peptides on a nonadhesive glass substrate and two polymeric biomaterials. J Biomed Mater Res. 1991;25:223–42. doi: 10.1002/jbm.820250209. [DOI] [PubMed] [Google Scholar]
- 129.Conte MS, Choudhury RP, Shirakowa M, Fallon JT, Birinyi LK, Choudhry RP. Endothelial cell seeding fails to attenuate intimal thickening in balloon-injured rabbit arteries. J Vasc Surg. 1995;21:413–21. doi: 10.1016/s0741-5214(95)70283-0. [DOI] [PubMed] [Google Scholar]
- 130.Conklin BS, Richter ER, Kreutziger KL, Zhong DS, Chen C. Development and evaluation of a novel decellularized vascular xenograft. Med Eng Phys. 2002;24:173–83. doi: 10.1016/s1350-4533(02)00010-3. [DOI] [PubMed] [Google Scholar]
- 131.Conklin BS, Wu H, Lin PH, Lumsden AB, Chen C. Basic fibroblast growth factor coating and endothelial cell seeding of a decellularized heparin-coated vascular graft. Artif Organs. 2004;28:668–75. doi: 10.1111/j.1525-1594.2004.00062.x. [DOI] [PubMed] [Google Scholar]
- 132.Hile DD, Amirpour ML, Akgerman A, Pishko MV. Active growth factor delivery from poly(D,L-lactide-co-glycolide) foams prepared in supercritical CO(2) J Control Release. 2000;66:177–85. doi: 10.1016/s0168-3659(99)00268-0. [DOI] [PubMed] [Google Scholar]
- 133.Desire L, Mysiakine E, Bonnafous D, Couvreur P, Sagodira S, Breton P, Fattal E. Sustained delivery of growth factors from methylidene malonate 2.1.2-based polymers. Biomaterials. 2006;27:2609–20. doi: 10.1016/j.biomaterials.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 134.Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci USA. 2006;103:8155–60. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Clowes AW, Karnowsky MJ. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977;265:625–6. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- 136.Ip JH, Fuster V, Badimon L, Badimon J, Taubman MB, Chesebro JH. Syndromes of accelerated atherosclerosis: role of vascular injury and smooth muscle cell proliferation. J Am Coll Cardiol. 1990;15:1667–87. doi: 10.1016/0735-1097(90)92845-s. [DOI] [PubMed] [Google Scholar]
- 137.McBride W, Lange RA, Hillis LD. Restenosis after successful coronary angioplasty. Pathophysiology and prevention. N Engl J Med. 1988;318:1734–7. doi: 10.1056/NEJM198806303182606. [DOI] [PubMed] [Google Scholar]
- 138.Liu MW, Roubin GS, King SB., 3rd Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation. 1989;79:1374–87. doi: 10.1161/01.cir.79.6.1374. [DOI] [PubMed] [Google Scholar]
- 139.Knetsch ML, Aldenhoff YB, Schraven M, Koole LH. Human endothelial cell attachment and proliferation on a novel vascular graft prototype. J Biomed Mater Res A. 2004;71:615–24. doi: 10.1002/jbm.a.30195. [DOI] [PubMed] [Google Scholar]
- 140.Edelman ER, Nathan A, Katada M, Gates J, Karnovsky MJ. Perivascular graft heparin delivery using biodegradable polymer wraps. Biomaterials. 2000;21:2279–86. doi: 10.1016/s0142-9612(00)00154-x. [DOI] [PubMed] [Google Scholar]
- 141.Verma S, Marsden PA. NO-eluting polyurethanes–vascular grafts of the future? N Engl J Med. 2005;353:730–1. doi: 10.1056/NEJMcibr052030. [DOI] [PubMed] [Google Scholar]
- 142.Loscalzo J. NO insufficiency, platelet activation, and arterial thrombosis. Circ Res. 2001;88:756–62. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- 143.Jun HW, Taite LJ, West JL. NO-producing polyurethanes. Biomacromolecules. 2005;6:838–44. doi: 10.1021/bm049419y. [DOI] [PubMed] [Google Scholar]
- 144.Hrabie JA, Keefer LK. Chemistry of the NO-releasing diazeniumdiolate (“nitroso-hydroxylamine”) functional group and its oxygen-substituted derivatives. Chem Rev. 2002;102:1135–54. doi: 10.1021/cr000028t. [DOI] [PubMed] [Google Scholar]
- 145.Keefer LK, Nims RW, Davies KM, Wink DA. “NONOates” (1-substituted diazen-1-ium-1,2-diolates) as NO donors: convenient NO dosage forms. Methods Enzymol. 1996;268:281–93. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 146.Taite LJ, Yang P, Jun HW, West JL. NO-releasing polyurethane-PEG copolymer containing the YIGSR peptide promotes endothelialization with decreased platelet adhesion. J Biomed Mater Res B Appl Biomater. 2008;84:108–16. doi: 10.1002/jbm.b.30850. [DOI] [PubMed] [Google Scholar]
- 147.Deible CR, Beckman EJ, Russell AJ, Wagner WR. Creating molecular barriers to acute platelet deposition on damaged arteries with reactive polyethylene glycol. J Biomed Mater Res. 1998;41:251–6. doi: 10.1002/(sici)1097-4636(199808)41:2<251::aid-jbm10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 148.Gombotz WR, Wang GH, Horbett TA, Hoffman AS. Protein adsorption to poly(ethylene oxide) surfaces. J Biomed Mater Res. 1991;25:1547–62. doi: 10.1002/jbm.820251211. [DOI] [PubMed] [Google Scholar]
- 149.Han DK, Park KD, Hubbell JA, Kim YH. Surface characteristics and biocompatibility of lactide-based poly(ethylene glycol) scaffolds for tissue engineering. J Biomater Sci Polym Ed. 1998;9:667–80. doi: 10.1163/156856298x00082. [DOI] [PubMed] [Google Scholar]
- 150.Kim S. Nonthrombogenic treatments and strategies. San Diego: Academic Press; 1996. pp. 297–308. [Google Scholar]
- 151.Park KD, Kim YS, Han DK, Kim YH, Lee EH, Suh H, Choi KS. Bacterial adhesion on PEG modified polyurethane surfaces. Biomaterials. 1998;19:851–9. doi: 10.1016/s0142-9612(97)00245-7. [DOI] [PubMed] [Google Scholar]
- 152.Suggs LJ, West JL, Mikos AG. Platelet adhesion on a bioresorbable poly (propylene fumarate-co-ethylene glycol) copolymer. Biomaterials. 1999;20:683–90. doi: 10.1016/s0142-9612(98)00226-9. [DOI] [PubMed] [Google Scholar]
- 153.Jun HW, West JL. Modification of polyurethaneurea with PEG and YIGSR peptide to enhance endothelialization without platelet adhesion. J Biomed Mater Res B Appl Biomater. 2005;72:131–9. doi: 10.1002/jbm.b.30135. [DOI] [PubMed] [Google Scholar]
- 154.Sata MSA, Kunisato A. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002:403–9. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 155.Kushibiki TTY. Future Direction of Gene Therapy in Tissue Engineering. In: Reis NARL, editor. Topics in Tis sue Engineering. 2005. pp. 1–33. [Google Scholar]
- 156.Madeddu P. Therapeutic angiogenesis and vasculogenesis for tissue regeneration. Exp Physiol. 2005;90:315–26. doi: 10.1113/expphysiol.2004.028571. [DOI] [PubMed] [Google Scholar]
- 157.Quarck R, Holvoet P. Gene therapy approaches for cardiovascular diseases. Curr Gene Ther. 2004;4:207–23. doi: 10.2174/1566523043346499. [DOI] [PubMed] [Google Scholar]
- 158.Melo LG, Pachori AS, Kong D, Gnecchi M, Wang K, Pratt RE, Dzau VJ. Gene and cell-based therapies for heart disease. FASEB J. 2004;18:648–63. doi: 10.1096/fj.03-1171rev. [DOI] [PubMed] [Google Scholar]
- 159.Audouy SA, De Leij LF, Hoekstra D, Molema G. In vivo characteristics of cationic liposomes as delivery vectors for gene therapy. Pharm Res. 2002;19:1599–605. doi: 10.1023/a:1020989709019. [DOI] [PubMed] [Google Scholar]
- 160.Voinea M, Simionescu M. Designing of ‘intelligent’ liposomes for efficient delivery of drugs. J Cell Mol Med. 2002;6:465–74. doi: 10.1111/j.1582-4934.2002.tb00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pedroso de Lima MC, Neves S, Filipe A, Duzgunes N, Simoes S. Cationic liposomes for gene delivery: from biophysics to biological applications. Curr Med Chem. 2003;10:1221–31. doi: 10.2174/0929867033457430. [DOI] [PubMed] [Google Scholar]
- 162.Nah JW, Yu L, Han SO, Ahn CH, Kim SW. Artery wall binding peptide-poly(ethylene glycol)-grafted-poly(L-lysine)-based gene delivery to artery wall cells. J Control Release. 2002;78:273–84. doi: 10.1016/s0168-3659(01)00499-0. [DOI] [PubMed] [Google Scholar]
- 163.Lee H, Jeong JH, Park TG. PEG grafted polylysine with fusogenic peptide for gene delivery: high transfection efficiency with low cytotoxicity. J Control Release. 2002;79:283–91. doi: 10.1016/s0168-3659(02)00002-0. [DOI] [PubMed] [Google Scholar]
- 164.Molas M, Bartrons R, Perales JC. Single-stranded DNA condensed with poly-L-lysine results in nanometric particles that are significantly smaller, more stable in physiological ionic strength fluids and afford higher efficiency of gene delivery than their double-stranded counterparts. Biochim Biophys Acta. 2002;1572:37–44. doi: 10.1016/s0304-4165(02)00276-3. [DOI] [PubMed] [Google Scholar]
- 165.Jeon E, Kim HD, Kim JS. Pluronic-grafted poly-(L)-lysine as a new synthetic gene carrier. J Biomed Mater Res A. 2003;66:854–9. doi: 10.1002/jbm.a.10012. [DOI] [PubMed] [Google Scholar]
- 166.Fischer D, Bieber T, Li Y, Elsasser HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res. 1999;16:1273–9. doi: 10.1023/a:1014861900478. [DOI] [PubMed] [Google Scholar]
- 167.Marschall P, Malik N, Larin Z. Transfer of YACs up to 2.3 Mb intact into human cells with polyethylenimine. Gene Ther. 1999;6:1634–7. doi: 10.1038/sj.gt.3300975. [DOI] [PubMed] [Google Scholar]
- 168.Campeau P, Chapdelaine P, Seigneurin-Venin S, Massie B, Tremblay JP. Transfection of large plasmids in primary human myoblasts. Gene Ther. 2001;8:1387–94. doi: 10.1038/sj.gt.3301532. [DOI] [PubMed] [Google Scholar]
- 169.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gosselin MA, Guo W, Lee RJ. Efficient gene transfer using reversibly cross-linked low molecular weight polyethylenimine. Bioconjug Chem. 2001;12:989–94. doi: 10.1021/bc0100455. [DOI] [PubMed] [Google Scholar]
- 171.Ferrari S, Moro E, Pettenazzo A, Behr JP, Zacchello F, Scarpa M. ExGen 500 is an efficient vector for gene delivery to lung epithelial cells in vitro and in vivo. Gene Ther. 1997;4:1100–6. doi: 10.1038/sj.gt.3300503. [DOI] [PubMed] [Google Scholar]
- 172.Coll JL, Chollet P, Brambilla E, Desplanques D, Behr JP, Favrot M. In vivo delivery to tumors of DNA complexed with linear polyethylenimine. Hum Gene Ther. 1999;10:1659–66. doi: 10.1089/10430349950017662. [DOI] [PubMed] [Google Scholar]
- 173.Wightman L, Kircheis R, Rossler V, Carotta S, Ruzicka R, Kursa M, Wagner E. Different behavior of branched and linear polyethylenimine for gene delivery in vitro and in vivo. J Gene Med. 2001;3:362–72. doi: 10.1002/jgm.187. [DOI] [PubMed] [Google Scholar]
- 174.Brunner S, Furtbauer E, Sauer T, Kursa M, Wagner E. Overcoming the nuclear barrier: cell cycle independent nonviral gene transfer with linear polyethylenimine or electroporation. Mol Ther. 2002;5:80–6. doi: 10.1006/mthe.2001.0509. [DOI] [PubMed] [Google Scholar]
- 175.Rainov NG, Kramm CM. Vector delivery methods and targeting strategies for gene therapy of brain tumors. Curr Gene Ther. 2001;1:367–83. doi: 10.2174/1566523013348445. [DOI] [PubMed] [Google Scholar]
- 176.Pooga M, Langel U. Targeting of cancer-related proteins with PNA oligomers. Curr Cancer Drug Targets. 2001;1:231–9. doi: 10.2174/1568009013334142. [DOI] [PubMed] [Google Scholar]
- 177.Gosselin MA, Lee RJ. Folate receptor-targeted liposomes as vectors for therapeutic agents. Biotechnol Annu Rev. 2002;8:103–31. doi: 10.1016/s1387-2656(02)08006-7. [DOI] [PubMed] [Google Scholar]
- 178.Wickham TJ. Ligand-directed targeting of genes to the site of disease. Nat Med. 2003;9:135–9. doi: 10.1038/nm0103-135. [DOI] [PubMed] [Google Scholar]
- 179.Mir LM, Moller PH, Andre F, Gehl J. Electric pulse-mediated gene delivery to various animal tissues. Adv Genet. 2005;54:83–114. doi: 10.1016/S0065-2660(05)54005-7. [DOI] [PubMed] [Google Scholar]
- 180.Murphy WL, Mooney DJ. Controlled delivery of inductive proteins, plasmid DNA and cells from tissue engineering matrices. J Periodontal Res. 1999;34:413–9. doi: 10.1111/j.1600-0765.1999.tb02275.x. [DOI] [PubMed] [Google Scholar]
- 181.Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17:551–4. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 182.Wang D, Robinson DR, Kwon GS, Samuel J. Encapsulation of plasmid DNA in biodegradable poly(D, L-lactic-co-glycolic acid) microspheres as a novel approach for immunogene delivery. J Control Release. 1999;57:9–18. doi: 10.1016/s0168-3659(98)00099-6. [DOI] [PubMed] [Google Scholar]
- 183.Capan Y, Jiang G, Giovagnoli S, Na KH, DeLuca PP. Preparation and characterization of poly(D,L-lactide-co-glycolide) microspheres for controlled release of human growth hormone. AAPS PharmSciTech. 2003;4:1–10. doi: 10.1208/pt040228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Luo D, Woodrow-Mumford K, Belcheva N, Saltzman WM. Controlled DNA delivery systems. Pharm Res. 1999;16:1300–8. doi: 10.1023/a:1014870102295. [DOI] [PubMed] [Google Scholar]
- 185.Hedley ML. Formulations containing poly (lactide-co-glycolide) and plasmid DNA expression vectors. Expert Opin Biol Ther. 2003;3:903–10. doi: 10.1517/14712598.3.6.903. [DOI] [PubMed] [Google Scholar]
- 186.Houchin-Ray T, Swift LA, Jang JH, Shea LD. Patterned PLG substrates for localized DNA delivery and directed neurite extension. Biomaterials. 2007;28:2603–11. doi: 10.1016/j.biomaterials.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jang JH, Shea LD. Intramuscular delivery of DNA releasing microspheres: microsphere properties and transgene expression. J Control Release. 2006;112:120–8. doi: 10.1016/j.jconrel.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jang JH, Bengali Z, Houchin TL, Shea LD. Surface adsorption of DNA to tissue engineering scaffolds for efficient gene delivery. J Biomed Mater Res A. 2006;77:50–8. doi: 10.1002/jbm.a.30643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jang JH, Shea LD. Controllable delivery of non-viral DNA from porous scaffolds. J Control Release. 2003;86:157–68. doi: 10.1016/s0168-3659(02)00369-3. [DOI] [PubMed] [Google Scholar]
- 190.Ismail FA, Napaporn J, Hughes JA, Brazeau GA. In situ gel formulations for gene delivery: release and myotoxicity studies. Pharm Dev Technol. 2000;5:391–7. doi: 10.1081/pdt-100100555. [DOI] [PubMed] [Google Scholar]
- 191.Perez C, Sanchez A, Putnam D, Ting D, Langer R, Alonso MJ. Poly(lactic acid)-poly(ethylene glycol) nanoparticles as new carriers for the delivery of plasmid DNA. J Control Release. 2001;75:211–24. doi: 10.1016/s0168-3659(01)00397-2. [DOI] [PubMed] [Google Scholar]
- 192.Wang J, Zhang PC, Mao HQ, Leong KW. Enhanced gene expression in mouse muscle by sustained release of plasmid DNA using PPE-EA as a carrier. Gene Ther. 2002;9:1254–61. doi: 10.1038/sj.gt.3301794. [DOI] [PubMed] [Google Scholar]
- 193.Lim YB, Han SO, Kong HU, Lee Y, Park JS, Jeong B, Kim SW. Biodegradable polyester, poly[alpha-(4-aminobutyl)-L-glycolic acid], as a non-toxic gene carrier. Pharm Res. 2000;17:811–6. doi: 10.1023/a:1007552007765. [DOI] [PubMed] [Google Scholar]
- 194.Park JS, Oh YK, Yoon H, Kim JM, Kim CK. In situgelling and mucoadhesive polymer vehicles for controlled intranasal delivery of plasmid DNA. J Biomed Mater Res. 2002;59:144–51. doi: 10.1002/jbm.1227. [DOI] [PubMed] [Google Scholar]
- 195.Shen H, Goldberg E, Saltzman WM. Gene expression and mucosal immune responses after vaginal DNA immunization in mice using a controlled delivery matrix. J Control Release. 2003;86:339–48. doi: 10.1016/s0168-3659(02)00354-1. [DOI] [PubMed] [Google Scholar]
- 196.Megeed Z, Cappello J, Ghandehari H. Controlled release of plasmid DNA from a genetically engineered silk-elastinlike hydrogel. Pharm Res. 2002;19:954–9. doi: 10.1023/a:1016406120288. [DOI] [PubMed] [Google Scholar]
- 197.Perlstein I, Connolly JM, Cui X, Song C, Li Q, Jones PL, Lu Z, DeFelice S, Klugherz B, Wilensky R, Levy RJ. DNA delivery from an intravascular stent with a denatured collagen-polylactic-polyglycolic acid-controlled release coating: mechanisms of enhanced transfection. Gene Ther. 2003;10:1420–8. doi: 10.1038/sj.gt.3302043. [DOI] [PubMed] [Google Scholar]
- 198.Ochiya T, Takahama Y, Nagahara S, Sumita Y, Hisada A, Itoh H, Nagai Y, Terada M. New delivery system for plas-mid DNA in vivo using atelocollagen as a carrier material: the Minipellet. Nat Med. 1999;5:707–10. doi: 10.1038/9560. [DOI] [PubMed] [Google Scholar]
- 199.Fukunaka Y, Iwanaga K, Morimoto K, Kakemi M, Tabata Y. Controlled release of plasmid DNA from cationized gelatin hydrogels based on hydrogel degradation. J Control Release. 2002;80:333–43. doi: 10.1016/s0168-3659(02)00026-3. [DOI] [PubMed] [Google Scholar]
- 200.Kushibiki T, Matsumoto K, Nakamura T, Tabata Y. Suppression of tumor metastasis by NK4 plasmid DNA released from cationized gelatin. Gene Ther. 2004;11:1205–14. doi: 10.1038/sj.gt.3302285. [DOI] [PubMed] [Google Scholar]
- 201.Huang YC, Simmons C, Kaigler D, Rice KG, Mooney DJ. Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4) Gene Ther. 2005;12:418–26. doi: 10.1038/sj.gt.3302439. [DOI] [PubMed] [Google Scholar]
- 202.Bonadio J. Tissue engineering via local gene delivery. J Mol Med. 2000;78:303–11. doi: 10.1007/s001090000118. [DOI] [PubMed] [Google Scholar]
- 203.Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5:753–9. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- 204.Ikada Y, Tabata Y. Protein release from gelatin matrices. Adv Drug Deliv Rev. 1998;31:287–301. doi: 10.1016/s0169-409x(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 205.Yamamoto M, Tabata Y, Hong L, Miyamoto S, Hashimoto N, Ikada Y. Bone regeneration by transforming growth factor beta1 released from a biodegradable hydrogel. J Control Release. 2000;64:133–42. doi: 10.1016/s0168-3659(99)00129-7. [DOI] [PubMed] [Google Scholar]
- 206.Mullen PM, Lollo CP, Phan QC, Amini A, Banaszczyk MG, Fabrycki JM, Wu D, Carlo AT, Pezzoli P, Coffin CC, Carlo DJ. Strength of conjugate binding to plasmid DNA affects degradation rate and expression level in vivo. Biochim Biophys Acta. 2000;1523:103–10. doi: 10.1016/s0304-4165(00)00104-5. [DOI] [PubMed] [Google Scholar]
- 207.Park S, Healy KE. Nanoparticulate DNA packaging using terpolymers of poly(lysine-g-(lactide-b-ethylene glycol)) Bioconjug Chem. 2003;14:311–9. doi: 10.1021/bc025623b. [DOI] [PubMed] [Google Scholar]
- 208.Moret I, Esteban Peris J, Guillem VM, Benet M, Revert F, Dasi F, Crespo A, Alino SF. Stability of PEI-DNA and DOTAP-DNA complexes: effect of alkaline pH, heparin and serum. J Control Release. 2001;76:169–81. doi: 10.1016/s0168-3659(01)00415-1. [DOI] [PubMed] [Google Scholar]
- 209.Kofidis T, Lebl DR, Martinez EC, Hoyt G, Tanaka M, Robbins RC. Novel injectable bioartificial tissue facilitates targeted, less invasive, large-scale tissue restoration on the beating heart after myocardial injury. Circulation. 2005;112:173–7. doi: 10.1161/CIRCULATIONAHA.104.526178. [DOI] [PubMed] [Google Scholar]
- 210.Kofidis T, De Bruin JL, Hoyt G, Lebl DR, Tanaka M, Yamane T, Chang CP, Robbins RC. Injectable bioartificial myocardial tissue for large-scale intramural cell transfer and functional recovery of injured heart muscle. J Thorac Cardiovasc Surg. 2004;128:571–8. doi: 10.1016/j.jtcvs.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 211.Cummings CL, Gawlitta D, Nerem RM, Stegemann JP. Properties of engineered vascular constructs made from collagen, fibrin, and collagen-fibrin mixtures. Biomaterials. 2004;25:3699–706. doi: 10.1016/j.biomaterials.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 212.Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci USA. 2003;100:12741–6. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.He W, Ma Z, Teo WE, Dong YX, Robless PA, Lim TC, Ramakrishna S. Tubular nanofiber scaffolds for tissue engineered small-diameter vascular grafts. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.32081. [DOI] [PubMed] [Google Scholar]
- 214.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–93. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 215.Pankajakshan D, Krishnan VK, Krishnan LK. Vascular tissue generation in response to signaling molecules integrated with a novel poly(epsilon-caprolactone)-fibrin hybrid scaffold. J Tissue Eng Regen Med. 2007;1:389–97. doi: 10.1002/term.48. [DOI] [PubMed] [Google Scholar]
- 216.Williamson MR, Black R, Kielty C. PCL-PU composite vascular scaffold production for vascular tissue engineering: attachment, proliferation and bioactivity of human vascular endothelial cells. Biomaterials. 2006;27:3608–16. doi: 10.1016/j.biomaterials.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 217.Davis ME, Motion JP, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang S, Lee RT. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111:442–50. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Motlagh D, Allen J, Hoshi R, Yang J, Lui K, Ameer G. Hemocompatibility evaluation of poly(diol citrate) in vitro for vascular tissue engineering. J Biomed Mater Res A. 2007;82:907–16. doi: 10.1002/jbm.a.31211. [DOI] [PubMed] [Google Scholar]
- 219.Yang J, Webb AR, Pickerill SJ, Hageman G, Ameer GA. Synthesis and evaluation of poly(diol citrate) biodegradable elastomers. Biomaterials. 2006;27:1889–98. doi: 10.1016/j.biomaterials.2005.05.106. [DOI] [PubMed] [Google Scholar]
- 220.Wang Y, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat Biotechnol. 2002;20:602–6. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 221.Day RM, Maquet V, Boccaccini AR, Jerome R, Forbes A. In vitro and in vivo analysis of macroporous biodegradable poly(D,L-lactide-co-glycolide) scaffolds containing bioactive glass. J Biomed Mater Res A. 2005;75:778–87. doi: 10.1002/jbm.a.30433. [DOI] [PubMed] [Google Scholar]
- 222.Zisch AH, Schenk U, Schense JC, Sakiyama-Elbert SE, Hubbell JA. Covalently conjugated VEGF–fibrin matrices for endothelialization. J Control Release. 2001;72:101–13. doi: 10.1016/s0168-3659(01)00266-8. [DOI] [PubMed] [Google Scholar]
- 223.Koch S, Yao C, Grieb G, Prevel P, Noah EM, Steffens GC. Enhancing angiogenesis in collagen matrices by covalent incorporation of VEGF. J Mater Sci Mater Med. 2006;17:735–41. doi: 10.1007/s10856-006-9684-x. [DOI] [PubMed] [Google Scholar]
- 224.Ravin AG, Olbrich KC, Levin LS, Usala AL, Klitzman B. Long- and short-term effects of biological hydrogels on capsule microvascular density around implants in rats. J Biomed Mater Res. 2001;58:313–8. doi: 10.1002/1097-4636(2001)58:3<313::aid-jbm1023>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 225.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65:389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 226.Thompson JA, Anderson KD, DiPietro JM, Zwiebel JA, Zametta M, Anderson WF, Maciag T. Site-directed neovessel formation in vivo. Science. 1988;241:1349–52. doi: 10.1126/science.2457952. [DOI] [PubMed] [Google Scholar]
- 227.Yamamoto M, Ikada Y, Tabata Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J Biomater Sci Polym Ed. 2001;12:77–88. doi: 10.1163/156856201744461. [DOI] [PubMed] [Google Scholar]
- 228.Ozeki M, Ishii T, Hirano Y, Tabata Y. Controlled release of hepatocyte growth factor from gelatin hydrogels based on hydrogel degradation. J Drug Target. 2001;9:461–71. doi: 10.3109/10611860108998780. [DOI] [PubMed] [Google Scholar]


