Abstract
The balance between lesion and regeneration of the endothelium is critical for the maintenance of vessel integrity. Exposure to cardiovascular risk factors (CRF) alters the regulatory functions of the endothelium that progresses from a quiescent state to activation, apoptosis and death. In the last 10 years, identification of circulating endothelial cells (CEC) and endothelial-derived microparticles (EMP) in the circulation has raised considerable interest as non-invasive markers of vascular dysfunction. Indeed, these endothelial-derived biomarkers were associated with most of the CRFs, were indicative of a poor clinical outcome in atherothrombotic disorders and correlated with established parameters of endothelial dysfunction. CEC and EMP also behave as potential pathogenic vectors able to accelerate endothelial dysfunction and promote disease progression. The endothelial response to injury has been enlarged by the discovery of a powerful physiological repair process based on the recruitment of circulating endothelial progenitor cells (EPC) from the bone marrow. Recent studies indicate that reduction of EPC number and function by CRF plays a critical role in the progression of cardiovascular diseases. This EPC-mediated repair to injury response can be integrated into a clinical endothelial phenotype defining the ‘vascular competence’ of each individual. In the future, provided that standardization of available methodologies could be achieved, multimarker strategies combining CEC, EMP and EPC levels as integrative markers of ‘vascular competence’ may offer new perspectives to assess vascular risk and to monitor treatment efficacy.
Keywords: circulating endothelial cells, endothelial microparticles, endothelial progenitor cells, endothelial injury/repair, endothelial biomarkers, vascular competence
Introduction
Strategically located between blood and tissues, the endothelium is exposed to blood-borne mediators, biomechanical stimuli and cells from circulating blood and neighbouring tissues, which determines a switch from a quiescent to an activated phenotype. Such activated phenotype, resulting from the physiological adaptation of a given region, can also occur pathophysiologically.
It is now well recognized that disruption of endothelial integrity and physiological functions represent a pivotal mechanism in the initiation and development of cardiovascular disorders (CVD) [1]. Consequently, exploration of endothelium has raised growing interest and has led to the development of a range of methods that can be divided into functional testing and surrogate biomarkers.
The former includes endothelium-dependent vasorelaxation, arterial stiffness and pulse wave propagation, and has recently been reviewed [2]. A second category is defined by established soluble molecules reflecting alterations of the main regulatory functions of the endothelium such as inflammation, haemostasis or permeability. However, these markers have been disappointing by their lack of specificity and clinical relevance at the individual level. The capacity of the adherent endothelium to undergo cellular alterations has revisited our vision of the endothelium as a dynamic tissue in equilibrium with a circulating compartment reflecting both lesion and regeneration of the vascular tree. This endothelial-derived compartment has led to delineate a third group of cellular markers, corresponding to circulating endothelial cells (CEC) and endothelial-derived microparticles (EMP) released from the injured vessels and progenitors endothelial cells (EPC) involved in vascular regeneration.
Since CEC, EMP and EPC have been individually reviewed [3–8], the present review aims to summarize the novel insight provided by these emerging cellular biomarkers (i) in the current understanding of the endothelial dynamics between injury and repair, (ii) in the evaluation of vascular integrity in a specific and non-invasive way and (ii) in the assessment of vascular risk. This review will also discuss the promises of multimarker strategies combining CEC, and EMP and EPC as integrative markers of vascular competence, an emerging concept for vascular risk stratification and therapeutic options.
Dynamics between endothelial injury and repair: the response to injury theory ‘revisited’
Initially viewed as a single-cell lining of the vascular system, the endothelium has emerged as a dynamically mutable interface locally responsive to environmental stimuli. As a consequence, reprogramming of the endothelium generates an impressive repertoire of biological responses playing a key role in the control of vascular homeostasis such as vasomotion, permeability, haemostasis, inflammation or angiogenesis [9]. According to the response to injury theory [10], mechanical damage or chronic exposure to cardiovascular risk factors (CRF) alters the regulatory functions of the endothelium, which progress towards pro-inflammatory activation, senescence and apoptosis (Fig. 1). As a consequence, the endothelium not only displays altered functions, but also loses its integrity. Microparticles released from activated or apoptotic endothelial cells [11] and endothelial cells detached from injured vessels [12] constitute a hallmark of these deleterious responses affecting the vessel wall. The response to injury theory has been enlarged by the discovery of a powerful physiological repair process based on EPC. In response to injury, regenerative mechanisms are triggered to restore endothelium integrity. In the past, endothelial repair was solely attributed to angiogenesis, involving adjacent mature EC able to replicate locally and replace the lost cells. Since the pioneering works by Asahara [13], it has become obvious that the recruitment of EPC represents an additional mechanism for endothelial repair. Recruited from the bone marrow, these cells are able to differentiate into mature cells and restore endothelial integrity at sites of vascular injury. The demonstration that patients with high EPC levels have preserved endothelial function, despite exposure to CRF, underlines their critical role in the ongoing maintenance of vascular integrity [14].
1.
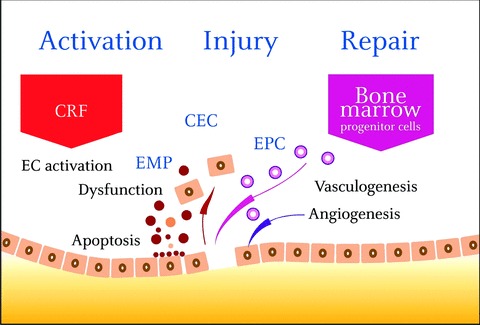
Mechanical damage or chronic exposure to CRF alters the regulatory functions of the endothelium which progress to apoptosis and dysfunction. Disruption of endothelial integrity is associated with a broad spectrum of responses including detachment of mature endothelial cells (CEC) and shedding of endothelial microparticles (EMP). In response to injury, endothelial progenitors cells (EPC), recruited from the bone marrow, are able to differentiate into mature cells and to restore endothelial integrity. These endothelial responses can be integrated into a dynamic ‘activation / lesion/regeneration triad’ CRF: cardiovascular risk factor, CEC: circulating endothelial cells, EMP: endothelial microparticles, EPC: endothelial progenitor cells.
As a whole, this EPC-mediated repair constitutes the third step of the response to injury initiated by chronic activation of the endothelium in response to CRF. This spectrum of endothelial responses can be integrated in a dynamic triad ‘activation/injury/ repair’ which has critically transformed our understanding of vascular biology (Fig. 1).
Emerging biomarkers reflecting the dynamics between endothelial injury and repair: from pathophysiology to clinical testing
Appreciation of the multifaceted biology of endothelial cells has been the basis of new assays providing the opportunity to measure endothelium integrity in a non-invasive and specific manner. Whereas the determination of CEC and EMP levels have raised considerable interest to appreciate the status of activated/ damaged endothelium, EPC levels have been used to evaluate the endogenous repair potential. The comparative characteristics of CEC, EMP and EPC have been summarized in Table 1.
1.
Comparative characteristics of CEC, EMP and EPC
 |
 |
 |
||||||
|---|---|---|---|---|---|---|---|---|
| Origin | Blood vessel wall | Blood vessel wall CEC ? EPC ? | Bone marrow, other niches | |||||
| Morphology | Mature cells of diameter 15–50 μm | Endothelial derived vesicles of diameter 0.1–1 μm | Immature cells of diameter less than 15 [xm | |||||
| Phenotype | CD31+, CD34+, CD105+, CD146+ | CD144+, CD146+, CD62E+, CD51+ | CD133+, CD34+, KDR+, CD117+ | |||||
| Ulex Europaeus lectin+, vWF+ | CD31+/CD42∼ | CD146 +/− | ||||||
| eNOS+ | CD31+/CD51 + | CD45 +/− | ||||||
| CD45−, CD133− | ||||||||
| Detection Methods | Density centrifugation | Flow cytometry | Flow cytometry | |||||
| CD146 IMS and fluorescence microscopy | ELISA | Clonogenic assays : | ||||||
| CD146 IMS and flow cytometry | Solid phase capture | - CFU-EC or Culture assay (‘early EPC’) | ||||||
| CD146 IMS and image analysis | - HPP-ECFC (‘late EPC’) | |||||||
| Flow cytometry | ||||||||
| Pathophysiopathology | Endothelial damage | Endothelial activation / apoptosis | Neovascularisation, repair | |||||
Abbreviations : CEC, Circulating endothelial cells, EMP, endothelial microparticles, EPC, endothelial progenitor cells, IMS, immuno-magnetic separation, CFU-EC, colony formit unit-endothelial cells, HPP-ECFC, high proliferation potential-endothelial colony forming cells.
Circulating endothelial cells
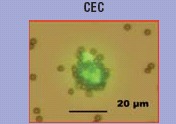
Current consensus supports the view that CEC are mature cells shed from the vessel wall in response to injury [15]. These cells present a heterogeneous size (from 10 to 50 micron), express endothelial markers (von Willebrand factor [vWF], CD31, CD144) but are negative for leucocyte markers. In contrast to EPC, they do not express immature markers such as CD133, and do not give rise to cell colonies with a high proliferative potential. Due to the extreme scarcity of CEC in peripheral blood, an important step in their identification has been the development of sensitive technologies for the detection of rare events based on the immunola-belling with monoclonal antibodies to novel endothelial antigens [16]. In 1992, an antibody recognizing the CD146 antigen combined with an immuno-magnetic separation assay (IMS) allowed the immunological characterization of CEC in the peripheral blood of patients submitted to coronary angioplasty chosen as a model of vascular injury. The marked elevation of CEC number found after angioplasty, confirmed that they result from endothelial trauma triggered by the catheter procedure itself [12].
IMS assay has become the consensus protocol for CEC enumeration [17]. This method combines a first step of enrichment using CD146 coated magnetic microbeads and a second step of cell numeration, according selected criteria, using fluorescent microscopy after cell staining with acridin orange or with the endothelial specific Ulex europaeus agglutinin 1 lectin. The rationale for cell enrichment comes from the very low number of CEC since, with IMS normal values lower than 10 cells/ml have been consensually reported [15]. Alternative methodologies for CEC counting include flow cytometry, which has been largely used to detect CEC in cancer patients [18]. However, despite an attempt of standardization recently proposed by Duda [19], normal values using flow cytometry are very heterogeneous and about 100–1000-fold higher compared to those from IMS. More recently, hybrid methods combining immunomagnetic enrichment using paramagnetic particles coated with CD146 mab followed by multiparametric identification of CEC by flow cytometry or by image analysis have been proposed [20, 21]. With these approaches CEC counts in healthy individuals are in the same order of magnitude than those reported by IMS.
Although it is clear that CEC represent mature cells, the potential mechanisms of endothelial cell detachment are probably multiple and not exclusive. Different experimental models have documented that denudation of the vessels can be triggered by mechanical injury, protease- or cytokine-mediated detachment, or activation of apoptosis. A potential mechanism is represented by anoikis, a cell death mechanism induced by lack of anchorage leading to a disruption of the interaction between cells and extracellular matrix. This can result from integrin disengagement that impairs their association with focal adhesion kinase in cells and inhibits survival signals [22]. In addition, endothelial cell detachment can also result from pericellular proteolysis and defective adhesion of cells to the extracellular matrix and to neighbouring cells that involves loss of VE-cadherin-mediated homotypic interactions [23].
Another important question is the vascular bed origin of CEC that varies according to the disease process and can be delineated using specific antigens. Besides its structural heterogeneity, the endothelium displays different functions linked to the expression of specific constitutive or inducible antigens. EphrinB2, delta-like 4 or neuropilin-1 are preferentially expressed by arterial endothe-lial cells whereas EphrinB4 and neuropilin-2 are markers of venous cells [24, 25]. In practice, CD36, a maker of microvessels, has been used to document the anatomical origin of CEC. In addition, E-selectin, VCAM-1 or tissue factor (TF) expression by CEC have been used to quantify pro-inflammatory and pro-coagulant activation of the vessels (Table 2). Assuming that CEC are representative of the vessel they derived from, analysis of their pheno-type may provide important insights not only on their anatomical origin but also on their pathogenic involvement (see below).
2.
Changes in blood level of CEC in patients with cardiovascular and other diseases
| Disease/condition | CEC changes and phenotypes | Methods | Main finding(s) | Reference | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anatomic origin | Activation | Apoptosis | ||||||||||||||||||||||
| Coronary angioplasty | ↑ | IMS | CEC elevation indicative of traumatic vessel wall injury | [12] | ||||||||||||||||||||
| Acute coronary syndromes | ↑ | CD36 | ICAM-1, TF+ | 10% | IMS | CEC elevation indicative of injury | [26] | |||||||||||||||||
| IMS | CEC elevation earlier and independent of troponin 1 | [27] | ||||||||||||||||||||||
| IMS | CEC level correlated with vWF, IL6 are predictive of MACE | [28] | ||||||||||||||||||||||
| Heart failure | ↑ | IMS | CEC correlated inversely with FMD and positively with vWF and sTF | [29] | ||||||||||||||||||||
| Diabetes mellitus | ↑ | IMS | CEC levels independent of plasma glucose level and HbA1c | [30] | ||||||||||||||||||||
| Thrombotic microangiopathy | ↑ | CD36 | IMS | CEC level correlated with prognosis | [31] | |||||||||||||||||||
| Stroke | ↑ | IMS | CEC level correlated with sE-selectin and vWF | [32] | ||||||||||||||||||||
| Pulmonary hypertension | ↑ | CD36 | E sel | IMS | CEC correlated with SBP and DBP | [33] | ||||||||||||||||||
| Sickle cell anaemia | ↑ | CD36 | ICAM-1, E sel, VCAM-1,TF+, | 80% | IMS | Elevated CEC correlated with crisis | [34] | |||||||||||||||||
| Haemodialysis | ↑ | IMS | CEC are predictive of cardiovascular events | [35] | ||||||||||||||||||||
| Kidney transplantation | ↑↓ | IMS | CEC are indicative of vascular rejection | [36] | ||||||||||||||||||||
| IMS | CEC decrease 1-year post-transplant and correlated with IS treatment | [37] | ||||||||||||||||||||||
| HSC transplantation | ↑ | IMS | CEC correlated with intensity of conditioning treatment | [38] | ||||||||||||||||||||
| Systemic sclerosis | ↑ | ICAM-1, E sel | FCM | Total and activated CEC correlated with disease activity score and the severity of pulmonary hypertension | [39] | |||||||||||||||||||
| Vasculitis | ↑ | TF+ | IMS | CEC correlated with disease severity and with IS treatment | [40] | |||||||||||||||||||
| Systemic lupus | ↑ | Nitrotyrosine | 100% | FCM | CEC level inversely correlated with FMD and positively with vWF and sTF | [41] | ||||||||||||||||||
| FCM | CEC correlated with disease severity; plasma c3a | [42] | ||||||||||||||||||||||
Several reviews indicated that increased CEC levels have been reported in cardiovascular diseases [43, 44] such as acute coronary syndromes (ACS) [26–29] or stroke [32] and in most of their associated risk factors like diabetes [30], hypertension [32] and chronic renal failure [35]. CEC were also increased in other pathological situations associated with atherosclerotic complications including inflammatory vasculitis [39], transplantation [45] or lupus [46]. These main clinical studies indicate that CEC levels are indicative of disease activity and generally correlate with functional or plasma markers of endothelial injury (Table 2). An interesting illustration of CEC relevance, both in pathophysiology and clinical testing, has been provided by ACS. Consistent data reported by several studies indicated that myocardial infarction and unstable angina are associated with increased CEC counts compared to normal persons or patients with stable angina. In clinical testing, a multimarker strategy combining CEC and cardiac Troponin markedly improved diagnosis accuracy in patients with unstable angina [27], because CEC elevation was earlier and independent of troponin level. Besides this diagnostic interest, CEC have been proposed as a novel prognostic indicator since raised CEC levels during the first 48 hrs of ACS independently predicted both death and major adverse cardiovascular events at 30 days and 1 year [28]. Analysis of the phenotype of CEC from patients with ACS indicated that these cells are positive for TF [26], suggesting that they could disseminate a pro-coagulant potential. Consistently, high CEC levels also correlated with soluble TF, a hallmark of activation of the coagulation pathways, and with vWF, a marker of endothelial dysfunction [29]. Moreover, increased CEC were found to be associated with abnormal vascular responses, because patients with the highest CEC values presented the lowest flow-mediated dilatation [47]. Taken together these data suggest a link between endothelial detachment, vascular dysfunction and prothrombotic status. A novel insight that could change current opinion on CEC relates to their recently described capacity of apoptotic CEC to induce inflammatory signals in quiescent endothelial cells [48]. This response further support their possible pathogenic role as well as a survival mechanism by which healthy endothelium could be alerted to ongoing cell death.
Endothelial microparticles
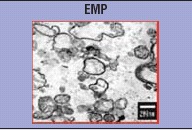
The first description of endothelial microparticles (EMP) was done in patients with lupus anticoagulant presenting thrombotic complications [11]. They were defined as small vesicular structures with a heterogeneous diameter (from 0.1 to 1 micron), resulting from the remodelling of membrane phospholipids and expressing phosphatidylserine (PS) and antigens representative of endothelial cells. Although commonly used to define microparticles, exposure of PS, has been shown to be undetectable in a significant proportion of circulating or in vitro generated EMP. The lack of PS expression may either involve its unavailability for annexin V binding due to PS engagement in other molecular interactions with plasma proteins, or suggest that a vesiculation process can occur independently of membrane asymmetry loss [49].
The current knowledge on EMP composition derives mainly from experiments performed on cultured endothelial cells. Therefore caution should be made because in vitro studies are not necessarily representative of the tissue specific features dictated by endothelial cell localization in the vascular tree. Extensive phe-notypic characterization indicated that EMP display the same molecules as their parent cells [11, 50]. For example, receptors such as TF, vascular cell adhesion molecule-1 or E-selectin were detected on MP shed from tumour necrosis factor activated human umbilical vein endothelial cells (HUVEC), suggesting that EMP represented a signature of endothelial pro-coagulant and pro-inflammatory responses. The expression of PS and TF the main activator of the extrinsic coagulation pathway indicate that EMP could provide pro-coagulant surfaces for the assembly of clotting enzymes promoting thrombin generation [51]. In vivo, the contribution of endothelial cells to the circulating pool of TF+ MP has been demonstrated in human endotoxemia [52] and patients with intravascular coagulopathies [53]. The presence of pro-coagulant EMP has also been found in atherosclerotic plaques [54] and in the circulation of patients with ACS [55]. However, MP generated from cultured endothelial cells stimulated with activated protein C harbour functional endothelial protein C receptor, protected from metalloproteinase cleavage, and display anticoagulant ability towards factor Va inactivation [56]. Although this mechanism is still undocumented in vivo, it may be indicative of potential anticoagulant properties that may counterbalance prothrombotic characteristics of EMP.
In normal persons, EMP represent a minority of total circulating MP. Flow cytometry is the most widely used method to characterize EMP, but the pro-coagulant function of EMP can also be measured using functional assays (based on coagulation activation by MP derived phospholipids and/or TF), two complementary approaches that have been reviewed in a forum [57, 58]. However as it is the case for the other MP subpopulations, their measurement remains a technical challenge due to the lack of standardization. First, several pre-analytical variables such as blood collection, sample processing, transportation and centrifugation may have a major impact on MP measurement and have not been studied in the literature. Although flow cytometry provides useful information, some limitations regarding MP measurement need to be considered such as threshold for particle size detection, standardized instrument settings or well-characterized antibodies against cell-associated antigens that are not expressed by other cell lineage.
The mechanisms controlling EMP formation remains mostly unknown. A recent study based on a gene profiling analysis has identified an original pathway of endothelial vesiculation induced by thrombin, involving the p-kinase ROCK-2 in the absence of cell death [59]. A better understanding of the mechanisms controlling the endothelial vesiculation is crucial for the pharmacological control of EMP release.
Taking into account the broad distribution of endothelial cells along the vascular tree, yet another open question is the anatomical site the EMP derived from, a question that relies on the availability of tissue specific endothelial markers. The possibility that they could also originate from apoptotic CEC cannot be excluded because both EMP and CEC are elevated in a number of CVD. In addition, EMP co-expressing immaturity and endothelial markers originating from apoptotic EPC have been recently identified [60].
A survey of the literature, summarized in Table 3, indicated that elevated EMP levels have been reported in CVD and other vascular settings associated with a thrombotic propensity [6, 74]. Elevated EMP levels are also associated with most of the CRF such as obesity [75], hypertension [63], diabetes [65, 66] and appear indicative of a poor clinical outcome. In vivo evidence that EMP are representative of the endothelial dysfunction came from convergent data demonstrating that EMP correlated with the severity of the endothelial dysfunction determined during angiography or flow-mediated dilatation in patients with end stage renal disease [67] and coronary artery disease [76]. EMP are not only a reflection of endothelial dysfunction but also may be deleterious by inducing or aggravating pre-existing vascular dysfunction, as shown by their ability to impair nitric oxide release from vascular endothelial cells [77]. The deleterious potential of EMP opens new pharmacological perspectives. Several therapies widely used for their beneficial effect in CVD, such as antioxidants, p-blockers or statins [6] also reduce EMP concentration.
3.
Changes in blood level of EMP in patients with cardiovascular and other diseases
| Disease/condition | EMP changes and phenotypes | Methods | Main finding(s) | Ref | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute Coronary syndrome | ↑ | CD146+, CD31 + | SPC | Elevated EMP levels with a pro-coagulant activity | [55] | ||||||||||||||
| ↑ | CD31+ | FCM | EMP levels correlated with high-risk lesions | [61] | |||||||||||||||
| Stroke | ↑ | CD105+, CD144+ | FCM | Link between EMP, severity lesions and clinical outcome | [62] | ||||||||||||||
| Hypertension | ↑ | CD31+/CD42− | FCM | EMP levels correlated with both SBP and DPB | [63] | ||||||||||||||
| Pulmonaryhypertension | ↑ | CD31 +/CD41−, CD144 | FCM | Levels of EMP predict haemodynamic severity of pulmonary hypertension | [64] | ||||||||||||||
| Type 2 diabetes | ↑ | CD144+ | FCM | EMP levels correlated with coronary artery disease | [65] | ||||||||||||||
| Type 1 diabetes | ↑ | CD51+ | FCM | EMP levels correlated with HbA1c | [66] | ||||||||||||||
| End-stage renal disease | ↑ | CD144+, CD31+/CD41− | FCM | High levels of EMP correlated with impaired vascular function | [67] | ||||||||||||||
| Antiphospholipidsyndrome | ↑ | CD51+ | FCM | EMP correlated with Lupus anticoagulant | [68] | ||||||||||||||
| Obesity | ↑ | CD31+/CD42−, | FCM | High levels of EMP correlated with altered FMD | [69] | ||||||||||||||
| Post-prandial hypertriglyceridemia | ↑ | CD31+/CD42− | FCM | EMP correlated with high fat meal | [70] | ||||||||||||||
| TTP | ↑ | CD31+, CD51+, CD54+, CD62E+, CD105+, CD106+ | FCM | Elevated EMP correlated with endothelial activation | [71] | ||||||||||||||
| Paroxysmal nocturnal haemoglo-binuria | ↑ | CD105+, CD144+ | FCM | Elevated EMP reflected the inflammatorystatus of endothelial cells | [72] | ||||||||||||||
| Pulmonary or venous embolism | ↑ | CD62E+, CD31+/CD42− | FCM | [73] | |||||||||||||||
| Sickle cell disease | ↑ | CD144+ | FCM | EMP levels correlated with crisis | [53] | ||||||||||||||
Abbreviations: TTP, thrombotic thrombocytopenic purpura, SPC, solid phase capture, SBP, systolic blood pressure, DPB, diastolic blood pressure, FMD, flow mediated dilation.
Knowledge accumulated from in vitro and in vivo experiments indicated that EMP provide a storage pool of bioactive effectors able to modulate the vascular homeostasis equation. EMP enriched in both PS and TF trigger monocytic TF-dependent pro-coagulant responses in vitro[78] and thrombus formation in vivo[54, 79]. In addition, EMP amplify endothelial dysfunction by impairing nitric oxide pathways, as shown by alteration to acetyl choline-induced relaxation using the rat aortic ring model [80].
Besides their role as pro-coagulant effectors, recent findings support the concept that MP also behave as vectors of plas-minogenolytic activity due to the expression of the uPA/uPAR system, a mechanism that modulates the angiogenic response of endothelial progenitors in vitro[81]. MP exposes other proteases such as metalloproteinase 2 and 9 that affect vascular remodelling and angiogenesis [82]. Such data have led to the concept that EMP are key factors at the crossroads between inflammation, coagulation, proteolysis and vascular repair.
Endothelial progenitor cells
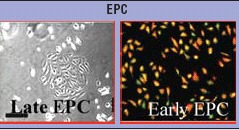
Since the pioneer's work of Asahara [83] 10 years ago, increasing data have extended our knowledge on EPC biology. Two functional criteria are recognized to distinguish between EPC and CEC. In vitro, EPC display the ability to form adherent colonies that proliferate, and differentiate into endothelial lineage as assessed by expression of various specific endothelial antigens including KDR, UEA-1, vWF, CD146 and CD144. In vivo, they contribute to neoan-giogenesis within ischemic sites or tumours or to vascular repair after vessel wall injury [13, 84]. Nevertheless, accumulating data demonstrate that these properties identify a heterogeneous pool of circulating cells related to their origin differentiation and functional characteristics. Within bone marrow, various stem cell populations may differentiate into endothelial progenitors including haemangioblast, a common precursor of haematopoietic stem cells and endothelial cells, multipotent adult progenitor cells or mesenchymal stem cells. Alternative sources of EPC may also include parenchymatous organ and blood vessels [85]. This heterogeneity is also demonstrated by the recognition of two distinct cell populations derived by culturing peripheral blood mononu-clear cells. These two different types are usually named by the generic term of EPC, despite highly differing characteristics. According to their development in culture within 4 to 7 days, a first type is called early outgrowth colonies and exhibits spindle-shaped morphology emanating from a central cluster of cells. These cells can be maintained in culture for 2 to 3 weeks but cannot be expanded by further passaging. They express endothelial markers but also myeloid markers like CD45, and CD14 attesting for a partial endothelial differentiation and a probable monocytic origin. Recently, Yoder et al. confirmed that this cell type displaying macrophage/monocyte characteristics is clonally related to the haematopoietic lineage [86]. Paracrine mechanisms mainly contribute to the capacity of these cells to facilitate new vessel formation in vivo. In addition these cells are not able to differentiate into functional endothelium. Based on these considerations, it has been proposed that these cells should not be considered as a ‘progenitor’ population. The second cell type, called late outgrowth colonies, do not appear in culture until 2 to 3 weeks, exhibits a ‘classic endothelial’ phenotype and an exponential growth. Late EPC appear far more capable of in vitro and in vivo morphogenesis into capillary structures. On this basis, the progenitor cells that give rise to this endothelial progeny may best refer to EPC. Their nature remains to be precisely defined although it has been proposed that such cells derive from a non-haematopoietic progenitor expressing CD34 [86, 87].
Despite the lack of consensual definition and phenotypic characterization, EPC detection has been intensively developed to appreciate cardiovascular risk, based on various methodologies. Consistent with the original description of Asahara, enumeration of putative EPC in clinical situation is most often based on flow cytometric analysis of circulating mononuclear cells expressing CD34 as immaturity marker, and KDR as marker of endothelial lineage, although this phenotype may overlap in part with that of mature endothelial cells [88, 89]. Association of CD133 to the previous markers, while strengthening the stemness phenotype, yields lower cell count hardly detectable in steady state conditions. In addition, it has been suggested that CD133+ cells include haematopoietic rather than endothelial progenitors. EPC can also be evaluated using ex vivo culture assays based on their clono-genic potential. A variety of culture protocols have been reported and referred as colony forming unit-endothelial cells including the commercially available Endocult™. However, most of such tests evaluate early EPC that are increasingly considered as manifestation of plasticity of the monocyte/macrophage lineage, prominently related to inflammation rather than endothelial biology. By contrast, quantification of late EPC reflecting probable ‘true angioblasts’ requires prolonged culture protocols, large volume of peripheral blood and is hardly used in large clinical studies [90]. These approaches are not correlated and may contribute to discrepant results [91]. So despite extensive research activities there is no accepted standard method to quantitate EPC. Such standardization may progress from an exact and consensual definition of EPC, including discrimination of cells with functionally heterogeneous pro-angiogenic activities.
Important insights from experimental models were to identify factors that regulate EPC-mediated endothelial repair [92]. EPC release from bone marrow, homing and recruitment are triggered by increased availability of angiogenic growth factors like VEFG or SDF1 produced by hypoxic areas or in response to vessel damage. Consistently, although present in the peripheral blood of healthy individuals at very low levels, this population can dramatically increase at times of acute pathophysiological stress such as myocardial infarction [93] or vascular injury (Table 4, panel A). Mobilization of EPC is also associated with atheroprotective strategies including regular physical training, and pharmacological substances like statins, estrogens or erythropoietin [115]. By contrast, classical CRF such as diabetes, age or uraemia negatively affect EPC.
4.
Clinical studies illustrating changes in blood levels of EPC in cardiovascular situations
| Disease/condition | Methods for EPC detection | Main finding(s) | Ref | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FCM | Culture assay | ||||||||||||
| Panel A : Disease/condition associated with increased EPC levels | |||||||||||||
| Acute ischemia | |||||||||||||
| Myocardial infarction | CD34+/CD117+, CD34+/KDR+ | EPC correlated plasma VEGF levels and CPK values | [94] | ||||||||||
| CD133+/KDR+ | + | EPC correlated with collateral vessel formation | [95] | ||||||||||
| CD34+/CD133+/KDR+ | + | EPC differentiation is associated with myocardial salvage | [96] | ||||||||||
| CD34+ | EPC mobilization correlated with favourable post-AMI remodelling | [97] | |||||||||||
| Unstable angina | + | Correlation with serum CRP levels | [98] | ||||||||||
| Stroke | + | EPC increment in the first week is associated with good outcome | [99] | ||||||||||
| CD31+/CD34+, CD34+/KDR+ | EPC mobilization predicted improvement of neurological outcome | [100] | |||||||||||
| Liver transplantation | CD34+/CD133+, CD34+/KDR+ | Host derived cells, associated with VEGF, SCF, G-CSF increase | [101] | ||||||||||
| Coroanary artery bypass grafting | + | EPC mobilization correlated with G-CSF levels | [102] | ||||||||||
| CD34+/CD133+ | EPC increase is associated with cytochemokine release and showed a negative age dependency | [103] | |||||||||||
| Vascular injury | |||||||||||||
| Coronary stent implantation | CD34+ | + | EPC increase is more marked in patients developing restenosis | [104] | |||||||||
| CD34+/CD45low | + | EPC mobilization inversely correlated with vascular injury assessed by CEC count | [105] | ||||||||||
| Drugs/Life style modifications | |||||||||||||
| Statins therapy | CD34+/CD133+/KDR+ | + | EPC increase in independent of VEGF | [106] | |||||||||
| CD45−/KDR+ | + (early and late EPC) | EPC increase is associated with enhanced EPC function in relation to IL-8 production by monocytes | [107] | ||||||||||
| + | In patients with chronic heart failure, EPC increase is associated with improvement of endothelial function (FMD) | [108] | |||||||||||
| CD34+/KDR+ | + (late EPC) | Long-term treatment in CAD patients | [109] | ||||||||||
| Erythropoletin | CD34+ | + | Standard EPO dose in patients with renal anaemia, increased EPC number is associated with increased EPC survival and function | [110] | |||||||||
| CD34+/CD45− | + | One bolus of EPO in patients with acute myocardial infarction | [111] | ||||||||||
| Physical training | CD34+/CD133+/KDR+ | EPC increase correlated with FMD change | [112] | ||||||||||
| + | EPC increase is associated with intensified school sports in children | [113] | |||||||||||
| CD34+/KDR+ | + | EPC increase is associated with reduced EPC apoptosis in CAD patients | [114] | ||||||||||
| Panel B: Disease/condition associated with decreased EPC levels | |||||||||||||
| Cardiovascular diseases | |||||||||||||
| Stable CAD | CD34+/KDR+ | EPC number inversely correlates with the presence of CVRF | [117] | ||||||||||
| CD34+/KDR+ | Low levels of EPC independently predict poor prognosis | [118] | |||||||||||
| CD133+/KDR+ | + | EPC number and migratory activity inversely correlate with the severity of coronary stenosis and CRP | [119] | ||||||||||
| Cerebrovascular disease | CD34+, CD133+ | EPC level correlates with regional blood flow | [120] | ||||||||||
| Pulmonary hypertension | CD133+/KDR+ | + | EPC reduction correlates with IL-6, vWF and BNP levels and is associated with impaired migration and adhesion to fibronectin | [121] | |||||||||
| CD34+/C133+/KDR+ CD34+, CD34+/KDR+, CD34+/CD133+ | EPC reduction is associated with raised inflammatory markers and meliorated by phosphodiesterase inhibitor Sildenafil | [122] | |||||||||||
| Heart failure | CD34+ | EPC inversely correlate with disease severity | [123] | ||||||||||
| CD34+ CD133+/KDR+ | + | EPC reduction is associated with functional exhaustion of EPC within bone marrow | [124] | ||||||||||
| + | EPC reduction is associated with the advances phases of the disease | [125] | |||||||||||
| In-stent restenos | + | Low EPC number and function is associated with diffuse restenosis | [126] | ||||||||||
| + | Increased EPC senescence is associated with in-stent restenosis | [127] | |||||||||||
| Cardiovascular risk factors | |||||||||||||
| Type 2 Diabetes | CD34+/CD117+, | EPC number negatively correlated with disease severity, and individually predict microvascular complications, | [128] | ||||||||||
| CD34+/CD133+/KDR | + | EPC reduction is associated with EPC dysfunction involving eNOS | [129] | ||||||||||
| + | EPC number is related to HbA1c levels in untreated patients and increased by pharmacological glycemic control | [130] | |||||||||||
| CD34+/KDR+ | EPC reduction is more marked in patients with peripheral vascular disease, EPC number correlates with ABI | [131] | |||||||||||
| Type 1 Diabetes | + | Low EPC number is associated with EPC dysfunction | [132] | ||||||||||
| Hypertension | CD34+/KDR+ | EPC inversely correlates with systolic blood pressure | [133] | ||||||||||
| CD34+/CD133+/CD45 | Refractory hypertension independantly determines EPC number | [134] | |||||||||||
| End stage renal disease | CD34+/KDR+ | + | EPC reduction and altered function are related to serum fetuin A levels, haematocrite and reticulocytes | [135] | |||||||||
| CD34+/CD144+ | + | EPC number and migration inversely correlates with uraemia and systolic blood pressure and is restored by nocturnal haemodialysis | [136] | ||||||||||
| CD34+/KDR+ | + | Uremic serum impaired normal EPC outgrown in vitro | [137] | ||||||||||
| CD34+/CD45+ | + | EPC number correlated with renal function and is normalized after renal transplantation | [138] | ||||||||||
| + | EPC Inversely correlates with framingham score and dose of dialysis | [139] | |||||||||||
| Dyslipidemia | + | EPC number and impaired functionality Inversely correlated with total and LDL cholesterol | [140] | ||||||||||
| CD34+/KDR+ | EMP/EPC Ratio correlates with LDL cholesterol and arterial stiffness | [141] | |||||||||||
| Smoking | + | Impaired EPC function related to increased oxidative stress | [142] | ||||||||||
| CD34+/CD133+/KDR+ | + | EPC levels increased after 2 week smoking cessation | [143] | ||||||||||
| aging | CD34+ CD34+/KDR+ | + | EPC reduction correlated with decreased arterial elasticity | [144] | |||||||||
| Increased EPC levels is childhood, inverse relation with age in healthy individuals | [145] | ||||||||||||
| Hyperhomocysteinemia | CD133+/KDR+ | + | EPC levels inversely correlated with Homocystein levels | [146] | |||||||||
Abbreviations : CAD, coronary artery disease, FMD, flow mediated dilatation, EPO, erythropïetin, CPK, creatine phosphokinase, AMI, acute myocar-dial infarction, CRP, C reactive protein, IL-8 : interleukin-8, VEGF, vascular endothelial growth factor, SCF, stem cell factor, G-SCF, granulocyte-stem cell factor, IL-6: interleukin-6, vWF, von Willebrant factor, BNP, type B natriuretic peptide; eNOs, endothelial Nitric oxide synthase, ABI, ankle brachial index, LDL, low-density lipoprotein.
The impact of CRF in the reduction of EPC availability is still poorly defined. However, several mechanisms have been put forward such as (i) exhaustion of the pool of stem/progenitor cells in the bone marrow, (ii) defective mobilization due to altered stem cell niche involving reduced nitric oxide bioavailabilty, (iii) impaired homing caused by reduction of chemoattractive factor synthesis or disturbance of their receptor-mediated signalling pathways and (iv) increased apoptosis and/or deregulated differentiation. This latter effect may involve intrinsic EPC defect or change in the local environment that determines cell fate either towards endothelial lineage or towards inflammatory cells or smooth muscle cells promoting atherosclerosis [116].
Early clinical studies, summarized in Table 4 (panel B), have reported reduced EPC levels and/or function in patients with chronic cardiovascular diseases, such as stable coronary artery disease, congestive heart failure, cerebrovascular disease, transplant vasculopathy or in stent restenosis [147, 148]. EPC defects have also been linked to situations that strengthen cardiovascular risk [149], such as diabetes mellitus [131, 132], hypertension [150], chronic renal failure [139], aging [151] or smoking [143]. The relationship between EPC reduction and CRF has been convincingly established in patients at various stages of atherosclerosis [152–154]. Decreased EPC levels and migratory activity were observed in CAD patients compared to controls and were found to inversely correlate with the number of CRF [117]. Together with significant correlation with the Framingham score reported in sub-clinical atherosclerosis, these data indicate that EPC may constitute surrogate marker of cumulative cardiovascular risk.
A further important step in the understanding of EPC role in cardiovascular disease was the demonstration that altered EPC numbers and functional capacities are major determinants in endothelial dysfunction [155]. In the absence of established atherosclerotic disease, as illustrated by aging or rheumatoid arthritis, EPC activity correlates with endothelial function evaluated by flow-mediated dilatation. Furthermore, in both healthy population and CAD patients, EPC levels have been recently shown to be the strongest predictor of endothelial dysfunction compared to traditional risk factors [156, 157]. Such data emphasize that EPC participate in the ongoing maintenance of endothelial integrity and that insufficient endothelial cell repair by EPC may play a causal role in vascular disease progression. Clinical evidence of this concept was provided by a recent study that investigated EPC-associated prognostic values. In more than 500 patients with CAD, followed for 12 months, the cumulative event-free survival rate increased with baseline levels of EPC, considering both the first major cardiovascular event or death from cardiovascular causes [158]. Thus, EPC-mediated vascular repair appeared as a protective mechanism able to counterbalance the impact of CRF, to modulate the clinical course of atherosclerosis and finally to determine cardiovascular outcome.
Endothelial lesion versus regeneration: towards the definition of ‘vascular competence’
Current evidence suggests that regenerative versus degenerative endothelial responses can be integrated in a clinical endothelial phe-notype, reflecting the net result between damage from risk factors and endogenous repair capacity. This endothelial phenotype characterizes a vascular status that could define the ‘vascular competence’ of each individual. Because CEC, EMP and EPC are key players of the endothelial homeostatic balance, their combined measurement offers a non-invasive and original way to estimate vascular competence at the individual level (Fig. 2). The interest in such multimarker strategies to identify patients with high vascular risk and their response to treatment has emerged from the recent literature.
2.
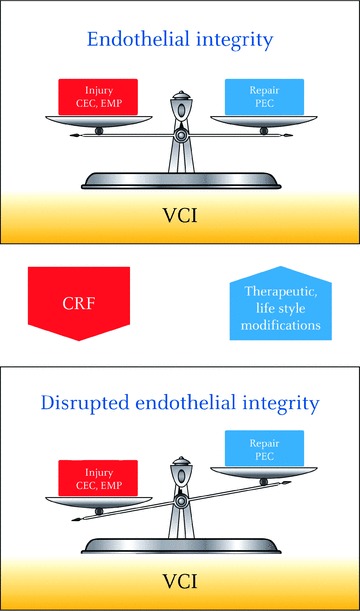
Endothelial integrity is viewed as a balance between endothelial injury reflected by CEC and EMP, and endogenous capacity for repair attested by EPC. The net result of this equilibrium can be integrated into a clinical endothelial phenotype defining vascular competence. Multimarker strategies combining CEC, EMP and EPC to define Vascular Competence Index are promising to evaluate at the individual level the impact of cardiovascular risk factors on disease progression. Such mul-timarker approach may also provide relevant tools for delineation and monitoring of therapeutic strategies or life style modifications aimed to improve endothelial function by limiting damage and/or reinforce regenerative mechanisms. CEC: circulating endothelial cells, EMP: endothelial microparticles, EPC: endothelial progenitor cells, VCI: vascular competence index.
If baseline levels of CEC, EMP and EPC represent the physiological equilibrium of endothelium in healthy situations, any deviation of this balance presumably indicates the existence of a deleterious process affecting endothelium integrity. Both increase in CEC and EMP may correlate with the extent of vascular injury both in acute or chronic endothelial suffering contexts. In addition, phenotypic analysis of these markers may provide insights into disease mechanism or aetiology. Indeed, EMP surface markers have been shown to depend on their production mode, CD62E+ EMP being mainly released during pro-inflammatory activation whereas CD144+ EMP is generated during apoptosis or structural damage [50, 65]. However, cautions are needed because these notions only rely on in vitro observations and have not been validated yet in vivo. Similarly, phenotype of elevated CEC gives clues on the pathogenic causes of detachment. CEC express inducible cell adhesion molecules in inflammatory pathologies associated with cytokine-induced detachment as documented in sickle cell anaemia models [159], or demonstrated apoptosis or necrosis of the endothelium as reported in lupus patients (Table 2). As a direct response to acute endothelial injury, like ischemia reperfusion injury, or pro-inflammatory stimulation, elevation of EMP and CEC is associated with increased number of circulating EPC (Table 4, panel A). Mobilization of functionally competent EPC indicates the existence of an endogenous compensatory repair mechanism that may efficiently contribute to restoration of endothelial integrity. Consequently a drive towards normalized levels of endothelial bio-markers, once repair has been effected, can be expected. Such endothelial biomarker profile, characterized by transient increase in EMP and EPC, has been recently described in healthy individuals exposed to brief second-hand smoke [160]. Insufficiency of this repair response is consistent with altered vascular competence and would argue for the development of vascular complications. In the first study reporting simultaneous measurement of CEC and EPC in patients with stable coronary artery disease who underwent percutaneous coronary intervention [105], both increased CEC and EPC mobilizations were observed after the procedure. Surprisingly, the extent of EPC mobilization was inversely related to the extent of injury reflected by CEC, suggesting that marked endothelial damage associated with poor endothelial repair could be useful to identify patients at risk for post-procedural complications.
By contrast to acute conditions, chronic exposure to CRF is associated with both endothelial-damaging process and depletion of cells efficient for repair. In that case, increased levels of EMP and/or CEC associated with reduced EPC levels or impaired EPC activity demonstrate the lack of compensatory responses that importantly contribute to the development and progression of atherosclerosis. Therefore, vascular index combining measurement of EPC and injury markers may offer better appreciation of vascular risk by evaluating both pathological processes involved in endothe-lial dysfunction as recently illustrated in patients with CAD patients with ischemic left ventricular dysfunction [161]. Also, calculation of EMP to EPC ratio has been proposed as a possible index reflecting the imbalance between endothelial damage and repair capacity. In comparison to normal persons, this ratio increased in hypercholes-terolaemic patients, as a result of both increased EMP and decreased EPC. Moreover, this ratio was highly correlated with low-density lipoprotein (LDL) cholesterol and pulse wave velocity, a measure of aortic stiffness [141]. Altogether, these data suggest that both increased endothelial injury and impaired repair may contribute to reduce aortic distensibility.
Despite great promises, integration of multimarker strategies based on CEC, EMP and EPC in the clinical practice is still in its infancy. Basically, a better knowledge of endothelial damage and repair processes is expected to define more precisely the relationship between CEC, EMP and EPC in physiological or pathological situations. Other limitations rely on the poor standardization of assays. Obviously, methodological issues and confusion delivered by the various definitions currently in use remain a major cause of apparent conflicting results notably regarding EPC. In addition, their physiological variations according to parameters such as age, gender or circadian rhythms has to be defined in large cohorts of healthy people. Standardization of methodologies available is therefore a crucial step for the full definition of the clinical significance of these markers. This standardization is one of the goals of the Scientific and Standardization Committee (SSC) on vascular biology of the International Society for Thrombosis and Haemostasis (ISTH).
Conclusion
The ability to explore the endothelium non-invasively has put in light a novel pathogenic understanding of CVD, defined by an imbalance between endothelial damage and repair capacity. In the future, multimarker approaches combining CEC, EMP and EPC measurement will be helpful as integrative markers of ‘vascular competence’. In clinical testing strategies, these cellular markers will certainly provide novel insights to detect endothelial dysfunction at early pre-clinical stages, to assess vascular risk in the later stages of atherothrombotic disorders and to delineate therapeutic options. Indeed, therapeutic strategies that are efficient to improve endothelial function could target the selective reduction of endothelial injury or the promotion of regenerative mechanisms. At the individual level, such therapeutic option may integrate, not only the predominant mechanism, but also the genetic predisposition and environmental risk factors. Important insights into disease monitoring and potential new therapeutic options will certainly emerge from this rapid developing field.
References
- 1.Landmesser U, Drexler H. Endothelial function and hypertension. Curr Opin Cardiol. 2007;22:316–20. doi: 10.1097/HCO.0b013e3281ca710d. [DOI] [PubMed] [Google Scholar]
- 2.Goligorsky MS. Clinical assessment of endothelial dysfunction: combine and rule. Curr Opin Nephrol Hypertens. 2006;15:617–24. doi: 10.1097/01.mnh.0000247497.62505.72. [DOI] [PubMed] [Google Scholar]
- 3.Boos CJ, Lip GY, Blann AD. Circulating endothelial cells in cardiovascular disease. J Am Coll Cardiol. 2006;48:1538–47. doi: 10.1016/j.jacc.2006.02.078. [DOI] [PubMed] [Google Scholar]
- 4.Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21:157–71. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Lynch SF, Ludlam CA. Plasma microparti-cles and vascular disorders. Br J Haematol. 2007;137:36–48. doi: 10.1111/j.1365-2141.2007.06514.x. [DOI] [PubMed] [Google Scholar]
- 6.Morel O, Toti F, Hugel B, Bakouboula B, Camoin-Jau L, Dignat-George F, Freyssinet JM. Procoagulant microparti-cles: disrupting the vascular homeostasis equation? Arterioscler Thromb Vasc Biol. 2006;26:2594–604. doi: 10.1161/01.ATV.0000246775.14471.26. [DOI] [PubMed] [Google Scholar]
- 7.Reed MJ, Karres N, Eyman D, Edelberg J. Endothelial precursor cells. Stem Cell Rev. 2007;3:218–25. doi: 10.1007/s12015-007-0007-5. [DOI] [PubMed] [Google Scholar]
- 8.Kawamoto A, Asahara T. Role of progenitor endothelial cells in cardiovascular disease and upcoming therapies. Catheter Cardiovasc Interv. 2007;70:477–84. doi: 10.1002/ccd.21292. [DOI] [PubMed] [Google Scholar]
- 9.Gimbrone MA, Jr, Nagel T, Topper JN. Biomechanical activation: an emerging paradigm in endothelial adhesion biology. J Clin Invest. 1997;100:S61–5. [PubMed] [Google Scholar]
- 10.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 11.Combes V, Simon AC, Grau GE, Arnoux D, Camoin L, Sabatier F, Mutin M, Sanmarco M, Sampol J, Dignat-George F. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest. 1999;104:93–102. doi: 10.1172/JCI4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George F, Brisson C, Poncelet P, Laurent JC, Massot O, Arnoux D, Ambrosi P, Klein-Soyer C, Cazenave JP, Sampol J. Rapid isolation of human endothelial cells from whole blood using S-Endo1 monoclonal antibody coupled to immuno-mag-netic beads: demonstration of endothelial injury after angioplasty. Thromb Haemost. 1992;67:147–53. [PubMed] [Google Scholar]
- 13.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 14.Murphy C, Kanaganayagam GS, Jiang B, Chowienczyk PJ, Zbinden R, Saha M, Rahman S, Shah AM, Marber MS, Kearney MT. Vascular dysfunction and reduced circulating endothelial progenitor cells in young healthy UK South Asian men. Arterioscler Thromb Vasc Biol. 2007;27:936–42. doi: 10.1161/01.ATV.0000258788.11372.d0. [DOI] [PubMed] [Google Scholar]
- 15.Blann AD, Woywodt A, Bertolini F, Bull TM, Buyon JP, Clancy RM, Haubitz M, Hebbel RP, Lip GY, Mancuso P. Circulating endothelial cells. Biomarker of vascular disease. Thromb Haemost. 2005;93:228–35. doi: 10.1160/TH04-09-0578. [DOI] [PubMed] [Google Scholar]
- 16.George F, Poncelet P, Laurent JC, Massot O, Arnoux D, Lequeux N, Ambrosi P, Chicheportiche C, Sampol J. Cytofluorometric detection of human endothelial cells in whole blood using S-Endo 1 monoclonal antibody. J Immunol Methods. 1991;139:65–75. doi: 10.1016/0022-1759(91)90352-g. [DOI] [PubMed] [Google Scholar]
- 17.Woywodt A, Blann AD, Kirsch T, Erdbruegger U, Banzet N, Haubitz M, Dignat-George F. Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: proposal of a definition and a consensus protocol. J Thromb Haemost. 2006;4:671–7. doi: 10.1111/j.1538-7836.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- 18.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothe-lial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6:835–45. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 19.Duda DG, Cohen KS, Scadden DT, Jain RK. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat Protoc. 2007;2:805–10. doi: 10.1038/nprot.2007.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowand JL, Martin G, Doyle GV, Miller MC, Pierce MS, Connelly MC, Rao C, Terstappen LW. Endothelial cells in peripheral blood of healthy subjects and patients with metastatic carcinomas. Cytometry A. 2007;71:105–13. doi: 10.1002/cyto.a.20364. [DOI] [PubMed] [Google Scholar]
- 21.Widemann A, Sabatier F, Arnaud L, Bonello L, Al-Massarani G, Paganelli F, Poncelet P, Dignat-George F. CD146-based immunomagnetic enrichment followed by multiparameter flow cytometry: a new approach to counting circulating endothelial cells. J Thromb Haemost. 2008;6:869–76. doi: 10.1111/j.1538-7836.2008.02931.x. [DOI] [PubMed] [Google Scholar]
- 22.Erdbruegger U, Haubitz M, Woywodt A. Circulating endothelial cells: a novel marker of endothelial damage. Clin Chim Acta. 2006;373:17–26. doi: 10.1016/j.cca.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Michel JB. Anoikis in the cardiovascular system : Known and unknown extracellular mediators. Arterioscl Vasc Biol. 2003;23:2146–54. doi: 10.1161/01.ATV.0000099882.52647.E4. [DOI] [PubMed] [Google Scholar]
- 24.Chi JT, Chang H, Haraldsen G, Jahnsen F, Troyanskaya O, Chang D, Wang Z, Rockson S, Van de Rijn M, Botsein D, Brown P. Endothelial cell diversity revealed by global expression profiling. PNAS. 2003;100:10623–8. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aird W. Phenotypic heterogeneity of the endothelium. Representative vascular beds. Circ Res. 2007;100:174–90. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 26.Mutin M, Canavy I, Blann A, Bory M, Sampol J, Dignat-George F. Direct evidence of endothelial injury in acute myocardial infarction and unstable angina by demonstration of circulating endothelial cells. Blood. 1999;93:2951–8. [PubMed] [Google Scholar]
- 27.Quilici J, Banzet N, Paule P, Meynard JB, Mutin M, Bonnet JL, Ambrosi P, Sampol J, Dignat-George F. Circulating endothelial cell count as a diagnostic marker for non-ST-elevation acute coronary syndromes. Circulation. 2004;110:1586–91. doi: 10.1161/01.CIR.0000142295.85740.98. [DOI] [PubMed] [Google Scholar]
- 28.Lee KW, Blann AD, Lip GY. Plasma markers of endothelial damage/dysfunction, inflammation and thrombogenesis in relation to TIMI risk stratification in acute coronary syndromes. Thromb Haemost. 2005;94:1077–83. doi: 10.1160/TH05-03-0179. [DOI] [PubMed] [Google Scholar]
- 29.Makin AJ, Blann AD, Chung NA, Silverman SH, Lip GY. Assessment of endothelial damage in atherosclerotic vascular disease by quantification of circulating endothelial cells. Relationship with von Willebrand factor and tissue factor. Eur Heart J. 2004;25:371–6. doi: 10.1016/j.ehj.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 30.McClung JA, Naseer N, Saleem M, Rossi GP, Weiss MB, Abraham NG, Kappas A. Circulating endothelial cells are elevated in patients with type 2 diabetes mellitus independently of HbA(1)c. Diabetologia. 2005;48:345–50. doi: 10.1007/s00125-004-1647-5. [DOI] [PubMed] [Google Scholar]
- 31.Erdbruegger U, Woywodt A, Kirsch T, Haller H, Haubitz M. Circulating andothe-lial cells as a prognostic marker in throm-botic microangiopathy. Am J Kidney Dis. 2006;48:564–70. doi: 10.1053/j.ajkd.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Nadar SK, Lip GY, Lee KW, Blann AD. Circulating endothelial cells in acute ischaemic stroke. Thromb Haemost. 2005;94:707–12. doi: 10.1160/TH04-12-0795. [DOI] [PubMed] [Google Scholar]
- 33.Bull TM, Golpon H, Hebbel RP, Solovey A, Cool CD, Tuder RM, Geraci MW, Voelkel NF. Circulating endothelial cells in pulmonary hypertension. Thromb Haemost. 2003;90:698–703. doi: 10.1160/TH03-04-0251. [DOI] [PubMed] [Google Scholar]
- 34.Solovey A, Lin Y, Browne P, Choong S, Wayner E, Hebbel RP. Circulating activated endothelial cells in sickle cell anemia. N Engl J Med. 1997;337:1584–90. doi: 10.1056/NEJM199711273372203. [DOI] [PubMed] [Google Scholar]
- 35.Koc M, Richards HB, Bihorac A, Ross EA, Schold JD, Segal MS. Circulating endothelial cells are associated with future vascular events in hemodialysis patients. Kidney Int. 2005;67:1078–83. doi: 10.1111/j.1523-1755.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 36.Woywodt A, Schroeder M, Gwinner W, Mengel M, Jaeger M, Schwarz A, Haller H, Haubitz M. Elevated numbers of circulating endothelial cells in renal transplant recipients. Transplantation. 2003;76:1–4. doi: 10.1097/01.TP.0000074569.65127.26. [DOI] [PubMed] [Google Scholar]
- 37.Al-Massarani H, Vacher-Coponat A, Widemann L, Arnaud P, Paula A, Loundou Robert S, Berland Y, Dignat-George F, Camoin-Jau L. Impact of immunosuppressive treatment on endothelial biomarkers after kidney transplantation. Am J Transplantation. 2008;8:2360–7. doi: 10.1111/j.1600-6143.2008.02399.x. [DOI] [PubMed] [Google Scholar]
- 38.Woywodt A, Scheer J, Hambach L, Buchholz S, Ganser A, Haller H, Hertenstein B, Haubitz M. Circulating endothelial cells as a marker of endothelial damage in allogeneic hematopoietic stem cell transplantation. Blood. 2004;103:3603–5. doi: 10.1182/blood-2003-10-3479. [DOI] [PubMed] [Google Scholar]
- 39.Del Papa N, Colombo G, Fracchiolla N, Moronetti LM, Ingegnoli F, Maglione W, Comina DP, Vitali C, Fantini F, Cortelezzi A. Circulating endothelial cells as a marker of ongoing vascular disease in systemic sclerosis. Arthritis Rheum. 2004;50:1296–304. doi: 10.1002/art.20116. [DOI] [PubMed] [Google Scholar]
- 40.Woywodt A, Streiber F, De Groot K, Regelsberger H, Haller H, Haubitz M. Circulating endothelial cells as markers for ANCA-associated small-vessel vasculitis. Lancet. 2003;361:206–10. doi: 10.1016/S0140-6736(03)12269-6. [DOI] [PubMed] [Google Scholar]
- 41.Rajagopalan S, Somers EC, Brook RD, Kehrer C, Pfenninger D, Lewis E, Chakrabarti A, Richardson BC, Shelden E, McCune WJ, Kaplan MJ. Endothelial cell apoptosis in systemic lupus erythe-matosus: a common pathway for abnormal vascular function and thrombosis propensity. Blood. 2004;103:3677–83. doi: 10.1182/blood-2003-09-3198. [DOI] [PubMed] [Google Scholar]
- 42.Clancy R, Marder G, Martin V, Belmont HM, Abramson SB, Buyon J. Circulating activated endothelial cells in systemic lupus erythematosus: further evidence for diffuse vasculopathy. Arthritis Rheum. 2001;44:1203–8. doi: 10.1002/1529-0131(200105)44:5<1203::AID-ANR204>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 43.Dignat-George F, Sampol J, Lip G, Blann AD. Circulating endothelial cells: realities and promises in vascular disorders. Pathophysiol Haemost Thromb. 2003;33:495–9. doi: 10.1159/000083851. [DOI] [PubMed] [Google Scholar]
- 44.Boos CJ, Lip GY, Blann AD. Circulating endothelial cells in cardiovascular disease. J Am Coll Cardiol. 2006;48:1538–47. doi: 10.1016/j.jacc.2006.02.078. [DOI] [PubMed] [Google Scholar]
- 45.Woywodt A. Circulating endothelial cells in vasculitis and transplantation. Pathophysiol Haemost Thromb. 2003;33:500–2. doi: 10.1159/000083852. [DOI] [PubMed] [Google Scholar]
- 46.Clancy RM. Circulating endothelial cells and vascular injury in systemic lupus ery-thematosus. Curr Rheumatol Rep. 2000;2:39–43. doi: 10.1007/s11926-996-0067-6. [DOI] [PubMed] [Google Scholar]
- 47.Chong AY, Blann AD, Patel J, Freestone B, Hughes E, Lip GY. Endothelial dysfunc tion and damage in congestive heart fail ure: relation of flow-mediated dilation to circulating endothelial cells, plasma indexes of endothelial damage, and brain natriuretic peptide. Circulation. 2004;110:1794–8. doi: 10.1161/01.CIR.0000143073.60937.50. [DOI] [PubMed] [Google Scholar]
- 48.Kirsch T, Woywodt A, Beese M, Wyss K, Park JK, Erdbruegger U, Hertel B, Haller H, Haubitz M. Engulfment of apoptotic cells by microvascular endothelial cells induces proinflammatory responses. Blood. 2007;109:2854–62. doi: 10.1182/blood-2006-06-026187. [DOI] [PubMed] [Google Scholar]
- 49.Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, Koopmeiners L, Key NS, Hebbel RP. Sickle blood contains tissue factor–positive microparticles derived from endothelial cells and mono-cytes. Blood. 2003;102:2678–83. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 50.Jimenez JJ, Jy W, Mauro LM, Soderland C, Horstman LL, Ahn YS. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb Res. 2003;109:175–80. doi: 10.1016/s0049-3848(03)00064-1. [DOI] [PubMed] [Google Scholar]
- 51.Falati S, Liu Q, Gross P, Merrill-Skoloff G, Chou J, Vandendries E, Celi A, Croce K, Furie BC, Furie B. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197:1585–98. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aras O, Shet A, Bach RR, Hysjulien JL, Slungaard A, Hebbel RP, Escolar G, Jilma B, Key NS. Induction of microparti-cle- and cell-associated intravascular tissue factor in human endotoxemia. Blood. 2004;103:4545–53. doi: 10.1182/blood-2003-03-0713. [DOI] [PubMed] [Google Scholar]
- 53.Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, Koopmeiners L, Key NS, Hebbel RP. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–83. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 54.Mallat Z, Hugel B, Ohan J, Leseche G, Freyssinet JM, Tedgui A. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation. 1999;99:348–53. doi: 10.1161/01.cir.99.3.348. [DOI] [PubMed] [Google Scholar]
- 55.Mallat Z, Benamer H, Hugel B, Benessiano J, Steg PG, Freyssinet JM, Tedgui A. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–3. doi: 10.1161/01.cir.101.8.841. [DOI] [PubMed] [Google Scholar]
- 56.Perez-Casal M, Downey C, Fukudome K, Marx G, Toh CH. Activated protein C induces the release of microparticle-asso-ciated endothelial protein C receptor. Blood. 2005;105:1515–22. doi: 10.1182/blood-2004-05-1896. [DOI] [PubMed] [Google Scholar]
- 57.Freyssinet JM, Dignat-George F. More on: Measuring circulating cell-derived microparticles. J Thromb Haemost. 2005;3:613–4. doi: 10.1111/j.1538-7836.2005.01169.x. [DOI] [PubMed] [Google Scholar]
- 58.Jy W, Horstman LL, Jimenez JJ, Ahn YS, Biró E, Nieuwland R, Sturk A, Dignat-George F, Sabatier F, Camoin-Jau L, Sampol J, Hugel B, Zobairi F, Freyssinet JM, Nomura S, Shet AS, Key NS, Hebbel RP. Measuring circulating cell-derived microparticles. J Thromb Haemost. 2004;2:1842–51. doi: 10.1111/j.1538-7836.2004.00936.x. [DOI] [PubMed] [Google Scholar]
- 59.Sapet C, Simoncini S, Loriod B, Puthier D, Sampol J, Nguyen C, Dignat-George F, Anfosso F. Thrombin-induced endothelial microparticle generation: identification of a novel pathway involving ROCK-II activation by caspase-2. Blood. 2006;108:1868–76. doi: 10.1182/blood-2006-04-014175. [DOI] [PubMed] [Google Scholar]
- 60.Pirro M, Schillaci G, Bagaglia F, Menecali C, Paltriccia R, Mannarino MR, Capanni M, Velardi A, Mannarino E. Microparticles derived from endothelial progenitor cells in patients at different cardiovascular risk. Atherosclerosis. 2008;197:757–67. doi: 10.1016/j.atherosclerosis.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 61.Bernal-Mizrachi L, Jy W, Jimenez JJ, Pastor J, Mauro LM, Horstman LL, De Marchena E, Ahn YS. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am Heart J. 2003;145:962–70. doi: 10.1016/S0002-8703(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 62.Simak J, Gelderman MP, Yu H, Wright V, Baird AE. Circulating endothelial micropar-ticles in acute ischemic stroke: a link to severity, lesion volume and outcome. J Thromb Haemost. 2006;4:1296–302. doi: 10.1111/j.1538-7836.2006.01911.x. [DOI] [PubMed] [Google Scholar]
- 63.Preston RA, Jy W, Jimenez JJ, Mauro LM, Horstman LL, Valle M, Aime G, Ahn YS. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension. 2003;41:211–7. doi: 10.1161/01.hyp.0000049760.15764.2d. [DOI] [PubMed] [Google Scholar]
- 64.Amabile N, Heiss C, Real WM, Minasi P, McGlothlin D, Rame EJ, Grossman W, De Marco T, Yeghiazarians Y. Circulating endothelial microparticle levels predict hemodynamic severity of pulmonary hypertension. Am J Respir Crit Care Med. 2008;177:1268–75. doi: 10.1164/rccm.200710-1458OC. [DOI] [PubMed] [Google Scholar]
- 65.Koga H, Sugiyama S, Kugiyama K, Watanabe K, Fukushima H, Tanaka T, Sakamoto T, Yoshimura M, Jinnouchi H, Ogawa H. Elevated levels of VE-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2005;45:1622–30. doi: 10.1016/j.jacc.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 66.Sabatier F, Darmon P, Hugel B, Combes V, Sanmarco M, Velut JG, Arnoux D, Charpiot P, Freyssinet JM, Oliver C, Sampol J, Dignat-George F. Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes. 2002;51:2840–5. doi: 10.2337/diabetes.51.9.2840. [DOI] [PubMed] [Google Scholar]
- 67.Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, London GM, Tedgui A, Boulanger CM. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005;16:3381–8. doi: 10.1681/ASN.2005050535. [DOI] [PubMed] [Google Scholar]
- 68.Dignat-George F, Camoin-Jau L, Sabatier F, Arnoux D, Anfosso F, Bardin N, Veit V, Combes V, Gentile S, Moal V, Sanmarco M, Sampol J. Endothelial microparticles: a potential contribution to the thrombotic complications of the antiphospholipid syndrome. Thromb Haemost. 2004;91:667–73. doi: 10.1160/TH03-07-0487. [DOI] [PubMed] [Google Scholar]
- 69.Esposito K, Ciotola M, Schisano B, Gualdiero R, Sardelli L, Misso L, Gianetti G, Giuliano D. Endothelial microparticles correlated with endothelial dysfunction in obese women. J Clin Endocrinol Metab. 2006;91:3676–9. doi: 10.1210/jc.2006-0851. [DOI] [PubMed] [Google Scholar]
- 70.Ferreira AC, Peter AA, Mendez AJ, Jimenez JJ, Mauro LM, Chirinos JA, Ghany R, Virani S, Garcia S, Horstman LL, Purow J, Jy W, Ahn YS, De Marchena E. Postprandial Hypertriglyceridemia increases circulating levels of endothelial cell microparticles. Circulation. 2004;110:3599–603. doi: 10.1161/01.CIR.0000148820.55611.6B. [DOI] [PubMed] [Google Scholar]
- 71.Jimenez JJ, Jy W, Mauro LM, Horstman LL, Soderland C, Ahn YS. Endothelial microparticles released in thrombotic thrombocytopenic purpura express von Willebrand factor and markers of endothe-lial activation. Br J Haematol. 2003;123:896–902. doi: 10.1046/j.1365-2141.2003.04716.x. [DOI] [PubMed] [Google Scholar]
- 72.Simak J, Holada K, Risitano AM, Zivny JH, Young NS, Vostal JG. Elevated circulating endothelial membrane micropar-ticles in paroxysmal nocturnal haemoglo-binuria. Br J Haematol. 2004;125:804–13. doi: 10.1111/j.1365-2141.2004.04974.x. [DOI] [PubMed] [Google Scholar]
- 73.Chirinos JA, Heresi GA, Velasquez H, Jy W, Jimenez JJ, Ahn E, Horstman LL, Soriano AO, Zambrano JP, Ahn YS. Elevation of endothelial microparticles platelets, and leukocyte activation in patients with venous thromboembolism. J Am Coll Cardiol. 2005;45:1467–71. doi: 10.1016/j.jacc.2004.12.075. [DOI] [PubMed] [Google Scholar]
- 74.Boulanger CM, Amabile N, Tedgui A. Circulating microparticles: a potential prognostic marker for atherosclerotic vascular disease. Hypertension. 2006;48:180–6. doi: 10.1161/01.HYP.0000231507.00962.b5. [DOI] [PubMed] [Google Scholar]
- 75.Goichot B, Grunebaum L, Desprez D, Vinzio S, Meyer L, Schlienger JL, Lessard M, Simon C. Circulating procoag-ulant microparticles in obesity. Diabetes Metab. 2006;32:82–5. doi: 10.1016/s1262-3636(07)70251-3. [DOI] [PubMed] [Google Scholar]
- 76.Werner N, Wassmann S, Ahlers P, Kosiol S, Nickenig G. Circulating CD31 +/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:112–6. doi: 10.1161/01.ATV.0000191634.13057.15. [DOI] [PubMed] [Google Scholar]
- 77.Brodsky SV, Zhang F, Nasjletti A, Goligorsky MS. Endothelium-derived microparticles impair endothelial function in vitro. Am J Physiol Heart Circ Physiol. 2004;286:H1910–5. doi: 10.1152/ajpheart.01172.2003. [DOI] [PubMed] [Google Scholar]
- 78.Sabatier F, Roux V, Anfosso F, Camoin L, Sampol J, Dignat-George F. Interaction of endothelial microparticles with monocytic cells in vitro induces tissue factor-dependent procoagulant activity. Blood. 2002;99:3962–70. doi: 10.1182/blood.v99.11.3962. [DOI] [PubMed] [Google Scholar]
- 79.Abid Hussein MN, B”ing AN, Bird E, Hoek FJ, Vogel GM, Meuleman DG, Sturk A, Nieuwland R. Phospholipid composition of in vitro endothelial microparticles and their in vivo thrombogenic properties. Thromb Res. 2008;121:865–71. doi: 10.1016/j.thromres.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 80.Mezentsev A, Merks RM, O'Riordan E, Chen J, Mendelev N, Goligorsky MS, Brodsky SV. Endothelial microparticles affect angiogenesis in vitro: role of oxida-tive stress. Am J Physiol Heart Circ Physiol. 2005;289:H1106–14. doi: 10.1152/ajpheart.00265.2005. [DOI] [PubMed] [Google Scholar]
- 81.Lacroix R, Sabatier F, Mialhe A, Basire A, Pannell R, Borghi H, Robert S, Lamy E, Plawinski L, Camoin-Jau L, Gurewich V, Angles-Cano E, Dignat-George F. Activation of plasminogen into plasmin at the surface of endothelial microparticles: a mechanism that modulates angiogenic properties of endothelial progenitor cells in vitro. Blood. 2007;110:2432–9. doi: 10.1182/blood-2007-02-069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taraboletti G, D'Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol. 2002;160:673–80. doi: 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Asahara T, Murohara T, Sullivan A, Silver M, Van Der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 84.Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol. 2004;287:C572–9. doi: 10.1152/ajpcell.00330.2003. [DOI] [PubMed] [Google Scholar]
- 85.Smadja DM, Cornet A, Emmerich J, Aiach M, Gaussem P. Endothelial progenitor cells: characterization, in vitro expansion, and prospects for autologous cell therapy. Cell Biol Toxicol. 2007;23:223–39. doi: 10.1007/s10565-007-0177-6. [DOI] [PubMed] [Google Scholar]
- 86.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–18. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 88.George J, Shmilovich H, Deutsch V, Miller H, Keren G, Roth A. Comparative analysis of methods for assessment of circulating endothelial progenitor cells. Tissue Eng. 2006;12:331–5. doi: 10.1089/ten.2006.12.331. [DOI] [PubMed] [Google Scholar]
- 89.Fadini GP, Baesso I, Albiero M, Sartore S, Agostini C, Avogaro A. Technical notes on endothelial progenitor cells: ways to escape from the knowledge plateau. Atherosclerosis. 2008;197:496–503. doi: 10.1016/j.atherosclerosis.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 90.Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21:1141–9. doi: 10.1038/sj.leu.2404676. [DOI] [PubMed] [Google Scholar]
- 91.Xiao Q, Kiechl S, Patel S, Oberhollenzer F, Weger S, Mayr A, Metzler B, Reindl M, Hu Y, Willeit J, Xu Q. Endothelial progenitor cells, cardiovascular risk factors, cytokine levels and atherosclerosis-results from a large population-based study. PLoS ONE. 2007;10(2):e975. doi: 10.1371/journal.pone.0000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aicher A, Zeiher AM, Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension. 2005;45:321–5. doi: 10.1161/01.HYP.0000154789.28695.ea. [DOI] [PubMed] [Google Scholar]
- 93.Wojakowski W, Tendera M. Mobilization of bone marrow-derived progenitor cells in acute coronary syndromes. Folia Histochem Cytobiol. 2005;43:229–32. [PubMed] [Google Scholar]
- 94.Massa M, Rosti V, Ferrario M, Campanelli R, Ramajoli I, Rosso R, De Ferrari GM, Ferlini M, Goffredo L, Bertoletti A, Klersy C, Pecci A, Moratti R, Tavazzi L. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005;105:199–206. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- 95.Lev EI, Kleiman NS, Birnbaum Y, Harris D, Korbling M, Estrov Z. Circulating endothelial progenitor cells and coronary collaterals in patients with non-ST segment elevation myocardial infarction. J Vasc Res. 2005;42:408–14. doi: 10.1159/000087370. [DOI] [PubMed] [Google Scholar]
- 96.Numaguchi Y, Sone T, Okumura K, Ishii M, Morita Y, Kubota R, Yokouchi K, Imai H, Harada M, Osanai H, Kondo T, Murohara T. The impact of the capability of circulating progenitor cell to differentiate on myocardial salvage in patients with primary acute myocardial infarction. Circulation. 2006;4:I114–9. doi: 10.1161/CIRCULATIONAHA.105.000588. [DOI] [PubMed] [Google Scholar]
- 97.Leone AM, Rutella S, Bonanno G, Abbate A, Rebuzzi AG, Giovannini S, Lombardi M, Galiuto L, Liuzzo G, Andreotti F, Lanza GA, Contemi AM, Leone G, Crea F. Mobilization of bone marrow-derived stem cells after myocardial infarction and left ventricular function. Eur Heart J. 2005;26:1196–204. doi: 10.1093/eurheartj/ehi164. [DOI] [PubMed] [Google Scholar]
- 98.George J, Goldstein E, Abashidze S, Deutsch V, Shmilovich H, Finkelstein A, Herz I, Miller H, Keren G. Circulating endothelial progenitor cells in patients with unstable angina: association with systemic inflammation. Eur Heart J. 2004;25:1003–8. doi: 10.1016/j.ehj.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 99.Sobrino T, Hurtado O, Moro MA, Rodríguez-Yáñez M, Castellanos M, Brea D, Moldes O, Blanco M, Arenillas JF, Leira R, Dávalos A, Lizasoain I, Castillo J. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke. 2007;38:2759–64. doi: 10.1161/STROKEAHA.107.484386. [DOI] [PubMed] [Google Scholar]
- 100.Yip HK, Chang LT, Chang WN, Lu CH, Liou CW, Lan MY, Liu JS, Youssef AA, Chang HW. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2008;39:69–74. doi: 10.1161/STROKEAHA.107.489401. [DOI] [PubMed] [Google Scholar]
- 101.Lemoli RM, Catani L, Talarico S, Loggi E, Gramenzi A, Baccarani U, Fogli M, Grazi GL, Aluigi M, Marzocchi G, Bernardi M, Pinna A, Bresadola F, Baccarani M, Andreone P. Mobilization of bone marrow-derived hematopoietic and endothelial stem cells after orthotopic liver transplantation and liver resection. Stem Cells. 2006;24:2817–25. doi: 10.1634/stemcells.2006-0333. [DOI] [PubMed] [Google Scholar]
- 102.Roberts N, Xiao Q, Weir G, Xu Q, Jahangiri M. Endothelial progenitor cells are mobilized after cardiac surgery. Ann Thorac Surg. 2007;83:598–605. doi: 10.1016/j.athoracsur.2006.09.087. [DOI] [PubMed] [Google Scholar]
- 103.Scheubel RJ, Zorn H, Silber RE, Kuss O, Morawietz H, Holtz J, Simm A. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–80. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 104.Inoue T, Sata M, Hikichi Y, Sohma R, Fukuda D, Uchida T, Shimizu M, Komoda H, Node K. Mobilization of CD34-positive bone marrow-derived cells after coronary stent implantation: impact on restenosis. Circulation. 2007;115:553–61. doi: 10.1161/CIRCULATIONAHA.106.621714. [DOI] [PubMed] [Google Scholar]
- 105.Bonello L, Basire A, Sabatier F, Paganelli F, Dignat-George F. Endothelial injury induced by coronary angioplasty triggers mobilization of endothelial progenitor cells in patients with stable coronary artery disease. J Thromb Haemost. 2006;4:979–81. doi: 10.1111/j.1538-7836.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 106.Spiel AO, Mayr FB, Leitner JM, Firbas C, Sieghart W, Jilma B. Simvastatin and rosuvastatin mobilize Endothelial Progenitor Cells but do not prevent their acute decrease during systemic inflammation. Thromb Res. 2008;123:108–13. doi: 10.1016/j.thromres.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 107.Park KW, Hwang KK, Cho HJ, Hur J, Yang HM, Yoon CH, Kang HJ, Oh BH, Park YB, Kim HS. Simvastatin enhances endothelial differentiation of peripheral blood mononuclear cells in hypercholesterolemic patients and induces pro-angiogenic cytokine IL-8 secretion from monocytes. Clin Chim Acta. 2008;388:156–66. doi: 10.1016/j.cca.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 108.Landmesser U, Bahlmann F, Mueller M, Spiekermann S, Kirchhoff N, Schulz S, Manes C, Fischer D, De Groot K, Fliser D, Fauler G, März W, Drexler H. Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation. 2005;111:2356–63. doi: 10.1161/01.CIR.0000164260.82417.3F. [DOI] [PubMed] [Google Scholar]
- 109.Deschaseaux F, Selmani Z, Falcoz PE, Mersin N, Meneveau N, Penfornis A, Kleinclauss C, Chocron S, Etievent JP, Tiberghien P, Kantelip JP, Davani S. Two types of circulating endothelial progenitor cells in patients receiving long term therapy by HMG-CoA reductase inhibitors. Eur J Pharmacol. 2007;562:111–8. doi: 10.1016/j.ejphar.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 110.Bahlmann FH, DeGroot K, Duckert T, Niemczyk E, Bahlmann E, Boehm SM, Haller H, Fliser D. Endothelial progenitor cell proliferation and differentiation is regulated by erythropoietin. Kidney Int. 2003;64:1648–52. doi: 10.1046/j.1523-1755.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 111.Lipsic E, Van Der Meer P, Voors AA, Westenbrink BD, Van Den Heuvel AF, De Boer HC, Van Zonneveld AJ, Schoemaker RG, Van Gilst WH, Zijlstra F, Van Veldhuisen DJ. A single bolus of a long-acting erythropoietin analogue darbepo-etin alfa in patients with acute myocardial infarction: a randomized feasibility and safety study. Cardiovasc Drugs Ther. 2006;20:135–41. doi: 10.1007/s10557-006-7680-5. [DOI] [PubMed] [Google Scholar]
- 112.Steiner S, Niessner A, Ziegler S, Richter B, Seidinger D, Pleiner J, Penka M, Wolzt M, Huber K, Wojta J, Minar E, Kopp CW. Endurance training increases the number of endothelial progenitor cells in patients with cardiovascular risk and coronary artery disease. Atherosclerosis. 2005;181:305–10. doi: 10.1016/j.atherosclerosis.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 113.Walther C, Adams V, Bothur I, Drechsler K, Fikenzer S, Sonnabend M, Bublitz B, Körner A, Erbs S, Busse M, Schuler G. Increasing physical education in high school students: effects on concentration of circulating endothelial progenitor cells. Eur J Cardiovasc Prev Rehabil. 2008;15:416–22. doi: 10.1097/HJR.0b013e3282fb2df1. [DOI] [PubMed] [Google Scholar]
- 114.Laufs U, Werner N, Link A, Endres M, Wassmann S, Jürgens K, Miche E, Böhm M, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neoin-tima formation, and enhances angiogene-sis. Circulation. 2004;109:220–6. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 115.Brixius K, Funcke F, Graf C, Bloch W. Endothelial progenitor cells: a new target for the prevention of cardiovascular diseases. Eur J Cardiovasc Prev Rehabil. 2006;13:705–10. doi: 10.1097/01.hjr.0000221862.34662.31. [DOI] [PubMed] [Google Scholar]
- 116.Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med. 2004;82:671–7. doi: 10.1007/s00109-004-0580-x. [DOI] [PubMed] [Google Scholar]
- 117.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 118.Schmidt-Lucke C, Rössig L, Fichtlscherer S, Vasa M, Britten M, Kämper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–7. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 119.Wang HY, Gao PJ, Ji KD, Shen WF, Fan CL, Lu L, Zhu DL. Circulating endothelial progenitor cells, C-reactive protein and severity of coronary stenosis in Chinese patients with coronary artery disease. Hypertens Res. 2007;30:133–41. doi: 10.1291/hypres.30.133. [DOI] [PubMed] [Google Scholar]
- 120.Taguchi A, Matsuyama T, Moriwaki H, Hayashi T, Hayashida K, Nagatsuka K, Todo K, Mori K, Stern DM, Soma T, Naritomi H. Circulating CD34-positive cells provide an index of cerebrovascular function. Circulation. 2004;109:2972–5. doi: 10.1161/01.CIR.0000133311.25587.DE. [DOI] [PubMed] [Google Scholar]
- 121.Junhui Z, Xingxiang W, Guosheng F, Yunpeng S, Furong Z, Junzhu C. Reduced number and activity of circulating endothe-lial progenitor cells in patients with idio-pathic pulmonary arterial hypertension. Respir Med. 2008;102:1073–9. doi: 10.1016/j.rmed.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 122.Diller GP, Van Eijl S, Okonko DO, Howard LS, Ali O, Thum T, Wort SJ, Bédard E, Gibbs JS, Bauersachs J, Hobbs AJ, Wilkins MR, Gatzoulis MA, Wharton J. Circulating endothelial progenitor cells in patients with Eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation. 2008;117:3020–30. doi: 10.1161/CIRCULATIONAHA.108.769646. [DOI] [PubMed] [Google Scholar]
- 123.Nonaka-Sarukawa M, Yamamoto K, Aoki H, Nishimura Y, Tomizawa H, Ichida M, Eizawa T, Muroi K, Ikeda U, Shimada K. Circulating endothelial progenitor cells in congestive heart failure. Int J Cardiol. 2007;119:344–8. doi: 10.1016/j.ijcard.2006.07.191. [DOI] [PubMed] [Google Scholar]
- 124.Kissel CK, Lehmann R, Assmus B, Aicher A, Honold J, Fischer-Rasokat U, Heeschen C, Spyridopoulos I, Dimmeler S, Zeiher AM. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postin-farction heart failure. J Am Coll Cardiol. 2007;49:2341–9. doi: 10.1016/j.jacc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 125.Valgimigli M, Rigolin GM, Fucili A, Porta MD, Soukhomovskaia O, Malagutti P, Bugli AM, Bragotti LZ, Francolini G, Mauro E, Castoldi G, Ferrari R. CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation. 2004;110:1209–12. doi: 10.1161/01.CIR.0000136813.89036.21. [DOI] [PubMed] [Google Scholar]
- 126.George J, Herz I, Goldstein E, Abashidze S, Deutch V, Finkelstein A, Michowitz Y, Miller H, Keren G. Number and adhesive properties of circulating endothelial progenitor cells in patients with in-stent restenosis. Arterioscler Thromb Vasc Biol. 2003;23:e57–60. doi: 10.1161/01.ATV.0000107029.65274.db. [DOI] [PubMed] [Google Scholar]
- 127.Matsuo Y, Imanishi T, Hayashi Y, Tomobuchi Y, Kubo T, Hano T, Akasaka T. The effect of senescence of endothelial progenitor cells on in-stent restenosis in patients undergoing coronary stenting. Intern Med. 2006;45:581–7. doi: 10.2169/internalmedicine.45.1663. [DOI] [PubMed] [Google Scholar]
- 128.Egan CG, Lavery R, Caporali F, Fondelli C, Laghi-Pasini F, Dotta F, Sorrentino V. Generalised reduction of putative endothelial progenitors and CXCR4-positive peripheral blood cells in type 2 diabetes. Diabetologia. 2008;51:1296–305. doi: 10.1007/s00125-008-0939-6. [DOI] [PubMed] [Google Scholar]
- 129.Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, Tsikas D, Ertl G, Bauersachs J. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56:666–74. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- 130.Kusuyama T, Omura T, Nishiya D, Enomoto S, Matsumoto R, Takeuchi K, Yoshikawa J, Yoshiyama M. Effects of treatment for diabetes mellitus on circulating vascular progenitor cells. J Pharmacol Sci. 2006;102:96–102. doi: 10.1254/jphs.fp0060256. [DOI] [PubMed] [Google Scholar]
- 131.Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, De Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–57. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 132.Loomans CJ, De Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, De Boer HC, Verhaar MC, Braam B, Rabelink TJ, Van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–9. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 133.Pirro M, Schillaci G, Menecali C, Bagaglia F, Paltriccia R, Vaudo G, Mannarino MR, Mannarino E. Reduced number of circulating endothelial progenitors and HOXA9 expression in CD34+ cells of hypertensive patients. J Hypertens. 2007;25:2093–9. doi: 10.1097/HJH.0b013e32828e506d. [DOI] [PubMed] [Google Scholar]
- 134.Oliveras A, Soler MJ, Martfnez-Estrada OM, Vázquez S, Marco-Feliu D, Vila JS, Vilard S, Lloveras J. Endothelial progenitor cells are reduced in refractory hypertension. J Hum Hypertens. 2008;22:183–90. doi: 10.1038/sj.jhh.1002304. [DOI] [PubMed] [Google Scholar]
- 135.Schlieper G, Hristov M, Brandenburg V, Krjer T, Westenfeld R, Mahnken AH, Yagmur E, Boecker G, Heussen N, Gladziwa U, Ketteler M, Weber C, Floege Predictors of low circulating endothelial progenitor cell numbers in haemodialysis patients. J Nephrol Dial Transplant. 2008;23:2611–8. doi: 10.1093/ndt/gfn103. [DOI] [PubMed] [Google Scholar]
- 136.Chan CT, Li SH, Verma S. Nocturnal hemodialysis is associated with restoration of impaired endothelial progenitor cell biology in end-stage renal disease. Am J Physiol Renal Physiol. 2005;289:F679–84. doi: 10.1152/ajprenal.00127.2005. [DOI] [PubMed] [Google Scholar]
- 137.Westerweel PE, Hoefer IE, Blankestijn PJ, De Bree P, Groeneveld D, Van Oostrom O, Braam B, Koomans HA, Verhaar MC. End-stage renal disease causes an imbalance between endothelial and smooth muscle progenitor cells. Am J Physiol Renal Physiol. 2007;292:F1132–40. doi: 10.1152/ajprenal.00163.2006. [DOI] [PubMed] [Google Scholar]
- 138.De Groot K, Bahlmann FH, Bahlmann E, Menne J, Haller H, Fliser D. Kidney graft function determines endothelial progenitor cell number in renal transplant recipients. Transplantation. 2005;79:941–5. doi: 10.1097/00007890-200504270-00012. [DOI] [PubMed] [Google Scholar]
- 139.Choi JH, Kim KL, Huh W, Kim B, Byun J, Suh W, Sung J, Jeon ES, Oh HY, Kim DK. Decreased number and impaired angio-genic function of endothelial progenitor cells in patients with chronic renal failure. Arterioscler Thromb Vasc Biol. 2004;24:1246–52. doi: 10.1161/01.ATV.0000133488.56221.4a. [DOI] [PubMed] [Google Scholar]
- 140.Chen JZ, Zhang FR, Tao QM, Wang XX, Zhu JH, Zhu JH. Number and activity of endothelial progenitor cells from peripheral blood in patients with hyper-cholesterolaemia. Clin Sci. 2004;107:273–80. doi: 10.1042/CS20030389. [DOI] [PubMed] [Google Scholar]
- 141.Pirro M, Schillaci G, Paltriccia R, Bagaglia F, Menecali C, Mannarino MR, Capanni M, Velardi A, Mannarino E. Increased ratio of CD31+/CD42- micropar-ticles to endothelial progenitors as a novel marker of atherosclerosis in hypercholes-terolemia. Arterioscler Thromb Vasc Biol. 2006;26:2530–5. doi: 10.1161/01.ATV.0000243941.72375.15. [DOI] [PubMed] [Google Scholar]
- 142.Michaud SE, Dussault S, Haddad P, Groleau J, Rivard A. Circulating endothelial progenitor cells from healthy smokers exhibit impaired functional activities. Atherosclerosis. 2006;187:423–32. doi: 10.1016/j.atherosclerosis.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 143.Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Lino S, Inden Y, Murohara T. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004;24:1442–7. doi: 10.1161/01.ATV.0000135655.52088.c5. [DOI] [PubMed] [Google Scholar]
- 144.Tao J, Wang Y, Yang Z, Tu C, Xu MG, Wang JM. Circulating endothelial progenitor cell deficiency contributes to impaired arterial elasticity in persons of advancing age. J Hum Hypertens. 2006;20:490–5. doi: 10.1038/sj.jhh.1001996. [DOI] [PubMed] [Google Scholar]
- 145.Hoetzer GL, Van Guilder GP, Irmiger HM, Keith RS, Stauffer BL, DeSouza CA. Aging, exercise, and endothelial progenitor cell clonogenic and migratory capacity in men. J Appl Physiol. 2007;102:847–52. doi: 10.1152/japplphysiol.01183.2006. [DOI] [PubMed] [Google Scholar]
- 146.Zhu J, Wang X, Chen J, Sun J, Zhang F. Reduced number and activity of circulating endothelial progenitor cells from patients with hyperhomocysteinemia. Arch Med Res. 2006;37:484–9. doi: 10.1016/j.arcmed.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 147.Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. 2007;49:741–52. doi: 10.1016/j.jacc.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 148.Werner N, Nickenig G. Endothelial progenitor cells in health and atherosclerotic disease. Ann Med. 2007;39:82–90. doi: 10.1080/07853890601073429. [DOI] [PubMed] [Google Scholar]
- 149.Balbarini A, Barsotti MC, Di Stefano R, Leone A, Santoni T. Circulating endothelial progenitor cells characterization, function and relationship with cardiovascular risk factors. Curr Pharm Des. 2007;13:1699–713. doi: 10.2174/138161207780831329. [DOI] [PubMed] [Google Scholar]
- 150.Imanishi T, Moriwaki C, Hano T, Nishio I. Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with essential hypertension. J Hypertens. 2005;23:1831–7. doi: 10.1097/01.hjh.0000183524.73746.1b. [DOI] [PubMed] [Google Scholar]
- 151.Thum T, Hoeber S, Froese S, Klink I, Stichtenoth DO, Galuppo P, Jakob M, Tsikas D, Anker SD, Poole-Wilson PA. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ Res. 2007;100:434–43. doi: 10.1161/01.RES.0000257912.78915.af. [DOI] [PubMed] [Google Scholar]
- 152.Chironi G, Walch L, Pernollet MG, Gariepy J, Levenson J, Rendu F, Simon A. Decreased number of circulating CD34+KDR+ cells in asymptomatic subjects with preclinical atherosclerosis. Atherosclerosis. 2007;191:115–20. doi: 10.1016/j.atherosclerosis.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 153.Hughes AD, Coady E, Raynor S, Mayet J, Wright AR, Shore AC, Kooner JS, Thorn SA, Chaturvedi N. Reduced endothelial progenitor cells in European and South Asian men with atherosclerosis. Eur J Clin Invest. 2007;37:35–41. doi: 10.1111/j.1365-2362.2007.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kunz GA, Liang G, Cuculi F, Gregg D, Vata KC, Shaw LK, Goldschmidt-Clermont PJ, Dong C, Taylor DA, Peterson ED. Circulating endothelial progenitor cells predict coronary artery disease severity. Am Heart J. 2006;152:190–5. doi: 10.1016/j.ahj.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 155.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 156.Murphy C, Kanaganayagam GS, Jiang B, Chowienczyk PJ, Zbinden R, Saha M, Rahman S, Shah AM, Marber MS, Kearney MT. Vascular dysfunction and reduced circulating endothelial progenitor cells in young healthy UK South Asian men. Arterioscler Thromb Vasc Biol. 2007;27:936–42. doi: 10.1161/01.ATV.0000258788.11372.d0. [DOI] [PubMed] [Google Scholar]
- 157.Werner N, Wassmann S, Ahlers P, Schiegl T, Kosiol S, Link A, Walenta K, Nickenig G. Endothelial progenitor cells correlate with endothelial function in patients with coronary artery disease. Basic Res Cardiol. 2007;102:565–71. doi: 10.1007/s00395-007-0680-1. [DOI] [PubMed] [Google Scholar]
- 158.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 159.Solovey AA, Solovey AN, Harkness J, Hebbel RP. Modulation of endothelial cell activation in sickle cell disease: a pilot study. Blood. 2001;97:1937–41. doi: 10.1182/blood.v97.7.1937. [DOI] [PubMed] [Google Scholar]
- 160.Heiss C, Amabile N, Lee AC, May Real W, Schick SF, Lao D, Wong ML, Jahn S, Angeli FS, Minasi P, Springer ML, Hammond SK, Glantz SA, Grossman W, Balmes JR, Yeghiazarians Y. Brief secondhand smoke exposure depresses endothelial progenitor activity and endothelial function. Journal Am Coll Cardiol. 2008;51:1760–71. doi: 10.1016/j.jacc.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 161.Bulut D, Maier K, Bulut-Streich N, Borgel J, Hanefeld C, Mugge A. Circulating endothelial microparticles correlate inversely with endothelial dysfunction in patients with ischemic left ventricular dysfunction. J Card Failure. 2008;14:336–40. doi: 10.1016/j.cardfail.2007.11.002. [DOI] [PubMed] [Google Scholar]


