Abstract
Lysyl hydroxylase (LH) isoform 3 is a post-translational enzyme possessing LH, collagen galactosyltransferase (GT) and glucosyltransferase (GGT) activities. We have demonstrated that LH3 is found not only intracellularly, but also on the cell surface and in the extracellular space, suggesting additional functions for LH3. Here we show that the targeted disruption of LH3 by siRNA causes a marked reduction of both glycosyltransferase activities, and the overexpression of LH3 in HT-1080 cells increases hydroxylation of lysyl residues and the subsequent galactosylation and glucosylation of hydroxylysyl residues. These data confirm the multi-functionality of LH3 in cells. Furthermore, treatment of cells in culture medium with a LH3 N-terminal fragment affects the cell behaviour, rapidly leading to arrest of growth and further to lethality if the fragment is glycosyltransferase-deficient, and leading to stimulation of proliferation if the fragment contains LH3 glycosyltransferase activities. The effect is reversible, the cells recovering after removal of the glycosyltransferase-deficient fragment. The findings were confirmed by overexpressing the full-length LH3 in native or mutated forms in the cells. The data indicate that the increase in proliferation depends on the glycosyltransferase activity of LH3. The overexpression of a glycosyltransferase-deficient mutant or targeted disruption of LH3 by siRNA in cells results in abnormal cell morphology followed by cell death. Our data clearly indicate that the deficiency of LH3 glycosyltransferase activities, especially in the extracellular space, causes growth arrest revealing the importance of the glycosyltransferase activities of LH3 for cell growth and viability, and identifying LH3 as a potential target for medical applications, such as cancer therapy.
Keywords: lysyl hydroxylase, hydroxylysyl glycosyltransferase, hydroxylysyl glycosylation, post-translational modification, collagen biosynthesis, cell growth, cell viability
Introduction
Collagens constitute a highly specialized family of extracellular matrix proteins, which are universally distributed in the animal body. In addition to the maintenance of the architecture of tissues, they have regulatory functions important for cell behaviour [1–3]. To date, more than 40 vertebrate collagen genes have been described, the products of which combine to form at least 29 distinct homo- and/or heterotrimeric molecules [4, 5]. The biosynthesis of collagen is a multi-step process characterized by a large number of co- and post-translational modifications, many of which are unique to collagens and collagen-like proteins. These modifications, which typically occur within the rough endoplasmic reticulum of the cell, include hydroxylation of lysyl residues catalysed by lysyl hydroxylase (EC 1.14.11.4, LH). Hydroxylysyl residues participate in the formation of collagen cross-links, which stabilize the extracellular matrix. They also serve as attachment sites for sugar units. The carbohydrates linked to hydroxylysyl residues are either a monosaccharide galactose or a disaccharide glucosylgalactose [6–11]. The formation of hydroxylysyl-linked carbohydrate units is catalysed by two specific enzyme activities, hydroxylysyl galacto-syltransferase (EC 2.4.1.50, GT) and galactosylhydroxylysyl glucosyltransferase (EC 2.4.1.66, GGT). The extent of hydroxylation and glycosylation varies considerably among different collagen types and within the same collagen type from various sources or even in the same tissue under different physiological and pathological conditions [11, 12]. The function of the hydroxylysyl-linked carbohydrate units remains poorly understood.
Three LH isoforms, LH1, LH2a/2b and LH3, originating from three different genes, have been characterized so far from human, mouse, rat and zebrafish [13–21]. We have demonstrated that LH3 and C. elegansLH, the only ortholog for lysyl hydroxylase in the nematode, are multi-functional enzymes possessing LH, GT and GGT activities in vitro[21–24]. The amino acids important for the catalytic activity of glycosyltransferases associated with LH3 are localized at the amino-terminal part of the molecule, being separate from the active site of LH [23, 24]. Our previous results demonstrate that LH3 resides not only in the ER, but also in the extracellular space [25, 26] such as in serum, and on cell surfaces associated with collagenous proteins. Furthermore, transgenic mouse studies show that a knock-out of the LH3 gene causes lethality at an early embryonic stage [27, 28], and that the dramatic reduction of GGT activity, not LH activity, of LH3 disrupts the formation of basement membranes during mouse embryogenesis [27]. The absence of the multi-functional LH in C. elegansresults in retention of type IV collagen within the cells and in failure to complete embryogenesis [29]. Schneider and Granato showed recently [19] that the glycosyltransferase domain of myotomal diwanka (LH3 in zebrafish) plays a critical role in growth cone migration. It acts through myotomal type XVIII collagen, a ligand for neural-receptor protein tyrosine phosphatases that guide motor axons.
In this study we have used HT-1080 cells to obtain more information about LH3, especially its extracellular role in cells. We demonstrate that treatment of cells with a mutated 30 kD LH3 N-terminal fragment (DXD fragment, see later) lacking glycosyltransferase activity in medium dramatically affects cell behaviour, slowing the growth rate and changing cell morphology, whereas treatment with the glycosyltransferase active fragment stimulates cell growth. The phenomenon is rapid, cell type specific and reversible, demonstrating for the first time that LH3-dependent glycosyltransferase activities in the extracellular space are important for cell growth and proliferation. The requirement of LH3 for cell viability was also demonstrated by the targeted disruption of LH3 by siRNA in cells, and the importance of the glycosyltransferase activity of LH3 for cell viability was confirmed by overexpression of the full-length LH3 in native and mutated forms in HT-1080 cells.
Experimental procedures
Cell culture
The following cell lines were used: human fibrosarcoma cells (HT-1080, ATCC CCL-121), African green monkey kidney cells (Cos7, ATCC CRL-1651), human embryonic kidney cells (293, ATCC CRL-1573), human lung fibroblasts (CCD-19LU, ECACC 90112708), locally established human skin fibroblasts (MR and NAF) and human osteosarcoma cells (MG-63, ATCC CRL-1427). The cells were grown in Minimum Essential Medium (MEM) or Dulbecco's Modified Eagle Medium (DMEM) (Invitrogen, Carlsbad, CA, USA) plus 10% foetal calf serum (FCS) (Promocell, Heidelberg, Germany) at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Overexpression of full-length LH3 cDNA and its mutants in HT-1080 cells
The human LH3 coding sequence covering the nucleotides 214-2447 [GenBank™/EBI Bank with accession number AF046889, 14] was subcloned into BamHI and XhoI sites of a pcDNA3 vector (Invitrogen, Carlsbad, CA, USA). Six histidines were inserted at the N-terminus after the signal peptide. The glycosyltransferase-defi-cient mutant was constructed by mutating a short conserved DDDDD motif in the sequence at position 187-191 to ADAAA [23]. The LH-deficient mutant was made by mutating the aspartate at position 669 to alanine [22]. Nucleofector (Amaxa, Koeln, Germany) was used for delivery of LH3 and the mutated cDNAs directly into the cell. 2 × 105 to 2 × 106 HT-1080 cells were plated at least 1 day before the transfection. To establish the stably transfected clones, the cells were kept under constant selection pressure with G418 at 750 |xg/ml and those originated from the single clones were finally picked up and transferred to new culture dishes.
Hydroxylation and glycosylation analysis
HT-1080 cells stably transfected with the pcDNA3 vector (clone 9), full-length LH3 cDNA (clone H0-12) and the LH-deficient mutant (clone H14-3) were grown under standard conditions for 48 hrs. The collagenous proteins in the cells and media were collected and digested by highly purified collagenase (Sigma, St. Louis, MO, USA) as described elsewhere [25]. After being hydrolysed in 2M NaOH, the amount of galactosylhydroxylysine (GH) and glucosylgalactosylhy-droxylysine (GGH) as fluorescent dansyl derivatives were measured by slightly modifying the high-performance liquid chromatography (HPLC) method presented by Moro et al.[30]. GH and GGH standards were kindly obtained from Professor R. Tenni, Italy. The amount of hydroxylysine in collagenous proteins was measured in the culture medium of cells using an amino acid analyser, after hydrolysing the samples at 110°C overnight in 6 M HCl.
Gene silencing by RNA interference
A Silencer™ siRNA cocktail kit (RNase III) was purchased from Ambion (Austin, TX, USA). Two 500 bp-long target sequences, LH3 siRNAs-1 and LH3 siRNAs-2, corresponding to 378–878 nt and 1111-1611 nt (54–55% identity with the corresponding nucleotide sequence of LH1 and 52–63% identity with that of LH2) located at the 5’ and medial regions of human LH3 cDNA [14], were selected as templates. Long dsRNAs were generated by in vitro transcription. The dsRNAs were then cleaved by RNase III into a mixture or cocktail of siRNA molecules capable of inducing the RNAi effect very specifically in mammalian cells. In order to observe the long-term effect of LH3 siRNAs on cell behaviour nucleotides corresponding to 380–398 nt, 630–648 nt and 1125–1243 nt (37–73% identity with the corresponding nucleotides of the other LH isoforms) of the human LH3 sequence [14, 21] were synthesized and cloned into pRNA U6.1/Neo (Genscript, Piscataway, NJ, USA) and pSilencer 2.1-U6 neo (Ambion), respectively. Empty pRNA U6.1/Neo vector and pSilencer 2.1-U6 neo negative control were used as controls. RNAi expression vectors were introduced into HT-1080 and Cos7 cells by Nucleofector transfection (Amaxa, Koeln, Germany). G418 was used for selection (750 μg/ml for the first 3 days and 375 μg/ml for the rest of the selection process).
LH3 N-terminal fragment with or without glycosyltransferase activities
A 30 kD His-tagged amino-terminal fragment of human LH3 with GGT/GT activities [22–24] corresponding to nucleotides encoding amino acids 25-290, was cloned into a pET-15b vector (Novagen, Madison, WI, USA). The fragment, produced in an E. coli system, was purified with Ni-NTA agarose (Qiagen, Hilden, Germany) [24]. In order to avoid any cytotoxic effect from the elution buffer (30 mM MES, 300 mM imidazole, 400 mM NaCl, 10% glycerol, pH 6.5), the eluate was equilibrated in culture medium by using Amicon Ultra centrifugal filter devices [10,000 molecular weight cut off (MWCO), Millipore, Billarica, MA, USA]. The same fragment with DDDDD187–191 ADADD mutations, designated the DXD fragment having 98% of the glycosyltransferase activities eliminated [23], was constructed and purified in the same way. The purity of both fragments after equilibration in culture medium was checked by 10% SDS-PAGE, the results indicating one 30 kD band in the gel. The GGT activity assay revealed that 1 μg of the LH3 N-terminal fragment has GGT activity of about 80,000 dpm, which corresponds to GGT activity measured in 1 ml of mouse serum [25]. Fragment analysis by CD spectroscopy (Jasco J-715 spectropolarimeter equipped with a microprocessor for spectral accumulation and data manipulation) indicated that the mutations of the LH3 N-terminal fragment did not cause secondary structural changes.
Immunofluorescence and light microscopy
HT-1080 cells transfected with the LH3 siRNA expression vector pRNA U6.1/Neo or pSilencer 2.1-U6 neo, were observed by light microscopy (Nikon, Tokyo, Japan). For the cell morphology study HT-1080 cells, either overexpressing full-length human LH3 or mutants, or treated with the LH3 fragments in medium, were grown on glass coverslips, fixed with 4% paraformaldehyde at room temperature for 15 min. and stained as described elsewhere [22] with a monoclonal antibody against either the polyhistidine tag (Sigma) or collagen IV (DAKO, Glostrup, Denmark) at a dilution of 1:100. Alexa Fluor 488 or 594 conjugated antimouse antibody (1:100, Invitrogen, Carlsbad, CA, USA) was used as the secondary antibody, and the staining was detected by fluorescence microscopy (Nikon, Tokyo, Japan). Cells transfected with the empty pcDNA3 vector or untreated cells were used as controls. Other cell lines treated with the LH3 fragment in medium were stained with antibodies against pro-collagen type I [31] or collagen type IV (DAKO). The recovery experiment was carried out by seeding 1×105 HT-1080 cells on coverslips in 35 mm plates and treating the cells for 24 hrs with media or media containing the DXD fragment. All media were then changed either to 10% foetal calf serum (FCS) DMEM or to 10% FCS DMEM containing the LH3 N-terminal fragment. The cells were fixed as described above after 24, 48 and 72 hrs, respectively, and stained with a type IV collagen antibody. For cytoskeletal protein staining, 1 × 105 of HT-1080 cells were grown on coverslips and treated with the LH3 fragments for 48 hrs without changing the medium. The cells were fixed and stained as above with a monoclonal antibody against tubulin or vimentin (Sigma), at a 1:100 dilution. Actin cytoskeleton was stained with Alexa Fluor 568 phalloidin (1:20, Invitrogen) that binds specifically to F-actin. Confocal microscopy images were acquired with an Olympus Fluoview 1000 confocal microscope (Olympus, Tokyo, Japan).
Flow cytometric analysis
The proliferation assay was carried out with HT-1080 cells incubated with the LH3 N-terminal fragment or the DXD fragment (about 3 μg/ml), and with HT-1080 cells stably overexpressing the LH-deficient mutant of LH3 (clone H14-3) using the cells transfected with empty pcDNA3 vector (clone 9) as a control. The cells (about 1 x105) were grown in 6×35 mm dishes and maintained in 10% FCS DMEM. The medium was changed every 24 hrs. The cells were trypsinized and counted daily using the absolute counting function of a CyFlow Space flow cytometer (Partec, Münster, Germany) for up to 4 days.
In cell cycle analysis HT-1080 cells were seeded at a density of 1 × 105 cells/35 mm plate in triplicate in 10% FCS DMEM containing either the LH3 N-terminal fragment or the DXD fragment (3μg/ml of the purified fragment). The treatment was maintained for 48 hrs with one medium change at 24 hrs. Untreated cells were used as the control. The cells were harvested and re-suspended in 100 μl of 3.4 mM sodium citrate buffer, pH 7.6, containing 0.1% Nonidet P40 and 1.5 mM spermine tetrahydrochloride. The DNA was stained by propidium iodide and analysed with a CyFlow Space flow cytometer (Partec, Münster, Germany). The fraction of every cell cycle phase was calculated.
Cell surface binding analysis
A total of 1 × 106 HT-1080 cells /10 cm plates were grown for 18 hrs to about 50% confluency. The cells were treated with the LH3 N-terminal fragment or the DXD fragment (3 μg/ml of the purified fragment) added in culture medium for 3 hrs. The cells were washed twice by PBS and homogenized in buffer containing 20 mM Tris-HCl, pH 8, 0.4 M NaCl, 1 mM DTT. The cell lysates were collected after centrifugation at 14,000 rpm for 30 min. at +4°C. Any his-tagged proteins in the lysates and the added fragments in media were concentrated by Nickel purification as described elsewhere [22]. The cell pellet fractions were re-suspended in the same buffer and separated by 15% SDS-PAGE for Western blotting analysis.
Non-permeabilized cell staining was carried out by using the affinity purified polyclonal antibody PLOD3 (ProteinTech Group, Inc., Chicago, IL, USA) against the human LH3 at a working solution of 10 (μg/ml. A total of 1×105 HT-1080 cells were inoculated into 35 mm plates with coverslips and cultivated with the LH3 N-terminal fragments as described above for 7 hrs. The cells were washed twice with phosphate buffered saline (PBS), fixed with 4% paraformaldehyde in PBS, pH 7.4, for 3 min. at room temperature, then blocked in 1% bovine serum albumin in PBS, pH 7.4, for 1 hr at room temperature. Goat anti-rabbit Alexa Fluor 488 (Invitrogen) was used as the secondary antibody at a 1:100 dilution. Wheat germ agglutinin (WGA), Texas Red-X conjugate (10 μg/ml, Invitrogen), was used as a cell surface marker. Hoechst 33258 (1 μg/ml, Sigma, St. Louis, MO, USA) was used for nuclei staining. The cell surface staining was checked by Olympus Fluoview 1000 confocal microscope.
Other methods
LH, GT and GGT activity assays were done as described elsewhere [32, 33]. Western blot analysis was carried out using monoclonal antibodies against the His tag (Sigma) or polyclonal antibodies, hLH3N1, against the purified human LH3 N-terminal fragment (Davids Biotechnologie, Regensburg, Germany). The proteins were fractionated under reducing conditions by 10% or 15% SDS-PAGE, blotted onto an Immobilon-P membrane (Millipore), and incubated with the primary antibody. Antimouse (Invitrogen) or anti-rabbit (P.A.R.I.S., Compiègne, France) IgG peroxidase conjugate was used as the secondary antibody. Bound antibodies were visualized using the enhanced chemiluminescence (ECL) detection system (Amersham Pharmacia Biotech, Upsala, Sweden) and x-ray film (Eastman Kodak Co, Rochester, NY, USA). The QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) was used to make mutations in the cDNA sequences. The nucleotide changes of the mutations were confirmed by sequencing.
Results
LH3 functions as LH, GT and GGT in HT-1080 cells
In order to verify that LH3 modifies lysyl residues in collagens in cellulo, we have generated wild-type, LH-deficient and glycosyltransferase-deficient human LH3 cDNA constructs in a mammalian pcDNA3 expression vector, and successfully transfected them separately into HT-1080 cells. These cells produce mainly type IV collagen [34, 35] that is highly hydroxylated and glycosylated [6, 10], and thus a good read-out molecule for the study. Western blot analysis showed clearly the overexpression of human LH3 and its mutated variants in the transfected HT-1080 cells under constant selection pressure (Fig. 1A) or with stably transfected clones (Fig. 1B). Stably transfected clones were easily established with all constructs except the glycosyltransferase-deficient mutant (Fig. 1B). The transfected cell lines were used to analyse cellular LH, GT and GGT activities as well as the amount of lysyl modifications in collagenous proteins.
1.
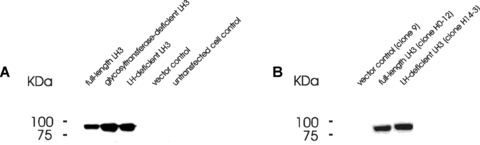
Overexpression of LH3 cDNAs in HT-1080 cells. Cells were transfected with different human LH3 cDNAs in a pcDNA3 vector with a 6×His-tag at the N-terminal after the signal peptide. His-tag antibodies were used to detect the overexpressed LH3 and its mutated variants by Western blot analysis in HT-1080 cell lysates after Nickel purification [24]. The molecular weight of the LH3 bands corresponds to about 85 kD. (A) The transfected cells were kept under constant selection pressure with G418 at 750 μg/ml. (B) The LH3 protein produced from the single stably transfected clones.
LH activity was measured by the assay not separating LH3 from the other two LH isoforms, and thus the activity observed represents the total LH activity of the cells. Our results indicate that LH activity was increased about 1.30-fold in cells stably transfected with the wild-type LH3 (clone H0-12), and about 1.34-fold in cells transfected with the glycosyltransferase-deficient LH3 under selection pressure. In cells stably transfected with a LH-deficient construct (clone H14-3), the LH activity was reduced to about 54% compared to that of a pcDNA3 transfected clone (clone 9). Analysis of the amounts of the hydroxylysyl residues in collagenous proteins from cell culture medium by amino acid analysis revealed that the overexpression of wild-type LH3 resulted in about a 1.5-fold increase in the hydroxylation of lysyl residues compared to that in the vector control. No increase was found in the hydroxylysine content of the medium of cells transfected with the LH-deficient clone (not shown). The results indicate that the increase of hydroxylysine in collagenous proteins is due to the overexpression of the LH activity of LH3. Overexpression of LH3 also slightly increased the glycosylation of hydroxylysyl residues in collagenous sequences in cells (Table 1). Approximately a 10% increase in the content of GGH and GH was observed, when the cells were stably expressing wild-type LH3 (clone H0-12), the increase was also found with LH-deficient LH3 (clone H14-3) revealing that LH3-dependent glycosyltransferases are able to increase the glycosylation of hydroxylysyl residues in cells. GGT activity increased about 6-fold and GT activity 2-fold in the HT-1080 cells stably overexpressing wild-type human LH3 or the LH-deficient LH3 when compared with control cells transfected with the empty vector (clone 9) (Table 1).
1.
GT/GGT activities and galactosylation (GH) and glucosylation (GGH) of hydroxylysyl residues in HT-1080 cells stably overexpressing LH3 and the LH-deficient mutant of LH3*
| Construct | GT% | GGT% | GH%† | GGH%‡ |
|---|---|---|---|---|
| pcDNA3 (clone 9) | 100 | 100 | 70.6 ± 2.8 | 42.0 ± 9.0 |
| Non-mutated LH3 (clone H0-12) | 201 ± 56 | 647 ± 218 | 75.9 ± 6.1 | 52.7 ± 30.1 |
| LH-deficient mutant (clone H14-3) | 218 ± 126 | 603 ± 293 | 79.0 ± 4.9 | 46.6 ± 16.1 |
Mean value from three experiments ± SD, assayed as described in experimental procedures.
Galactosylhydrosylysine as percentage of hydroxylated lysyl residues.
Glucosylgalactosylhydroxylysine as percentage of galactosylated hydroxylysine residues.
RNA interference analysis was carried out in order to confirm that LH3 is responsible for GT and GGT activities of cells. This is a very specific method to target the knock-down to a certain gene. As shown elsewhere [36] one nucleotide change in the targeting sequence is enough to fail the suppression of the gene, and thus it is probable that homologous genes like the other LH isoforms remain unaffected in the treatment. Knocking-down of the LH3 gene by siRNA cocktails, a vector-free system consisting of a mixture of specifically targeted small interference RNAs, resulted in efficient reduction of GGT and GT activities after delivery of siRNAs into the cells. A difference was observed between the knocking-down efficacy of the two siRNA cocktails (LH3 siRNAs-1 and LH3 siRNAs-2), suggesting that the more effective targeting sequence of LH3 is located in the medial region of the molecule (not shown).
The effect of RNA interference was transient, GGT/GT activities returned to normal levels after 24 hrs to 48 hrs. The effect could be prolonged when the targeted sequences were used sequentially in double transfections. Only 20% of GGT and 17% of GT activities were obtained, compared to that of the negative control, when the cells were transfected with LH3 siRNAs-1 and LH3 siRNAs-2 in a consecutive manner with a 24 hrs interval and followed by a further 48 hrs cultivation. The negative control siRNA, which had no significant homology to any known gene sequences from mouse, rat or human, was composed of a 19 bp scrambled sequence with 3’dT overhangs. In order to prolong the effect of RNAi we also used a vector-based siRNA and cotransfected three LH3 siRNA-expression constructs in pRNAU6.1/ Neo into HT-1080 cells. This enabled us to target three different sequences at the 5’ and medial regions of the LH3 transcript simultaneously, thus making the knock-down more specific. After 10 days (Fig. 2) the GT and GGT activities were reduced to the level of about 15–18% of the untreated cells, the cells appearing round and dying very quickly within a further 2 days. It should be noted that transfection of the vector alone (Fig. 2) as negative control also caused reduction of GGT and GT activities in the cells compared to untreated cells; however, the reduction was less than that in LH3 siRNAs transfected cells and both activities were recovered after culturing the cells for a longer time, whereas the LH3 siRNA expression maintained the activities at a very low level leading finally to the cell death. Taken all together, our data clearly show that disruption of LH3 reduces both glycosyltransferase activities in HT-1080 cells, indicating that LH3 is responsible for GGT and GT activities in these cells.
2.
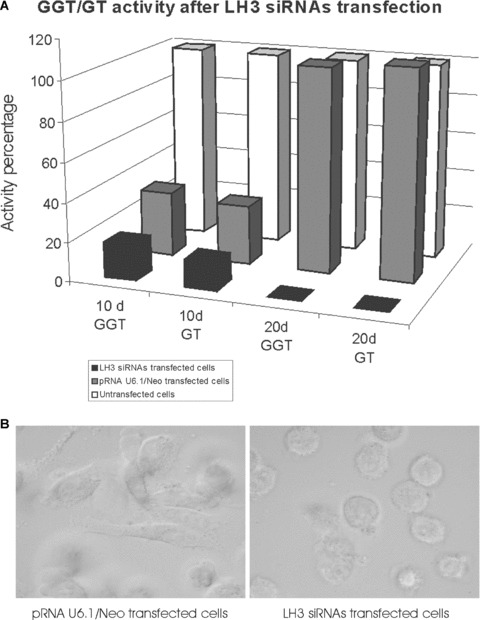
The effect of LH3 siRNAs on HT-1080 cells. (A) Galactosyltransferase (GT) and glucosyltransferase (GGT) activities in untransfected (white), vector transfected (pRNA U6.1/Neo) (grey) and LH3 siRNA (black) expressing HT-1080 cells after 10 days and 20 days of the treatment. The untreated cells were taken as 100%. The data presented were the mean values from two or three assays. (B) Light microscopic view at 10 days after transfection of HT-1080 cells transfected either with empty pRNA U6.1/Neo vector (control) or with LH3 siRNA-expressing constructs. The vector transfected cells grew normally with no obvious morphological change, whereas the LH3 siRNA transfected cells showed growth retardation, an abnormal rounded shape (a sign of cell detachment), and finally cell death.
The addition of a glycosyltransferase-deficient LH3 fragment to the culture medium changes the cell morphology and slows cell growth
As demonstrated recently [25, 26], LH3 is secreted from cells and is located on the cell surface and in the extracellular space in some tissues. This extracellular localization differentiates LH3 from the other LH isoforms, although the functions of LH3 in the extracellular space are not known. In order to study the extracellular functions we exposed cell surfaces to medium containing an excess amount of the glycosyltransferase-deficient LH3 N-terminal fragment, and then determined the consequences of the treatment to the cell. The same amount of a LH3 N-terminal fragment with GGT activity was used as a control. We treated HT-1080 cells with a purified 30 kD LH3 N-terminal fragment deficient in the glycosyltransferase activities (the DXD fragment) by adding the fragment (0–4 μg/ml) into the medium. Our preliminary experiments revealed that the effect was concentration dependent, a 3–4 μg/ml concentration of the DXD fragment arresting cell growth already after 24 hrs incubation (see later in Fig. 5A), 3 μg/ml reducing the cell number by 12% and 4 μg/ml by 34%, when compared to the LH3 N-terminal fragment. A fragment concentration of 3 μg/ml was used in further studies.
5.
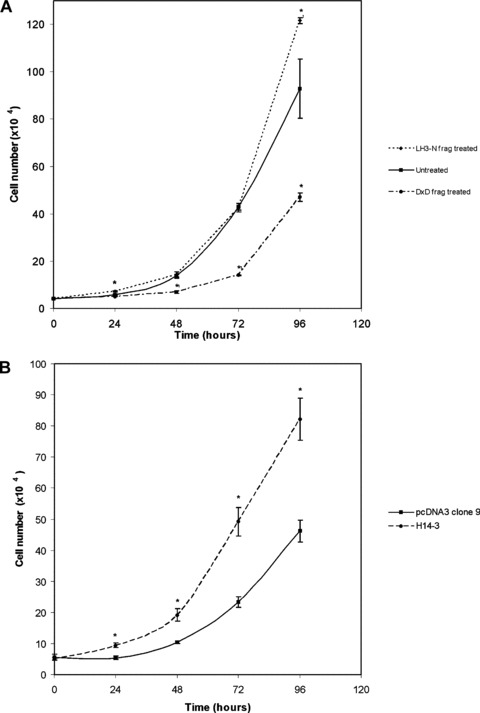
The effect of the glycosyltransferase activity of LH3 on HT-1080 cell proliferation. The cells were grown under the conditions described in experimental procedures. Student's t-test (one-tailed) was used for the statistical analysis, P< 0.05 was taken as a significant change. The changes of cell numbers deviating significantly from control cells are indicated by stars. (A) Extracellular effect in the presence of the wild-type LH3 N-terminal fragment or its glycosyltransferase-deficient form (DXD fragment) in cell medium. Untreated cells were used as controls. Significant inhibition of cell proliferation was observed with the DXD fragment-treated cells. (B) Intracellular effect by stably overexpressing full-length LH-deficient LH3 (clone H14-3). Remarkable acceleration of cell growth was seen, compared to the pcDNA3 vector (clone 9) trans-fected cells, when the glycosyltransferase activities of LH3 (not LH activity of LH3) increased in HT-1080 cells (clone H14-3).
We first determined whether the LH3 N-terminal fragment and/or its DXD mutated form bound to the cell surface when added to the cell medium. HT-1080 cells were incubated with the fragments (3 μg/ml) for 3 hrs at 37°C. The fragments remaining in the medium served as a molecular weight control. The cell lysate and the cell pellet were collected after sonication and centrifugation. The his-tagged proteins in lysate and medium were concentrated by Ni-NTA column. All fractions were re-suspended in a SDS sample loading buffer, and analysed by Western blotting with the antibody hLH3N1 against the purified human LH3 N-terminal fragment (Fig. 3A). The antibody detected a major 30 kD band in the cell pellet fraction, indicating that the fragments were bound to the surface of the HT-1080 cells (Fig. 3A, lane 1–3). The same-sized bands in medium confirmed that similar amounts of both fragments were used in the experiment (Fig. 3A, lane 4–6). A faint band was also observed in the cell lysate after Nickel purification, indicating that a trace amount of the fragment was internalized in the cell (Fig. 3A, lane 7–9) and suggesting that endocytosis is associated with the phenomenon. Both fragments, mutated and wild-type, behaved similarly. Furthermore, immuno-fluorescence stainings of non-permeabilized HT-1080 cells treated with the DXD fragment and with the LH3 N-terminal fragment revealed an overlay of the LH3 staining with the cell surface marker, so confirming that the fragments were bound to the cell surface (Fig. 3B, F, I).
3.
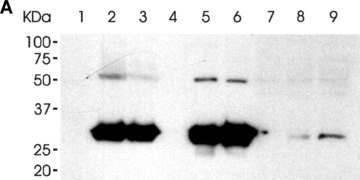
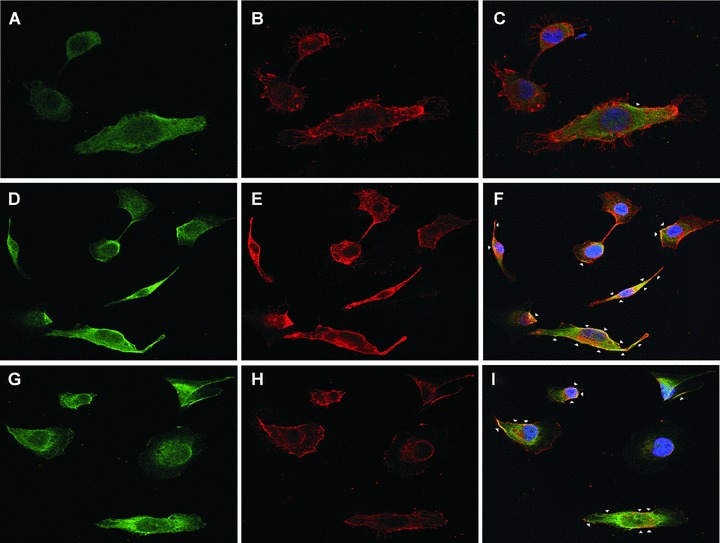
The binding of the LH3 N-terminal fragment and its glycosyltransferase-deficient counterpart on HT-1080 cell surfaces. (A) Western blot analysis after incubation with the hLH3N1 antibody: lanes 1–3 are cell pellets from untreated cells, DXD fragment treated cells and wild-type LH3 N-terminal fragment treated cells, respectively, demonstrating that both fragments (30 kD) bound to cell membranes; lanes 4–6 are medium samples purified on a Nickel column from the untreated cells, DXD fragment-treated cells and LH3 N-terminal fragment-treated cells, respectively, showing the same amount of the fragments (30 kD) used in all experiments. Lanes 7–9 are cell lysates purified on a Nickel column from untreated cells, DXD fragment treated cells and LH3 N-terminal fragment-treated cells, indicating trace amount of the fragments (30 kD) taken into the cells. (B) Immunofluorescence staining of non-permeabilized cells (A–C: untreated; D–F: DXD treatment; G–I: LH3N treatment) by the affinity-purified PLOD3 antibody (green colour, A, D and G), Wheat germ agglutinin (cell surface marker, red colour, B, E and H), and Hoechst 33258 (nuclei marker, blue colour). Parts C, F and I show the overlapping of the LH3 staining with the WGA staining on the cell surface (yellow colour, indicated by arrowheads). Untreated cells (C) are used as a background control, showing a small amount of endogenous LH3 on the cell surface. The cells treated with the DXD fragment (F) or the LH3N fragment (I) show much more overlap of the LH3 with the WGA, indicating that both fragments are bound to the cell surface.
As seen in Figs 4 and 5A, the DXD fragment in HT-1080 cell medium affected the cells very rapidly inducing cell stretching (Fig. 4) as well as a significant inhibition of cell proliferation. The number of cells in DXD treated plates was significantly lower when compared with untreated cells (Fig. 5A). As seen in Fig. 5A, treatment of HT-1080 cells with the wild-type N-terminal fragment of LH3 (3 μg/ml of the purified fragment) showed a tendency to stimulate cell proliferation. In order to confirm that the glycosyltransferase activities, not LH activity, of LH3 are responsible for stimulating cell proliferation, we overexpressed the full-length LH-deficient LH3 (clone H14–3) in HT-1080 cells, and compared the cell growth with the vector transfected cells (clone 9). The data given in Fig. 5B indicate that overexpression of the glycosyltransferase activities in the cells cause an increase in cell proliferation. Our additional data (not shown) demonstrate that overexpressing LH-deficient LH3 in cells produces a marked increase of LH-defi-cient LH3 protein and GGT activity in the cell medium. Taken together, the data suggest that the glycosyltransferase activities of LH3 promote cell proliferation, and the presence of LH3 glycosyltransferase activities in the extracellular space is required for cell growth and viability.
4.
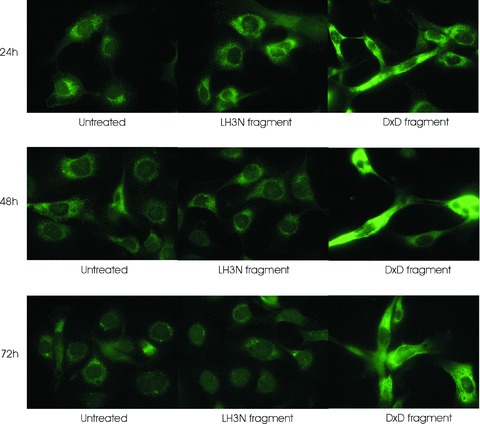
Visualization of the permeabilized cell bodies by type IV collagen staining after incubation of HT-1080 cells with media containing the LH3 N-terminal fragment with (LH3N fragment) or without (DXD fragment) glycosyltransferase activities. The cells were incubated for 24, 48 and 72 hrs with the fragments before the staining. The morphological change of the cells treated with DXD fragment occurred within 24 hrs and remained the same during the treatment; whereas no change was observed in untreated cells or cells treated with the active LH3N fragment.
To test whether the reduction of cell proliferation caused by the DXD fragment of LH3 was due to arrest of cell division at a specific stage in the cell cycle, we analysed the DNA content by flow cytometry. There was, however, no obvious difference (not shown) in DNA content between the untreated cells and those incubated with the LH3 N-terminal fragment or the DXD fragment in medium.
Actin filaments and microtubules are involved in the cell morphology change after the addition of the glycosyltransferase-deficient LH3 fragment in the culture medium
Actin and vimentin are the most widely distributed proteins to form cytoskeletal networks in cells. The networks are frequently associated with microtubules. The integrins are the cell surface receptors that are the main mediators in cell-extracellular matrix adhesions. The cytoplasmic domains of integrins interact with actin filaments. Therefore remodeling of the actin cytoskeleton represents a key element of the response to extracellular stimuli. Microtubules also play a crucial role in many cellular functions, including maintenance of cell shape, cell signalling and cell division. Different microtubule-associated proteins (MAPs) influence the assembly and stability of microtubules and the association of microtubules with other cell structures. MAPs are targets of many extracellular signals, participating in many signal transduction pathways and their binding to microtubules being regulated by phosphorylation.
In an attempt to establish whether cell morphology change is due to the disorganization of the cytoskeletal components in cells, we examined the cytoskeleton, a dynamic structure that maintains cell shape, enables cellular motion and plays important roles in both intracellular transport and cellular division. The organization of actin, tubulin and vimentin, the major types of protein filaments mechanically supporting cellular membranes, was studied in HT-1080 cells by using immuno-fluorescence staining after the treatment of the cells by addition of the DXD fragment or the LH3 N-terminal fragment to the cell culture medium. Our results showed that the vimentin cytoskeleton remained unchanged in the treated cells compared to the controls (Fig. 6C). However, actin networks underwent a clear re-organization following the DXD treatment (Fig. 6A). The filopodia, the rod-like cell surface projections filled with bundles of parallel actin filaments, were severely disrupted in the DXD fragment-treated cells with almost no cell–cell contact seen at the cell periphery; whereas, the protrusions were well-developed in the untreated controls and the LH3N fragment treated cells (Fig. 6A). As shown in Fig. 6B, the overall architecture of the microtubule network was not considerably altered. However, brighter perinuclear tubulin staining around the centrosome, with much less staining extending towards the filopodia, was observed in the DXD-treated cells than in the untreated controls and in the LH3N fragment-treated cells, thus confirming the lack of cell–cell contacts observed in the F-actin staining. Thus, the data clearly revealed cytoskeletal changes in the HT-1080 cells after the DXD fragment had been added to the cell culture medium, suggesting that LH3-associated glycosyltransferase activity is an important extracellular factor in maintaining cell shape, in controlling cell migration and in regulating cell-extracellular matrix adhesions.
6.
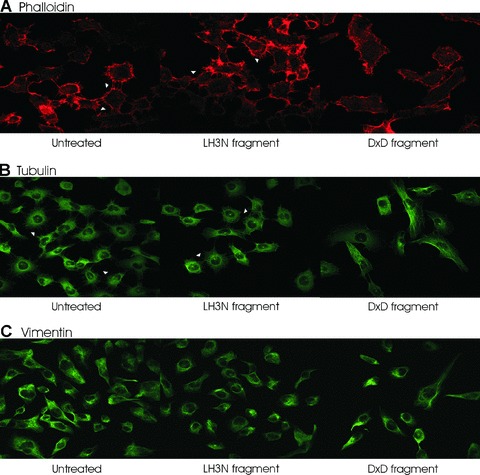
Cytoskeleton protein staining of HT-1080 cells after treatment of the LH3 N-terminal fragment with (LH3N fragment) or without (DXD fragment) glycosyltransferase activities. (A) Filamentous actin was visualized by Alexa Fluor 568 phalloidin (red). The filopodia indicated by arrowheads were well-developed in the control and the LH3N fragment treated cells, whereas the filopodia protrusions at the cell periphery were severely disrupted in the DXD fragment treated cells. (B) The overall architecture of the microtubule network was not considerably altered. However, brighter perinuclear tubulin staining around the centrosome, with much less staining extending towards the filopodia, was observed in the DXD-treated cells than in the untreated controls and in the LH3N fragment treated cells (indicated by arrowheads). (C) No obvious difference of vimentin staining was seen in the treated cells compared to the controls.
The morphological change caused by the DXD fragment in the extracellular space is a reversible process and dependent on cell type
To examine whether the change in cell morphology caused by treatment with the DXD fragment of LH3 can be reversed, we incubated the DXD treated HT-1080 cells for 24–72 hrs either with normal culture medium or with medium containing the LH3 N-terminal fragment (3 μg/ml of the purified fragment). Interestingly, after the DXD fragment was withdrawn from the medium, the morphology of the cells gradually returned to normal and the cells started to grow and divide. The morphology of most of the cells was normal 24 hrs after removal of the DXD fragment of LH3, and no abnormal, stretched cells were observed at 48 hrs (not shown). The addition of the LH3 N-terminal fragment with glycosyltransferase activities to the medium did not accelerate the process, compared to cell medium without any additions. We also carried out a competition experiment by adding LH3 N-terminal fragment to the medium simultaneously with the DXD fragment. When the fragments were added in equal amounts (3 mu;g/ml) to the medium, the LH3 N-terminal fragment did not prevent the morphology change caused by the DXD fragment. More studies are required to study whether the fragments are bound to different partners, have different binding affinities or whether the DXD fragment has a higher impact on cell behaviour than the LH3 N-terminal fragment.
In order to see if the DXD fragment of LH3 has a similar effect on various cultured cells, we treated other cell lines with the DXD fragment in culture medium (3 μg/ml of the purified fragment). As seen in Fig. 7A, for 293 cells, the DXD treatment clearly prevented cell proliferation and changed the cell morphology within 48 hrs, whereas only a very mild effect, if any, was detected for adult lung fibroblasts (Fig. 7B), adult skin fibroblasts and the osteosarcoma cells (not shown). The results indicate that certain cells are more sensitive to the treatment than others, revealing some cell type specificity.
7.
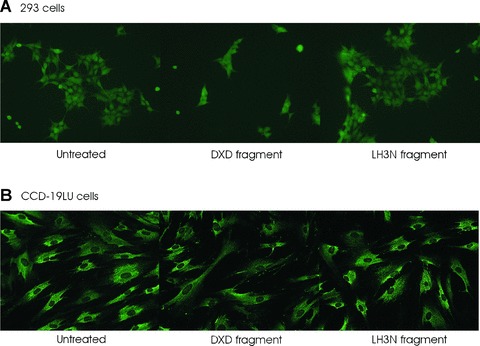
The effect of the LH3 N-terminal fragment and the DXD fragment on 293 and CCD-19LU cells. The cells were incubated with the fragments for 48 hrs. (A) Permeabilized 293 cells were stained with type IV collagen antibody. Cell morphology changes and growth arrest were observed in the DXD fragment treated cells. (B) Permeabilized CCD-19LU cells were stained with pro-collagen I antibody. No obvious change was observed.
Disturbance of the glycosyltransferase activities of LH3 inside the cell results in cell death
In order to confirm that a lack of glycosyltransferase activity of LH3 is responsible for cell growth arrest, we also investigated the effects of disturbing GGT activities inside the cells. We were not able to establish stably transfected cell lines overexpressing glycosyltransferase-deficient LH3, but we have data from transfected cells under constant selection pressure. The cells transfected with glycosyltransferase-deficient LH3 grew as a pool of cells, and, as shown earlier (Fig. 1A), they overexpressed glycosyltransferase-deficient LH3 under constant selection pressure. Immunofluorescence staining using an anti-polyhistidine antibody showed that the morphology of these cells was altered after about 1 month of transfection. They were much more stretched and required cell-cell attachment, which are features of stressed cells (Fig. 8C). Cells transfected with the empty vector or other constructs (wild-type LH3 or LH-deficient mutant) grew and divided normally (Fig. 8A, B and D). As described above RNAi was also used to disturb the glycosyltransferase activity of LH3 intracellularly. After being cotransfected with the three LH3 siRNA expression constructs in pRNAU6.1/Neo, cells showed growth retardation, an abnormal rounded shape as described above (Fig. 2B) and finally cell death, but after being transfected with the empty pRNAU6.1/Neo vector, cells grew normally with no obvious morphological change. The same experiments were carried out in Cos7 cells, and resulted in similar findings (not shown).
8.
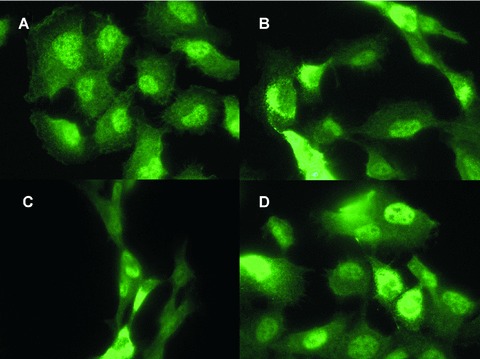
Immunofluorescence staining of His-tag proteins in permeabilized HT-1080 cells overexpressing full-length LH3 in native or mutated forms. Human LH3 and mutant LH3s were overexpressed using a pcDNA3 expression vector. The cells were constantly under selection pressure with 750 μg/ml G418 for one month, then stained by His-tag antibody. (A) pcDNA3 control. (B) Full-length human LH3 transfected cells. (C) Glycosyltransferase-deficient LH3 transfected cells. (D) LH-deficient LH3 transfected cells. A change in cell morphology was observed when the glycosyltransferase-deficient LH3 was overexpressed in the cells (C). Some non-specific staining was seen, as in A with His-tag antibodies.
These results indicate the importance of the glycosyltransferase activities of LH3 for cell growth and viability. The overexpression of glycosyltransferase-deficient LH3 intracellularly and the application of the mutated LH3 N-terminal fragment (the DXD fragment) extracellularly caused similar consequences: growth retardation, morphological change and cell death. However, a delay in the appearance of growth retardation was evident when glycosyltransferase-deficient LH3 was inside the cells.
Discussion
In this study, we explored the role of LH3, in particular its glycosyltransferase activities in the extracellular space of cultured HT-1080 cells. The data from the cells provide direct evidence that LH3 is able to hydroxylate lysyl residues and further glycosylate hydroxylysyl residues in these cells. HT-1080 cells synthesize mainly type IV collagen [34, 35], in which hydroxylysyl residues are highly glycosylated, about 75% in α1(IV) [11]. The increase of hydroxylysine and glycosylated hydroxylysines caused by overex-pression of LH3 is quite small, which is probably explained by the fact that type IV collagen in HT-1080 cells has already been highly hydroxylated and glycosylated, nearly reaching the saturating limit of modifications in collagens. Importantly, knock-down of the LH3 gene by the LH3 siRNA resulted in a great reduction of GGT and GT activities in HT-1080 cells compared to the control. Thus the results suggest that LH3 functions in vivo, not only as lysyl hydroxylase but also as both glycosyltransferases (GGT and GT). This finding confirms our recent data [40] showing that LH3 knock-out cells produce type I, IV and VI collagens that lack all the hydroxylysine-linked disaccharides, revealing that other cellular galactosyltransferases and glucosyltransferases are not able to compensate for the function of LH3 in ER.
Our current study of the extracellular role of LH3, especially the glycosyltransferase portion of the molecule, revealed it as important to cell growth and survival. The overexpression of a glycosyltransferase-deficient mutant, but not the full-length LH3 or the LH-deficient mutant, and knock-down of the LH3 gene by siRNA resulted in abnormal cell morphology, growth retardation and cell activity, is necessary for HT-1080 cell survival. Furthermore, our data indicate that morphology changes and cell lethality occur very quickly after adding glycosyltransferase-deficient LH3 N-terminal fragment to the extracellular space. Similar results were observed in transformed embryonic kidney cells. It is remarkable that the cell morphology of the LH3 siRNA-expressing cells differed from those treated extracellularly with the glycosyltransferase-deficient LH3 fragment, suggesting that the cells may be implementing different mechanisms under different treatment conditions. It is also possible that in the LH3 siRNA transfection, the GT/GGT activities were reduced too much within a very short time with the result that there was no time for the cells to develop the stretched shape (a sign of cells under stress) as seen in the DXD-treated cells or in those over-expressing the glycosyltransferase-deficient mutant intracellularly.
The cells recovered if the glycosyltransferase-deficient fragment was removed from the medium, revealing that the phenomenon is reversible. The addition of the glycosyltransferase-deficient fragment to the extracellular space of some other cell lines has only a mild effect, if any, on cell behaviour, suggesting cell membrane structure, probably receptor composition, may affect the phenomenon. Interestingly, the most noticeable changes were observed in transformed cells, this possibly indicating LH3 as a potential target of cancer. The morphology change occurred in 1 day in HT-1080 cells, if the glycosyltransferase-deficient LH3 fragment was added to the extracellular space, whereas additional days were required, if the full-length mutant was overexpressed intracellularly. The delay in the overexpressed situation can be explained by the fact that some time is needed to synthesize and secrete the mutated LH3 into the cell medium.
The presence of carbohydrates linked to hydroxylysyl residues is a unique feature of collagens and the proteins with collagenous sequences. However, the function of the sugar moieties and the factors which determine the level of glycosylation remain elusive. It was reported that the urinary excretion levels of hydroxylysine glycosides, especially GH, in osteoporotic patients were indirectly related to fractures in bone in osteoporosis [37]. Studies on fibrillar collagens have indicated that the hydroxylysyl-linked sugar units in collagens may play a role in the control of organization of the fibrils [38, 39]. Our recent analysis [40] from LH3 knock-out cells revealed that hydroxylysyl glycosylation is required for secretion of type IV collagen thus explaining the lack of type IV collagen in knock-out embryos. In addition, our data indicated that unglycosylated type VI collagen cannot form tetramers inside the cell, hydroxylysyl glycosylation therefore affects type VI collagen oligomerization and secretion. In addition, some specific hydroxylysine glycosylations are needed for the correct distribution of type VI collagen and probably for the formation or interactions of microfibrils [40]. It has also been reported that melanoma cell CD44 interaction with the a1(IV) 1263-1277 region from basement membrane collagen is regulated by ligand glycosylation. A marked reduction in cell adhesion and spreading was observed due to the presence of the single galactose residue in the α1(IV) sequence, suggesting significant biological consequences of even subtle changes in collagen carbohydrate content [41]. The metastatic process involves a coordinated series of tumour cell actions, including adhesion to and migration on extracellular matrix components and invasion of the basement membranes. Such interactions may be mediated by a great variety of cell surface biomolecules, including integrins. The α3βP1 integrin from two tumour cell types (melanoma and ovarian carcinoma) has been shown to bind directly to a glycosylated region within type IV collagen [41, 42].
Currently nine members in the family of collagenous transmembrane proteins have been characterized [43]. They probably all act as membrane-bound receptors. Therefore the hydroxylysine-linked carbohydrates in their collageous sequences may have a regulatory role in their function as well as in their association with ligands. However, no data have been reported so far concerning the glycosylation of hydroxylysine residues in these molecules. Our recent data indicate that LH3 is localized to the ER as well as to the extracellular space including the cell surface. Cell surface localization was confirmed by the LH3 N-terminal fragments used in this study. Furthermore, we have indicated that LH3 is able to modify lysyl residues of extracellular proteins in their native, non-denatured state, implying that LH3 may have a widespread regulatory function in tissues [25]. The multi-functionality and intra- as well as extracellular localization of LH3 in vivo may enable the molecule to play roles on cell surfaces or in the extracellular space not only in modulating the post-transla-tional modifications of lysyl residues in a dynamic way but also in regulating cell behaviour via cell-matrix interactions or receptor activation. It is also possible that the LH3 glycosyltransferase moiety is functioning as a coreceptor, controlling receptor clustering and higher order oligomerization, similar to the kinase domain for epidermal growth factor receptors [44]. It is known that the receptor chains have to be arranged in the correct orientation with respect to each other for signalling to occur. Our results obtained from cytoskeleton stainings further support the possible role of LH3 in receptor activation or receptor clustering followed by a signalling pathway. Our data also reveal a re-modelling of the actin filaments and microtubules with corresponding lack of well-developed filopodia in HT-1080 cells after binding of the DXD fragment to the cell membrane, a re-modelling not found in the untreated cells or the cells treated with the active LH3 N-terminal fragment. It should be noted that under normal growing conditions LH3 is produced endogenously in the HT-1080 cells and secreted into the extracellular space with a big part of this protein bound to the cell surface [25]. By adding LH3 N-terminal fragment to the cell culture medium, we merely overstimulated the in vivo condition, whereas by adding the DXD fragment we changed the in vivo condition by dramatically reducing the LH3 glycosyltransferase activities on the cell membrane. The binding of the added fragments is probably associated with endocytosis as a band corresponding to the size of the fragment in the cell lysate fraction was observed on immunoblot stained with antibody against the purified LH3 N-terminal fragment. Intriguingly, both fragments behaved similarly in the staining. Precise regulation of the cytoskeleton is required for diverse cellular processes such as changes in cell shape, proliferation, adhesion, migration and polarity, and alterations in the organization, distribution and dynamics of cytoskeletal proteins mediate these processes. It is known that several extracellular factors trigger signalling cascades that modulate the cytoskeleton. In addition, it is known [45] that endocytosis regulates many cellular signalling processes by controlling the number of functional receptors available at the cell surface. Conversely, some signalling processes regulate the endocytic pathway.
Partners for the LH3 glycosyltransferase moiety on the cell surface are not known, but our data clearly indicate that binding to the cell surface occurs after the addition of the LH3 N-terminal fragment or its glycosyltransferase-deficient counterpart to the cell medium. The addition of these fragments to the cell medium affects cell behaviour, leading to arrest of cell growth and further to cell lethality if the fragment is glycosyltransferase-deficient, and leading to stimulation of cell proliferation if the fragment contains LH3 glycosyltransferase activities. These findings indicate that cell growth is dependent on the LH3 glycosyltransferase activities in the extracellular space. More studies are required to analyse the exact mechanisms or possible signalling events behind the phenomena. The results are highly significant because they demonstrate for the first time that LH3 dependent glycosylation in the extracellular space influences cell behaviour. These results also propose LH3 as a potential target for medical applications, such as cancer therapy. This therapeutic targeting could be achieved as shown here, either by generating DXD fragments of LH3, or by generating antibodies against LH3, or by developing inhibitory compounds and targeting them at tumour tissues to prevent LH3 glycosyltransferase activities.
Acknowledgments
This work was supported by grants from the Research Council for Biosciences and the Environment within the Academy of Finland, a grant from the Sigrid Juselius Foundation and a grant from the Biocenter Oulu. We thank Mervi Matero and Tanja Schroderus for expert technical assis-tance. Maija Risteli, Päivi Pirilä and Juha Risteli are gratefully acknowledged for their help in LH activity measurements, Circular dichroism spectroscopy and amino acid analysis. Kaisa-Leena Tulla is acknowledged for expert HPLC analysis. We thank Antti Salo for providing the hLH3N1 antibody, and Sakari Kellokumpu is acknowledged for fruitful discussions.
References
- 1.Reichenberger E, Olsen BR. Collagens as organizers of extracellular matrix during morphogenesis. Cell Dev Biol. 1996;7:631–8. [Google Scholar]
- 2.Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 3.Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke G, Yancopoulos GD. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1:25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- 4.Kadler KE, Baldock C, Bella J, Boot-Handford RP. Collagens at a glance. J Cell Sci. 2007;120:1955–8. doi: 10.1242/jcs.03453. [DOI] [PubMed] [Google Scholar]
- 5.Säderhäll C, Marenholz I, Kerscher T, Rüschendorf F, Esparza-Gordillo J, Worm M, Gruber C, Mayr G, Albrecht M, Rohde K, Schulz H, Wahn U, Hubner N, Lee YA. Variants in a novel epidermal collagen gene (COL29A1) are associated with atopic dermatitis. PLoS Biol. 2007;5:1952–61. doi: 10.1371/journal.pbio.0050242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kivirikko KI, Myllylä R, Pihlajaniemi T. Hydroxylation of proline and lysine residues in collagens and other animal and plant proteins. In: Harding JJ, Crabbe MJC, editors. Post-translational modifications of proteins. Boca Raton: CRC Press; 1992. pp. 1–51. [Google Scholar]
- 7.Kielty CM, Hopkinson I, Grant ME. Collagen: the collagen family: structure, assembly and organization in the extracellular matrix. In: Royce PM, Steinmann B, editors. Connective tissue and its heritable disorders. New York: Wiley-Liss; 1993. pp. 103–47. [Google Scholar]
- 8.Kadler K. Synthesis and degradation of collagen. In: Sheterline P, editor. Protein profile. London: Academic Press; 1994. pp. 525–34. [Google Scholar]
- 9.Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–34. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 10.Ayad S, Boot-Handford R, Humphries MJ, Kadler KE, Shuttleworth CA. The extracellular matrix facts book. 2nd ed. London: Academic Press; 1998. [Google Scholar]
- 11.Kivirikko KI, Myllylä R. Collagen glycosyltransferases. Int Rev Connect Tissue Res. 1979;8:23–72. doi: 10.1016/b978-0-12-363708-6.50008-4. [DOI] [PubMed] [Google Scholar]
- 12.Kivirikko KI, Pihlajaniemi T. Collagen hydroxylases and the protein disulfide isomerase subunit of prolyl 4hydroxylases. Adv Enzymol Related Areas Mol Biol. 1998;72:325–98. doi: 10.1002/9780470123188.ch9. [DOI] [PubMed] [Google Scholar]
- 13.Valtavaara M, Papponen H, Pirttilä AM, Hiltunen K, Helander H, Myllylä R. Cloning and characterization of a novel human lysyl hydroxylase isoform highly expressed in pancreas and muscle. J Biol Chem. 1997;272:6831–4. doi: 10.1074/jbc.272.11.6831. [DOI] [PubMed] [Google Scholar]
- 14.Valtavaara M, Szpirer C, Szpirer J, Myllylä R. Primary structure, tissue distribution, and chromosomal localization of a novel isoform of lysyl hydroxylase (lysyl hydroxylase 3) J Biol Chem. 1998;273:12881–6. doi: 10.1074/jbc.273.21.12881. [DOI] [PubMed] [Google Scholar]
- 15.Passoja K, Rautavuoma K, Ala-Kokko L, Kosonen T, Kivirikko KI. Cloning and characterization of a third human lysyl hydroxylase isoform. Proc Natl Acad Sci USA. 1998;95:10482–6. doi: 10.1073/pnas.95.18.10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruotsalainen H, Sipilä L, Kerkelä E, Pospiech H, Myllylä R. Characterization of cDNAs for mouse lysyl hydroxylase 1, 2 and 3, their phylogenetic analysis and tissue-specific expression in the mouse. Matrix Biol. 1999;18:325–9. doi: 10.1016/s0945-053x(99)00016-5. [DOI] [PubMed] [Google Scholar]
- 17.Yeowell HN, Walker LC. Tissue specificity of a new splice form of the human lysyl hydroxylase 2 gene. Matrix Biol. 1999;18:179–87. doi: 10.1016/s0945-053x(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 18.Mercer DK, Nicol PF, Kimbembe C, Robins SP. Identification, expression, and tissue distribution of the three rat lysyl hydroxylase isoforms. Biochem Biophys Res Commun. 2003;307:803–9. doi: 10.1016/s0006-291x(03)01262-2. [DOI] [PubMed] [Google Scholar]
- 19.Schneider VA, Granato M. The myotomal diwanka (lh3) glycosyltransferase and type XVIII collagen are critical for motor growth cone migration. Neuron. 2006;50:683–95. doi: 10.1016/j.neuron.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 20.Schneider VA, Granato M. Genomic structure and embryonic expression of zebrafish lysyl hydroxylase 1 and lysyl hydroxylase 2. Matrix Biol. 2007;26:12–9. doi: 10.1016/j.matbio.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myllylä R, Wang C, Heikkinen J, Juffer A, Lampela O, Risteli M, Ruotsalainen H, Salo A, Sipilä L. Expanding the lysyl hydroxylase toolbox: new insights into the localization and activities of lysyl hydroxylase 3 (LH3) J Cell Physiol. 2007;212:323–9. doi: 10.1002/jcp.21036. [DOI] [PubMed] [Google Scholar]
- 22.Heikkinen J, Risteli M, Wang C, Latvala J, Rossi M, Valtavaara M, Myllylä R. Lysyl hydroxylase 3 is a multifunctional protein possessing collagen glucosyltrans-ferase activity. J Biol Chem. 2000;275:36158–63. doi: 10.1074/jbc.M006203200. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Risteli M, Heikkinen J, Hussa AK, Uitto L, Myllylä R. Identification of amino acids important for the catalytic activity of the collagen glucosyltransferase associated with the multifunctional lysyl hydroxylase 3 (LH3) J Biol Chem. 2002;277:18568–73. doi: 10.1074/jbc.M201389200. [DOI] [PubMed] [Google Scholar]
- 24.Wang C, Luosujärvi H, Heikkinen J, Risteli M, Uitto L, Myllylä R. The third activity for lysyl hydroxylase 3: galactosy-lation of hydroxylysyl residues in collagens in vitro. Matrix Biol. 2002;21:559–66. doi: 10.1016/s0945-053x(02)00071-9. [DOI] [PubMed] [Google Scholar]
- 25.Salo AM, Wang C, Sipilä L, Sormunen R, Vapola M, Kervinen P, Ruotsalainen H, Heikkinen J, Myllylä R. Lysyl hydroxylase 3 (LH3) modifies proteins in the extracellular space, a novel mechanism for matrix remodeling. J Cell Physiol. 2006;27:644–53. doi: 10.1002/jcp.20596. [DOI] [PubMed] [Google Scholar]
- 26.Salo AM, Sipilä L, Sormunen R, Ruotsalainen H, Vainio S, Myllylä R. The lysyl hydroxylase isoforms are widely expressed during mouse embryogenesis, but obtain tissue- and cell-specific patterns in the adult. Matrix Biol. 2006;25:475–83. doi: 10.1016/j.matbio.2006.08.260. [DOI] [PubMed] [Google Scholar]
- 27.Ruotsalainen H, Sipilä L, Vapola M, Sormunen R, Salo AM, Uitto L, Mercer DK, Robin SP, Risteli M, Aszodi A, Fässler R, Myllylä R. Glycosylation catalyzed by lysyl hydroxylase 3 is essential for basement membranes. J Cell Sci. 2006;119:625–35. doi: 10.1242/jcs.02780. [DOI] [PubMed] [Google Scholar]
- 28.Rautavuoma K, Takaluoma K, Sormunen R, Myllyharju J, Kivirikko KI, Soininen R. Premature aggregation of type IV collagen and early lethality in lysyl hydroxylase 3 null mice. Proc Natl Acad Sci USA. 2004;28:14120–5. doi: 10.1073/pnas.0404966101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norman KR, Moerman DG. The let-268 locus of Caenorhabditis elegans encodes a procollagen lysyl hydroxylase that is essential for type IV collagen secretion. Dev Biol. 2000;227:690–705. doi: 10.1006/dbio.2000.9897. [DOI] [PubMed] [Google Scholar]
- 30.Moro LC, Modricky N, Stagni F, Vittur F, De Bernard B. High-performance liquid chromatographic analysis of urinary hydroxylysyl glycosides as indicators of collagen turnover. Analyst. 1984;109:1621–2. doi: 10.1039/an9840901621. [DOI] [PubMed] [Google Scholar]
- 31.Kellokumpu S, Suokas M, Risteli L, Myllylä R. Protein disulfide isomerase and newly synthesized procollagen chains form higher-order structures in the lumen of the endoplasmic reticulum. J Biol Chem. 1997;272:2770–7. doi: 10.1074/jbc.272.5.2770. [DOI] [PubMed] [Google Scholar]
- 32.Kivirikko KI, Myllylä R. Posttranslational enzymes in the biosynthesis of collagen: intracellular enzymes. Methods Enzymol. 1982;82:245–304. doi: 10.1016/0076-6879(82)82067-3. [DOI] [PubMed] [Google Scholar]
- 33.Myllylä R, Risteli L, Kivirikko KI. Assay of collagen-galactosyltransferase and collagen-glucosyltransferase activities and preliminary characterization of enzymic reactions with transferases from chick-embryo cartilage. Eur J Biochem. 1975;52:401–10. doi: 10.1111/j.1432-1033.1975.tb04008.x. [DOI] [PubMed] [Google Scholar]
- 34.Alitalo K, Vaheri A, Krieg T, Timpl R. Biosynthesis of two subunits of type IV procollagen and of other basement membrane proteins by a human tumor cell line. Eur J Biochem. 1980;109:247–55. doi: 10.1111/j.1432-1033.1980.tb04790.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang C, Valtavaara M, Myllylä R. Lack of collagen type specificity for lysyl hydroxylase isoforms. DNA Cell Biol. 2000;19:71–7. doi: 10.1089/104454900314582. [DOI] [PubMed] [Google Scholar]
- 36.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–3. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 37.Yoshihara K, Mochidome N, Hara T, Osada S, Takayama A, Nagata M. Urinary excretion levels of hydroxylysine glyco-sides in osteoporotic patients. Biol Pharm Bull. 1994;17:836–9. doi: 10.1248/bpb.17.836. [DOI] [PubMed] [Google Scholar]
- 38.Brinckmann J, Notbohm H, Tronnier M, Acil Y, Fietzek PP, Schmeller W, Müller PK, Batge B. Overhydroxylation of lysyl residues is the initial step for altered collagen cross-links and fibril architecture in fibrotic skin. J Invest Dermatol. 1999;113:617–21. doi: 10.1046/j.1523-1747.1999.00735.x. [DOI] [PubMed] [Google Scholar]
- 39.Notbohm H, Nokelainen M, Myllyharju J, Fietzek PP, Müller PK, Kivirikko KI. Recombinant human type II collagens with low and high levels of hydroxylysine and its glycosylated forms show marked differences in fibrillogenesis in vitro. J Biol Chem. 1999;274:8988–92. doi: 10.1074/jbc.274.13.8988. [DOI] [PubMed] [Google Scholar]
- 40.Sipilä L, Ruotsalainen H, Sormunen R, Baker NL, Lamande SR, Vapola M, Wang C, Sado Y, Aszodi A, Myllylä R. Secretion and assembly of type IV and VI collagens depend on glycosylation of hydroxylysines. J Biol Chem. 2007;282:33381–8. doi: 10.1074/jbc.M704198200. [DOI] [PubMed] [Google Scholar]
- 41.Lauer-Fields JL, Malkar NB, Richet G, Drauz K, Fields GB. Melanoma cell CD44 interaction with the alpha 1 (IV) 1263–1277 region from basement membrane collagen is modulated by ligand glycosylation. J Biol Chem. 2003;278:14321–30. doi: 10.1074/jbc.M212246200. [DOI] [PubMed] [Google Scholar]
- 42.Miles AJ, Knutson JR, Skubitz APN, Furcht LT, McCarthy JB, Fields GB. A pep-tide model of basement membrane collagen alpha 1 (IV) 531–543 binds the alpha 3 beta 1 integrin. J Biol Chem. 1995;270:29047–50. doi: 10.1074/jbc.270.49.29047. [DOI] [PubMed] [Google Scholar]
- 43.Franzke CW, Bruckner P, Bruckner-Tuderman L. Collagenous transmembrane proteins: recent insights into biology and pathology. J Biol Chem. 2005;280:4005–8. doi: 10.1074/jbc.R400034200. [DOI] [PubMed] [Google Scholar]
- 44.Clayton AHA, Tavarnesi ML, Johns TG. Unligated epidermal growth factor receptor forms higher order oligomers within microclusters on A431 cells that are sensitive to tyrosine kinase inhibitor binding. Biochemistry. 2007;46:4589–97. doi: 10.1021/bi700002b. [DOI] [PubMed] [Google Scholar]
- 45.Von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol. 2007;19:436–45. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


