Abstract
The existence of endothelial progenitor cells (EPC) with high cell-cycle rate in human umbilical cord blood has been recently shown and represents a challenging strategy for therapeutic neovascularization. To enhance knowledge for future cellular therapy, we compared the phenotypic, functional and gene expression differences between EPC-derived cells generated from cord blood CD34+ cells, and lymphatic and macrovascular endothelial cells (EC) isolated from human foreskins and umbilical veins, respectively. Under appropriate culture conditions, EPC developed into fully matured EC with expression of similar endothelial markers as lymphatic and macrovascular EC, including CD31, CD36, von Willebrand factor FVIII, CD54 (ICAM-1), CD105 (endoglin), CD144 (VE-cadherin), Tie-1, Tie-2, VEGFR-1/Flt-1 and VEGFR-2/Flk-1. Few EPC-derived cells became positive for LYVE-1, indicating their origin from haematopoietic stem cells. However they lacked expression of other lymphatic cell-specific markers such as podoplanin and Prox-1. Functional tests demonstrated that the cobblestone EPC-derived cells up-regulated CD54 and CD62E expression in response to TNF-α, incorporated DiI-acetylated low-density liproprotein and formed cord- and tubular-like structures with capillary lumen in three-dimensional collagen culture – all characteristic features of the vascular endothelium. Structures compatible with Weibel-Palade bodies were also found by electron microscopy. Gene microarray profiling revealed that only a small percentage of genes investigated showed differential expression in EPC-derived cells and lymphatic EC. Among them were adhesion molecules, extracellular matrix proteins and cytokines. Our data point to the close lineage relationship of both types of vascular cells and support the theory of a venous origin of the lymphatic system.
Keywords: stem cells, endothelial cell differentiation, lymphatic capillaries, angiogenesis, vasculogenesis
Introduction
Endothelial progenitor cells (EPC) are anticipated to propagate the formation of new blood vessels by promoting vasculogenesis and angiogenesis. The neovascular formation in adults has been considered to result exclusively from angiogenesis, a term defined as the sprouting of fully differentiated endothelial cells (EC) from pre-existing blood vessels, whereas vasculogenesis refers to the differentiation of EC from (hem)angioblasts during early embryogenesis [1]. The recent discovery of circulating EPC in human peripheral blood, however, led to the new concept that vasculogenesis can occur also during post-natal life [2].
Hitherto, EPC were obtained from various cellular sources, including bone marrow, foetal liver, umbilical cord blood and peripheral blood, albeit the number of haematopoietic stem cells in the peripheral circulation is very low [3–5]. The physiological role of circulating EPC is not yet clearly established. Based on the fact that the number of circulating EPC is inversely correlated with the risk factor for coronary artery disease, EPC are supposed to participate in vascular repair processes [6]. They are also known to play a critical role in tumour angiogenesis [7]. Hence, the use of EPC is a promising tool for the replacement of pathologically altered vessels in cardiovascular diseases as well as for targeted anti-angiogenic therapy of malignant tumours.
Angiogenesis and the growth of lymphatic vessels, lymphangiogenesis, are likely to involve similar processes, though formal evidence of this assertion has yet to be published. Studies on lymphatic ontogeny raised the hypothesis that the lymphatic system arises through the progressive sprouting of EC from embryonic veins [8]. Currently, there is much debate whether lymphatic progenitor cells may contribute to post-natal lymph vessel formation. In human beings, two potential candidates for lymphatic progenitor cells have been identified so far. Salven et al.[9] described the existence of circulating CD34+ progenitor cells in adults that co-express CD133 and VEGFR-3 and have the capacity to differentiate into mature lymphatic EC, while Schoppmann et al.[10] found evidence for CD14+VEGFR-3+CD31+VEGFR-2− monocytes of high developmental plasticity to participate in lymphangiogenesis.
Although the blood and the lymphatic vasculature operate in parallel and share anatomical properties, they display distinct structural and functional features. When compared with the blood vessel counterparts, the lymphatic capillaries are directly connected to the surrounding extracellular matrix through anchoring filaments and are lined by a single thin layer of overlapping and attenuated EC that lack a continuous basal membrane and pericyte coverage. Functionally, the lymphatic endothelium controls tissue fluid homeostasis by draining protein-rich lymph from tissues and organs and elicits an immune function by transporting antigens in a free form or associated with dendritic cells as carriers from the peripheral tissues to the lymphoid organs. Also, it serves as a major route for absorption of fat from the small intestine and provides a pathogenic pathway for tumour metastasis [8].
For future therapeutic use of EPC, further basic information is essential. In the present study, we aimed to increase knowledge of EPC-derived cells by comparing their phenotypic, functional and gene expression properties with those of human dermal lymphatic endothelial cells (HDLEC) and human umbilical vein endothelial cells (HUVEC). We have chosen human umbilical cord blood CD133+ CD34+ cells as EPC because they are easier to obtain both in a higher number and cell-cycle rate than it is the case for the isolation of haematopoietic progenitors from the adult peripheral blood.
Material and methods
Generation of EPC-derived cells from human umbilical cord blood
Human umbilical cord was obtained from the Department of Obstetrics and Gynaecology, Innsbruck Medical University, Austria, according to institutional guidelines. Samples were generally processed within 24 hrs of collection. Mononuclear cells were separated from umbilical cord blood by density gradient centrifugation using Lymphoprep™ (1.077 g/ml; Nycomed Pharma, Oslo, Norway). After centrifugation at 400 ×g for 30 min. at room temperature (RT), mononuclear cells were collected from the interface and washed twice in phosphate buffer solution (PBS) at 300 ×g for 8 min. at 4°C. Cells bearing CD34 antigen were then enriched from mononuclear fractions by a positive magnetic beads separation method following the manufacturer's instructions (‘CD34+ Progenitor Cell Isolation Kit’, now termed ‘Indirect CD34 MicroBead Kit’ Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, mononuclear cells were treated with an Fc-receptor-blocking agent and labelled with a mouse Ig anti-human CD34 antibody for 15 min. at 4°C. After washing in PBS containing 0.5% bovine serum albumin (BSA) and 2-mM EDTA (Cellgro, Mediatech, Inc., Hernon, VA, USA), the cells were incubated with microbeads conjugated to an anti-mouse antibody. Target cells were passed through a MiniMACS™ column in a magnetic field where they retained. The CD34+ fraction was recovered by releasing the magnetic field and by flushing the cells from the column. The purity of isolated CD34+ cells was generally greater than 90% as verified by flow cytometry using FITC-conjugated anti-CD34 monoclonal antibodies (mAbs). Freshly isolated CD34+ cells were immediately seeded onto tissue culture, pre-coated with 1% gelatin (Sigma Chemicals, St. Louis, MO, USA) and cultured in endothelial cell basal medium (EBM; Clonetics Corp., Walkersville, MD, USA), supplemented with 20% human serum, 5 ng/ml epidermal growth factor (EGF; Clonetics Corp.), 2-mM L-glutamine, 1 μg/ml hydrocortisone acetate, 5×10−5 M dibutyryl cyclic adenosine monophosphate (Sigma Chemicals), 100 U/ml penicillin, 100 μg/ml streptomycin, 250 μg/ml amphotericin B (all purchased from Irvine Scientific, Santa Ana, CA, USA) at 37°C with 5% CO2 in a humidified atmosphere. The following cytokines were added to the media: 10 ng/ml vascular endothelial growth factor (VEGF, BD Biosciences Pharmingen San Diego, CA, USA), 10 ng/ml basic fibroblast growth factor (bFGF Strathmann Biotec, Hamburg, Germany) and 25 ng/ml recombinant humanized stem cell factor (rhSCF, specific activity 5×105 U/mg; PeproTech, London, UK). At day one after plating, the non-adherent cells were removed and fresh EBM medium was applied with VEGF, bFGF and rhSCF in the required concentrations as mentioned above. To maintain optimal culture conditions, media were changed every third day. For morphological, immunophenotypic, functional and microarray analyses, EPC-derived cells at passage 3 (on the average at 8 weeks of culture) were used. Altogether, 20 EPC cultures were investigated.
In some experiments, EPC-derived cells were cultured in the presence of varying concentrations of TNF-α (100 U, 200 U and 500 U/ml) (PeproTech) for 4 and 24 hrs to stimulate expression of CD54 (ICAM-1) and CD62E (E-selectin).
Isolation and culture of HDLEC and HUVEC
HDLEC were isolated from surgically removed normal foreskins obtained from newborns and children up to 7 years old as described earlier [11]. The culture medium for both EC types was EBM (Clonetics Corp.), supplemented with 20% human serum, 5 ng/ml EGF (Clonetics Corp.), 2 mM L-glutamine, 1 μg/ml hydrocortisone acetate, 5×10−5 M dibutyryl cyclic adenosine monophosphate (Sigma Chemicals), 100 U/ml penicillin, 100 μg/ml streptomycin, and 250 μg/ml amphotericin B (Irvine Scientific). The resulting cultures were consistently pure without contaminating fibroblasts as assessed by morphological and immunologic criteria. HUVEC, a gift from Dr. G. Wick (Department of Experimental Pathophysiology and Immunology, Innsbruck Medical University, Austria), were isolated according to the standard method and were grown in EBM (Clonetics Corp.), supplemented with 20% human serum and with the additives noted above. HDLEC and HUVEC were propagated through passage 3.
Flow cytometric analysis of surface molecules on EPC-derived cells
Levels of expression of cell surface molecules on EPC-derived cells were assessed using flow cytometry and commercially available antibodies, which are listed in Table 1. EDTA-detached EPC-derived cells were harvested by centrifugation, washed in PBS containing 0.5% BSA (Boehringer Ingelheim, Germany) and incubated for 30 min. at 4°C with the appropriate diluted unconjugated or FITC-/PE-conjugated antibodies. For indirect fluorescent staining, EPC-derived cells were further incubated with FITC-/PE-labelled F(ab)2 fragments of sheep anti-mouse IgG for 30 min. For negative controls, EPC-derived cells were stained with the corresponding isotype-matched Igs. Cellular fluorescence was examined in a FACScalibur flow cytometer (Becton Dickinson, Mountain View, CA, USA).
1.
Monoclonal and polyclonal antibodies used for flow cytometry, immunofluorescence stainings and Western blotting
| Antibody | Clone | Isotype | Dilution | Source | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD14, PE-labelled | rmC5-3 | lgG1 | 1:5 | BD Pharmingen, San Diego, CA, USA | |||||||||
| CD31, PE-labelled | JC70A | lgG1 | 1:50 | Dako, Glostrup, Denmark | |||||||||
| WM-59 | lgGl | 1:5 | BD Pharmingen, San Diego, CA, USA | ||||||||||
| CD34, FITC-labelled | 581 | lgGl | 1:5 | BD Pharmingen, San Diego, CA, USA | |||||||||
| CD36, PE-labelled | SMO | lgM | 1:20 | Ancell Corp., Bayport, MN, USA | |||||||||
| CD54 (ICAM-1) FITC-labelled | HA58 | lgGl | 1:5 | BD Pharmingen, San Diego, CA, USA | |||||||||
| CD62E (E-selectin) FITC-labelled | 1.2B6 | lgGl | 1:25 | Dako, Glostrup, Denmark | |||||||||
| CD105 (endoglin) PE-labelled | 266 | lgGl | 1:20 | BD Pharmingen, San Diego, CA, USA | |||||||||
| CD133 FITC-labelled | AC133 | lgGl | 1:100 | Abcam Ltd., Cambridge, UK | |||||||||
| lgGl | 1:25 | Miltenyi Biotech, Bergisch Gladbach, Germany | |||||||||||
| CD144 (VE-cadherin) PE-labelled | 55-7H1 | lgGl | 1:25 | BD Pharmingen, San Diego, CA, USA | |||||||||
| β2-microglobulin PE-labelled | 246-E8.E7 | lgG2a | 1:25 | Neomarkers, Fremont, CA, USA | |||||||||
| HLA-I PE-labelled | G46-2.6 | lgGl | 1:5 | BD Pharmingen, San Diego, CA, USA | |||||||||
| HLA-II PE-labelled | G46-6 | lgG2a | 1:5 | BD Pharmingen, San Diego, CA, USA | |||||||||
| LYVE-1 | Polyclonal | Rabbit serum | 1:400 | DCS Hamburg, Germany | |||||||||
| Polyclonal | Rabbit serum | 1:100 | Fitzgerald Industries International Inc., Concord, MA, USA | ||||||||||
| Podoplanin | Polyclonal | lgG1 | 1:200 | Acris, Hiddenhausen, Germany | |||||||||
| gp36 | lgGl | 1:100 | Fitzgerald Industries International Inc., Concord, MA, USA | ||||||||||
| Prox-1 | Polyclonal | Rabbit serum | 1:200 | RELIA Tech, Braunschweig, Germany | |||||||||
| Ployclonal | Rabbit serum | 1:100 | Fitzgerald Industries International Inc., Concord, MA, USA | ||||||||||
| Tie-1 | Polyclonal | 1:200 | Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA | ||||||||||
| Tie-2 | Polyclonal | 1:200 | Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA | ||||||||||
| VEGFR-1/FU-1 | Polyclonal | 1:200 | Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA | ||||||||||
| VEGFR-2/Flk-1 | Monoclonal | 1:100 | Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA | ||||||||||
| VEGFR-3 | Monoclonal | 1:100 | Chemicon International, Temecula, CA, USA | ||||||||||
| Goat IgG | 1: 50 | RD Systems, Inc., Tustin, CA, USA | |||||||||||
| von Willebrand Factor (FVIII) | F8/86 | IgGl | 1:50 | Dako, Glostrup, Denmark | |||||||||
| Polyclonal | Rabbit serum | 1:100 | Dako, Glostrup, Denmark | ||||||||||
Immunostaining of EPC-derived cells on cytospin preparations
Cytospins with EPC-derived cells were air-dried for at least 24 hrs, fixed in acetone for 10 min. and immunostained for the expression of the following molecules: CD31, Willebrand factor FVIII (vWF), podoplanin Prox-1 and LYVE-1. In brief, slides were incubated in sequence with the primary antibody, biotinylated anti-mouse Ig (Amersham-Pharmacia; Amersham, UK) and Texas Red-conjugated Streptavidin (Amersham). After blocking of residual binding sites with an excess of mouse gamma globulin (100 μg/ml), sections were counterstained with the FITC-conjugated secondary antibody. Finally, cytospins were mounted in Vectashield (Vector, Burlingame, CA, USA) and viewed on a conventional fluorescence microscope.
Uptake of DiI-ac-LDL
To assess the ability of EPC-derived cells to incorporate ac-LDL, which is a characteristic function of cells in endothelial lineage, EPC-derived cells on gelatin-coated 8-chamber Lab-Tek slides were incubated in the culture medium containing 10 μg/ml DiI-Ac-LDL (Invitrogen/Molecular Probes Inc., Eugene, OR, USA) at 37°C for 24 hrs. After vigorous washing with PBS, EPC-derived cells were mounted in Vectashield (Vector, Burlingame) and fluorescence was visualized with a fluorescence microscope.
Endothelial network formation in Matrigel
For analysis of capillary tube formation, 150-μl Matrigel (Becton Dickinson, San Jose, CA, USA) was added into a 6-well plate and was allowed to solidify at 37°C for 30 min. 5×104 EPC-derived cells were suspended in 300 μl culture medium and plated onto Matrigel layer. Twenty-four hours later, the medium was removed and capillary tube formation of vessel-like tube in Matrigel was observed under an inverted microscope.
Transmission electron microscopy
EPC-derived cells were grown to confluence on gelatin-coated coverslips and fixed in a mixture of 2.5% glutaraldehyde and 2% formaldehyde, freshly prepared from paraformaldehyde, in 0.1-M cacodylate buffer at pH 7.4 for 15 min. at room temperature (i.e. half-strength Karnovsky's fixative). After washing in cacodylate buffer, the samples were post-fixed in OsO4 for 15 min., en bloc contrasted with 0.5% veronal-buffered uranyl acetate and dehydrated in ascending concentrations of ethanols followed by embedding in Epon 812 resin. Ultrathin sections were stained with lead citrate and viewed with a transmission electron microscope at 80 kV (Philips EM400, FEI Company, Eindhoven, The Netherlands).
Western blot analysis
Identical numbers of EPC-derived cells, HDLEC and HUVEC were lysed on ice in CelLytic™-M Mammalian Cell Lysis/Extraction Reagent (Sigma Chemicals) containing a protease inhibitor cocktail (Sigma Chemicals) and centrifuged at 14,000 rpm. The concentration of the protein was determined with ‘Protein Reagent’ (BioRad, Hercules, CA, USA). The supernatant was then mixed with 4× SSB containing 20%ß-mercaptoethanol and boiled. Samples were separated with SDS-PAGE on 7.5–10% polyacrylamide gels and transferred to nitrocellulose membranes (Whatman-Schleicher & Schuell, Dassel, Germany) by a NOVEX blotter apparatus (Invitrogen, Carlsbad, CA, USA). The membranes were blocked with PBS blocking buffer containing 0.1% Tween20 and 5% non-fat dry milk, incubated with primary antibodies specific for Tie-1, Tie-2, VEGFR-1/ Flt-1 VEGFR-2/Flk-1, podoplanin, Prox-1, VEGF-R3 (Table 1) and α-tubulin (Oncogene Research, Cambridge, MA, USA), washed and incubated with anti-mouse or anti-rabbit horseradish-peroxidase-conjugated secondary antibodies (GE Healthcare, Piscataway, NJ, USA). The blots were developed by enhanced chemoluminescence (GE Healthcare, USA) according to the manufacturer's instructions and analysed with the chemoluminescence system of ultraviolet laboratory products (UVP).
Microarray analysis
Sample preparation and microarray processing were conducted following the manufacturer's recommended protocols at the Tyrolean Cancer Research Institute, Innsbruck, Austria. Total cellular RNA was extracted from confluent third passage EPC-derived cells and HDLEC cultures (each n= 3) with Trizol Reagent (Invitrogen, Carlsbad, CA, USA) and RNeasy Mini Kit (Qiagen, Germantown, MD, USA). For gene expression profiling, probes were stained with streptavidin phycoerythrin (Invitrogen/Molecular Probes), hybridized to the Humane Genome U133 Plus 2.0 microarray and processed using GeneChip Fluidics Station 450 (Affymetrix GeneChip, Santa Clara, CA, USA). Arrays were scanned by the GeneChip Scanner 3000 (Affymetrix), and the readings from the quantitative scanning were analysed by Bioconductor version 2.4.1. Each EPC-derived cell array was normalized to the corresponding HDLEC array (in all 9 combinations), and to allow a reliable comparison of gene expression levels, EPC-derived cells and HDLEC arrays were also mutually normalized. As normalization method, the Robust multi-array average (RMA) was chosen. Genes that showed a more than twofold up- or down-regulation between the sample and control chip were filtered because data may not be credible for genes with low signal intensities. Otherwise, genes that were expressed in an equivalent manner in the EPC-derived cell- EPC-derived cell controls or HDLEC-HDLEC controls were excluded.
Results
Differentiation and morphology of EPC-derived cells from CD34+ cord blood progenitor cells
Initially, purified CD34+ cord blood progenitor cells were small and round. Within 24 hrs in culture with EBM containing VEGF, bFGF and rhSCF, a limited number of CD34+ cells promptly attached to gelatin-coated flasks. However, when non-adherent CD34+ cells were transferred to fresh gelatin-coated flasks, they did not become adherent. This phenomenon was also seen when flasks were not coated with gelatin. Small clusters of spindle-shaped EPC-derived cells were observed by 2–4 days of culture. Over the following 2–4 weeks, these elongated EPC-derived cells formed a confluent monolayer with cobblestone appearance similar to colonies obtained from HDLEC and HUVEC. Morphological analysis using light microscopy revealed a uniform cell population (Fig. 1). In the presence of 20% human serum alone without VEGF, bFGF and rhSCF, cord blood-derived EPC-derived cells failed to proliferate and died, indicating their dependency on exogenous growth factors.
1.
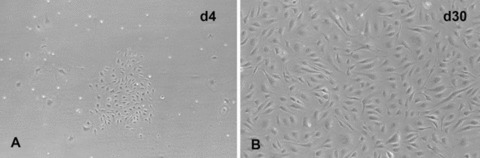
Differentiation of cord blood CD34+ progenitors into EPC-derived cells. Cluster formation of adherent cells was observed 4 days after plating (A) and confluent endothelial-like monolayers with characteristic cobblestone pattern were found at days 30 of culture (B). Outgrowth cell morphology was examined by phase contrast light microscopy. Original magnification: ×100.
Ultrastructurally, cells of variable sizes were found; some cells were extraordinarily large. They were often polarized in that a dense network of villi protruded from one pole of the cell. At the basis of these villi, micropinocytic vesicles could be detected. Sometimes they were abundant, thus resembling dermal EC in situ, but they were only found there in substantial numbers. The cells possessed large nuclei that were deeply indented one- or severalfold, similar to EC in situ, and contained many autophagic vacuoles filled with little membrane vesicles (reminiscent of mul-tivesicular bodies) and often large membrane whorls (myelinoid figures). Lipid droplets occasionally occurred, presumably a consequence of the long culture period. The cells were rich in ribosomes, both as free ribosomes and as ribosomes attached to the endoplasmic reticulum. Rarely, some stretches of rough endoplasmic reticulum were arranged in a parallel fashion, resembling ‘ergastoplasma’ as observed in plasma cells. Intermediate filaments were easily detected. Only rarely, they were arranged in the parallel, ‘spaghetti-like’ fashion as known from EC in situ. Unequivocal Weibel-Palade bodies were scarce, scarcer as compared to EC in situ. They could only be spotted in a fraction of all the section profiles (less than one in ten) (Fig. 2).
2.
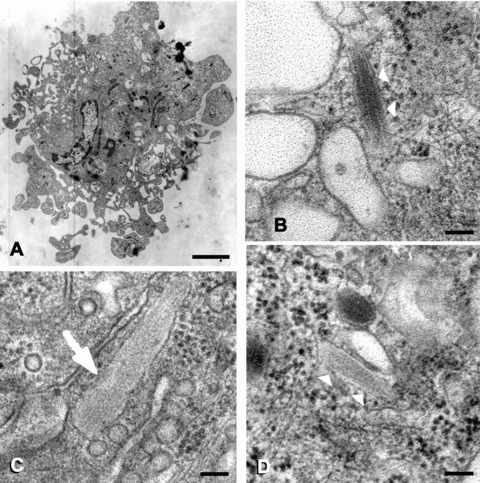
Weibel-Palade bodies in cord blood CD34+-derived EPC-derived cells. (B) and (D) show Weibel-Palade bodies in the cell that is depicted at low power in (A). Note the typical internal structure of the bodies. For comparison, (C) shows a Weibel-Palade body from a dermal EC in healthy human skin in situ. (B-D) are at the same magnification. Final magnifications: (A) ×6.200, (B-D) ×90.000; scale bars correspond to 2 μm in (A) and 100 nm in (B-D).
Phenotypic characterization of EPC-derived cells
In order to determine the phenotype of EPC-derived cells, we performed flow cytometry and immunohistochemistry analysis using a selected set of markers and compared it to that obtained in HDLEC and HUVEC. The cell surface expression of molecules is summarized in Figure 3 illustrating the major phenotypic differences between the EC types. By FACS analysis, EPC-derived cells were strongly positive for common endothelial markers such as CD31, CD54, CD105 and CD144. These results clearly indicate that the cord blood-derived CD34+ cells had undergone a complete EC differentiation process. In addition, EPC-derived cells were weakly positive for thrombomodulin and CD143 and failed to express CD36. Several cell surface endothelial markers were similarly expressed on HDLEC and HUVEC. Among them were CD31, CD54, CD105 and CD144. Other markers differentiated EPC-derived cells from the other EC types. Unlike EPC-derived cells, HDLEC abundantly expressed CD36, while CD36 was absent on HUVEC. In general, EPC-derived cell cultures did not contain monocytes or macrophages as attested by the absence of CD14+ cells. Differentiation into EC was further associated with the acquisition of HLA-class I antigens, but not HLA-class II antigens. In the line with this observation, EPC-derived cells efficiently expressed β2-microglobulin, which is the light chain of the HLA-class I antigen complex and was much more strongly expressed on HDLEC.
3.
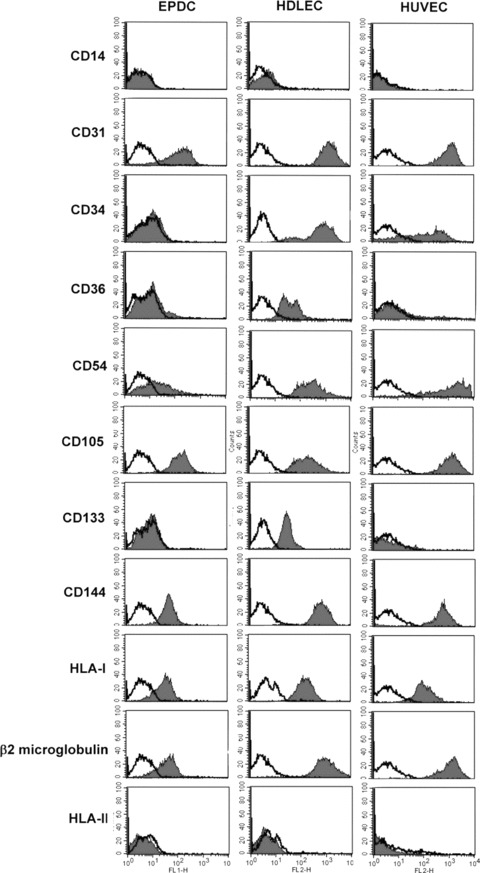
Comparative expression of surface markers in cultured EPC-derived cells, HDLEC and HUVEC by flow cytometric analysis. Cells were labelled with markers for EC, haematopoietic stem cells and monocytes. The black histograms outline the region of fluorescent intensity of the specific antibody and the white histograms that of the negative control antibody. These graphs are representative of five independent experiments.
Freshly isolated cord blood CD34+ cells expressed high levels of the progenitor cell marker CD133. During differentiation, the expression of CD133 and CD34 progressively decreased and was no longer detectable on terminally matured EPC-derived cells (Fig. 4). Interestingly, CD133 and CD34 were still present on the surface of HDLEC, although CD133 was detected at a significantly lower level than CD34 (Fig. 3). Contrarily, HUVEC showed weak expression of CD34 and loss of CD133 (Fig. 3).
4.
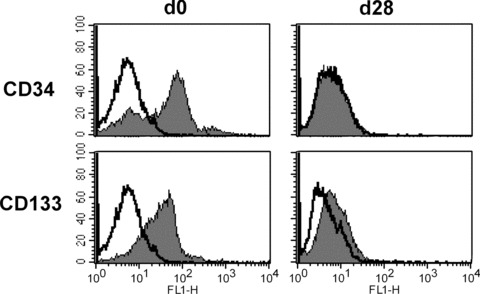
Expression of CD34 and CD133 on bone marrow-derived EPC during differentiation into EPC-derived cells. While freshly isolated bone marrow-derived EPC expressed high levels of CD34 and CD133, terminally matured EPC-derived cells were characterized by the loss of CD34 and CD133 expression.
To examine whether EPC-derived cells have phenotypes of lymphatic EC, EPC-derived cells were subjected to immunocytochemistry. Analyses on cytospins revealed that EPC-derived cells were positively stained for the panendothelial marker CD31 and vWF, but negatively stained for the lymphatic cell-specific markers podoplanin and Prox-1. Surprisingly, a minor subset of EPC-derived cells reacted with the lymphatic cell-specific marker LYVE-1, indicating their origin from haematopoietic stem cells (Fig. 5).
5.
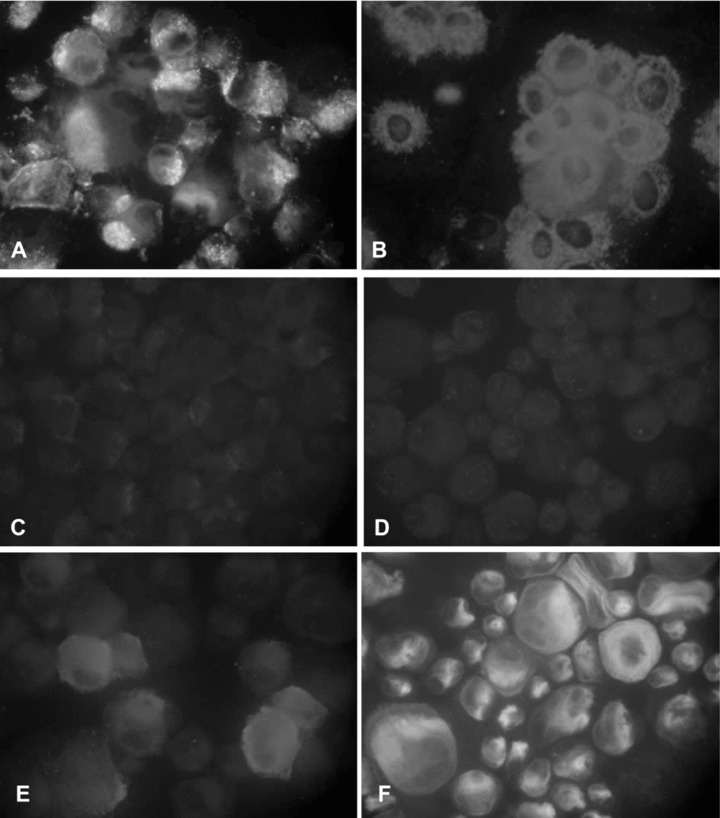
Immunofluorescence analysis of EPC-derived cells. Cells were positively stained for the panendothelial marker CD31 (A) and vWF (B), but negatively stained for the lymphatic cell-specific markers podoplanin and Prox-1 (C, D). Note that some few EPC-derived cells showed expression of the lymphatic cell-specific marker LYVE-1 (E, F). Original magnifications: (A, B, E, F) ×400, (B, C) ×200.
The endothelial phenotype was confirmed by Western blotting with antibodies specific for endothelial markers. Cultures of EPC-derived cells were extensively positive for Tie-1, Tie-2, VEGFR-1/ Flt-1 and VEGFR-2/Flk-1, whereas they lacked expression of podoplanin, Prox-1 and VEGFR-3. Conversely, these lymphatic cell-specific markers were observed in HDLEC (Fig. 6).
6.

Western blotting of cell lysates from EPC-derived cells at passage 3. EPC-derived cells expressed the endothelial markers Tie-1, Tie-2, VEGFR-1/ Flt-1 and VEGFR-2/Flk-1, but did not express the lymphatic cell-specific markers podoplanin, Prox-1 and VEGFR-3 (lane 1). HDLEC (lane 2) and HUVEC (lane 3) were used as controls. To demonstrate equal protein loading, the same blot was reprobed with anti-α-Tubulin antibody. Similar results were obtained in three independent experiments.
Functional characteristics of EPC-derived cells
We tested whether EPC-derived cells would up-regulate expression of the cell adhesion molecules CD54 and CD62E when exposed to TNF-α, which is a characteristic feature of EC. Incubation of EPC-derived cells with varying TNF-α concentrations (100 U/ml, 200 U/ml and 500 U/ml) for 4 and 24 hrs led to a markedly increased expression of both CD54 and CD62E (Fig. 7A). Treatment of HDLEC and HUVEC with TNF-α resulted in a similar dose and time response. However, in contrast to HDLEC and HUVEC, no decrease of CD62 expression was seen on EPC-derived cells after 5 hrs stimulation with TNF-α, indicating that the EPC-derived cells share similarities with mature EC, but are not alike (data not shown).
7.
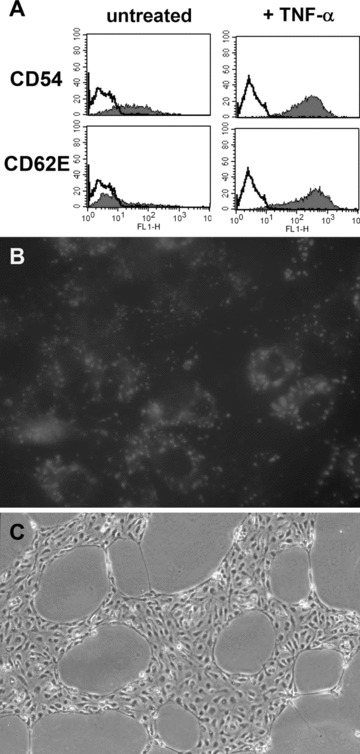
Functional characterization of EPC-derived cells. (A) EPC-derived cells, cultured for 24 hrs in differentiation medium with TNF-α (200 U/ml), up-regulated the surface expression of CD54 and CD62E. (B) A representative microscopic field of EPC-derived cells showing uptake of DiI-ac-LDL. Original magnification: ×400. (C) EPC-derived cells formed cord- and tubular-like structures after 24 hrs culture on Matrigel-coated wells, determined by phase contrast microscope. Original magnification: ×100. Each analysis is one representative example from a total of three donors.
Furthermore, EPC-derived cells efficiently incorporated DiI-ac-LDL, which is another important function of mature EC (Fig. 7B). The same results were observed in HDLEC and HUVEC.
The ability to form networks in Matrigel is also a hallmark of EC behaviour. Accordingly, we evaluated EPC-derived cells in network formation assays using Matrigel. Within 24 hrs of incubation, EPC-derived cells assembled into a capillary-like network on a Matrigel-coated surface, indistinguishable from those formed by HDLEC and HUVEC under the same conditions (Fig. 7C).
Comparative gene array analyses of cultured EPC-derived cells and HDLEC
Global gene expression patterns among EPC-derived cells and HDLEC populations were analysed to determine lineage relationships. Analyses were focused on a selected group of genes that are known to be involved in vascular development: growth factors, cytokines, chemokines, extracellular matrix proteins, adhesion and transmembrane molecules. Only genes showing at least a twofold difference between EPC-derived cells and HDLEC, but no activity in the EPC-derived cell-EPC-derived cell or HDLEC-HDLEC controls are listed in Table 2. Overall, the gene expression profiles of EPC-derived cells and HDLEC were quite similar. Of the 11.500 genes, 49 were up-regulated and 18 were down-regulated in the EPC-derived cells by a factor of twofold or greater, indicating the close lineage relationship of both types of vascular cells. In detail, EPC-derived cells expressed significantly higher levels of the vascular growth factors VEGF-C and FGF 16. Similarly, the angiogenic stimulators IL-6, annexin 3 and angiopoietin-like 4 as well as of the angiogenic inhibitor vasohibin were up-regulated in EPC-derived cells compared to HDLEC. Most strikingly, EPC-derived cells showed increased gene expression of adhesion and transmembrane molecules, including ECAM, E-selectin, NCAM, JAM-3 and VCAM, N-cadherin, integrin α1, α4 and α9 chains. Moreover, the extracellular matrix components type III, IV, V, VI, VIII, XII collagen and fibronectin were highly expressed in cultured EPC-derived cells as were the levels of the hyaluronan receptor CD44. Conversely, the expression of CD36 (thrombospondin receptor), reelin (extracellular matrix molecule) and podoplanin (transmembrane mucoprotein, present on lymphatic vessels) were strictly restricted to HDLEC. Furthermore, IL-7 that has significant impact on the lymphatic expression and the mannose receptor that directs the traffic of lymphocytes within the lymphatic vessels were one of the genes with the highest increase of expression in HDLEC.
2.
Genes showing significantly up- or down-regulated expression in EPC-derived cells compared to HDLEC*
| Fold increase in EPC-derived cells over HDLEC | Fold increase in HDLEC over EPC-derived cells | |
|---|---|---|
| Adhesion and transmembrane molecules | ||
| Endothelial cell adhesion molecule (ECAM) | 10.3 | |
| Junctional adhesion molecule-3 (JAM-3) | 11.6 | |
| Melanoma cell adhesion molecule (MCAM) | 12.5 | |
| Neural cell adhesion molecule-1 (NCAM-1) | 16 | |
| Neuroligin-1 | 14 | |
| Selectin E | 8.3 | |
| Vascular adhesion molecule-1 (VCAM-1) | 18.4 | |
| Integrin α1 | 12 | |
| Integrin α4 | 5.7 | |
| Integrin α9 | 12 | |
| Layilin | 8 | |
| N-cadherin | 37.5 | |
| Protocadherin α6 | 7 | |
| Protocadherin α9 | 7.2 | |
| Protocadherin α10 | 10.3 | |
| CD44 | 23.6 | |
| Mannose receptor 1 | 201 | |
| Podoplanin | 229 | |
| Cytoskeletal proteins | ||
| Desmoplakin | 24.8 | |
| Extracellular matrix molecules | ||
| Collagen type III α1 | 8.8 | |
| Collagen type IV α6 | 5.2 | |
| Collagen type Vα1 | 36.8 | |
| Collagen type VI α1 | 8 | |
| Collagen type VIII α1 | 43.4 | |
| Collagen type XII α1 | 16 | |
| Collagen type XIII α1 | 8.3 | |
| Collagen type XXVII α1 | 4.7 | |
| Fibronectin 1 | 19.7 | |
| Metallopeptidase inhibitor-3 (TIMP-3) | 16.1 | |
| Nidogen 2 | 14.4 | |
| Proteoglycan 1 | 10.8 | |
| Reelin | 40.8 | |
| Growth factors, cytokines, chemokines and their receptors Brain-derived neutropic factor (BDNF) | 9.85 | |
| Fibroblast growth factor 16 (FGF 16) | 8.8 | |
| Neuregulin 1 | 9 | |
| Transforming growth factor α (TGF α) | 12 | |
| Vascular endothelial growth factor-C (VEGF-C) | 16 | |
| Interleukin-1 (IL-1) | 29.9 | |
| Interleukin-1 receptor-like 1 | 90 | |
| Interleukin-6 (IL-6) | 7 | |
| Interleukin-7 (IL-7) | 20.8 | |
| Interleukin-17D (IL-17D) | 6.9 | |
| Chemokine ligand 3 | 7.2 | |
| Chemokine ligand 6 | 10.9 | |
| Chemokine ligand 14 | 16 | |
| Chemokine ligand 20 | 9 | |
| Chemokine receptor-like 2 | 5.5 | |
| Chemokine receptor 10 | 11.3 | |
| Hormones and their receptors | ||
| growth hormone receptor | 11.7 | |
| Inhibin βA | 36.8 | |
| Inhibin βB | 7 | |
| Relaxin 2 | 8.1 | |
| Retinoid acid receptor b | 6.8 | |
| Miscellaneous | ||
| Annexin A3 | 7.5 | |
| Angiopoitin-like 4 | 7 | |
| Apolipoprotein D | 11 | |
| Apolipoprotein L3 | 5.5 | |
| CD36 | 34 | |
| CD96 | 10 | |
| CD109 | 7.1 | |
| Clusterin | 8.9 | |
| Complement factor H | 7.1 | |
| Endothelial cell-specific molecule 1 | 4.7 | |
| Endothelin receptor type B | 15 | |
| Epithelial V-like antigen 1 | 64 | |
| Plasminogen activator, urokinase | 19.3 | |
| Vasohibin 1 | 6.6 |
Only genes showing a more than twofold up- or down-regulation between sample and control chip (in all 9 combinations) and no activity in the EPC-derived cell-EPC-derived cell or HDLEC-HDLEC controls were selected.
Discussion
In the present report, we undertook a comprehensive study of the phenotypic, functional and gene expression properties of EPC-derived cells propagated from the CD34+ cell fraction in umbilical cord blood to assess whether EPC-derived cells have particular in vitro behaviour compared with differentiated HDLEC and HUVEC.
The central finding of our study is that EPC-derived cells from umbilical cord blood share phenotypic and functional similarities with HDLEC, which corroborates the close relationship between blood vascular and lymphatic system [12]. In fact, both EC types expressed a similar profile of EC-specific antigens, including CD31, vWF, CD105 and CD144 [13, 14], and as shown in functional studies, they were indistinguishable in their EC behaviour too. Notably, EPC-derived cells failed to express CD34 and CD36, which correlates with the data from the microarray analysis and indicates that analogous to HUVEC, EPC-derived cells are rather macrovascular than microvascular EC [15, 16]. It is of further interest that our group could not document a differing expression of CD105 on EPC-derived cells and HDLEC. This finding was in contrast to other reports, showing that CD105 expression was increased in blood vascular endothelium, but was absent from or only sparsely expressed on lymphatic EC [13]. Such observed discrepancies may arise from differences in culture conditions employed. Specifically, growth factors and human serum may affect not only the selective growth of endothelial-like cells, but also the expression of different markers on their cell surface.
In accordance with published results, CD133 was lost as CD34+ EPC differentiated into mature EPC-derived cells [17]. CD133 is a marker of immature haematopoietic and progenitor cells and is present in a few other tissues such as kidney, pancreas and placenta, but not detectable on mature endothelium represented by HUVEC [18]. Gehling and co-workers [19] were the first who examined the capacity of CD133+ cells from granulocyte-stimulating factor-mobilized peripheral blood to differentiate into the endothelial lineage. Through the use of CD34 selection from umbilical cord blood, Eggermann et al.[20] defined EPC as VEGFR2+CD133+CD34+ cells that could be differentiated in culture to express markers of mature EC. Kim et al.[21], on the contrary, demonstrated that CD133−CD14+ cells from umbilical cord blood have the potential to form EC under endothelial growth factor stimulation in vitro.
That EPC-derived cells exhibit a classical phenotype of blood vascular EC, was demonstrated by the expression of Tie-1, Tie-2, VEGFR-1/ Flt-1 and VEGFR-2/Flk-1 [22, 23] and by the lack of lymphatic cell-specific markers podoplanin, Prox-1 and VEGFR-3 as well [9, 24, 25]. Since LYVE-1 expression is not restricted to lymphatic endothelium, but is found also on macrophages and dendritic cells, the sporadic detection of LYVE-1 on EPC-derived cells can be explained by their haematopoietic origin [26].
Microarray expression data were generally in agreement with previous reports and confirmed the vascular endothelial phenotype of EPC-derived cells [10, 15]. While podoplanin was exclusively expressed in HDLEC, EPC-derived cells showed no gene expression of any lymphatic cell-specific markers. Of particular interest was the finding that mainly adhesion molecules revealed an enhanced expression in cultured EPC-derived cells as compared with HDLEC, which elucidates the barrier function of blood vascular endothelium [27]. In contrast, HDLEC were characterized by a significant up-reg-ulation of the mannose receptor that is strictly restricted to lymphatic vessels where it supports lymphocyte adherence by binding to L-selectin on the lymphocyte surface [28]. Equally important was our finding that HDLEC expressed considerably higher levels of IL-7. This result supports the pivotal role of IL-7 in the regulation of lymphangiogenesis since it is known to induce the lymphangiogenic properties of EC by enhancing the expression of the lymphatic cell-specific markers podoplanin, Prox-1 and LYVE-1 [29].
By transmission electron microscopy, we identified Weibel-Palade bodies within EPC-derived cells, confirming that CD34+ cells had undergone a complete EC differentiation process. Consistent with our observation, Neumüeller et al.[30] also detected Weibel-Palade bodies in EPC-derived cells propagated from cord blood, whereas EPC-derived cells from peripheral blood did not display mature Weibel-Palade bodies, but organelles which they regarded as pre-Weibel-Palade bodies. These authors discriminated between spindle-like cell types with abundant Weibel-Palade bodies and cobblestone-like cell types with a fewer content of such organelles. The latter seem to be similar with our EPC-derived cells because they contained also few Weibel-Palade bodies.
In closing, we provided herein the first evidence for phenotypic and functional similarities among EPC-derived cells from CD34+ progenitors in umbilical cord blood and HDLEC. Most importantly, gene-profiling studies revealed that both vascular cell types differed in only a small percentage of gene expression patterns. Data presented in this study strengthen the hypothesis proposed by Florence Sabin a century ago that the lymphatic system develops shortly after the blood vascular system by sprouting from embryonic veins and then become through the formation of primitive sacs a mature lymphatic network.
Acknowledgments
This study was supported by the Tyrolean Provincial Hospital Company, Tilak Ges.m.b.H., and the Innsbruck Children's Cancer Research Institute. We thank all midwives from the Department of Obstetrics and Gynaecology, Innsbruck, for providing umbilical cord samples and Peter Fritsch, Chairman of the Department, for continued support.
References
- 1.Risau W, Sariola H, Zerwes HG, Sasse J, Ekblom P, Kemler R, Doetschman T. Vasculogenesis and angiogenesis in embryonic stem-cell-derived embryoid bodies. Development. 1998;102:471–8. doi: 10.1242/dev.102.3.471. [DOI] [PubMed] [Google Scholar]
- 2.Ashara T, Murohara T, Sullivan A, Silver M, Van Der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Ashara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 4.Nieda M, Nicol A, Denning-Kenndall P, Sweetenham J, Bradley B, Hows J. Endothelial cell precursors are normal components of human umbilical cord blood. Br J Haematol. 1997;98:775–7. doi: 10.1046/j.1365-2141.1997.2583074.x. [DOI] [PubMed] [Google Scholar]
- 5.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–7. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 7.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Raffii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 8.Saharinen P, Tammela T, Karkkainen MJ, Alitalo K. Lymphatic vasculature: development, moleculat regulation and role in tumor metastasis and inflammation. Trends Immunol. 2004;35:387–95. doi: 10.1016/j.it.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Salven P, Mustjoki S, Alitalo R, Alitalo K, Rafii S. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor. Blood. 2003;101:168–72. doi: 10.1182/blood-2002-03-0755. [DOI] [PubMed] [Google Scholar]
- 10.Schoppmann S, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–56. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen VA, Ebner S, Fürhapter C, Romani N, Kölle D, Fritsch P, Sepp N. Adhesion of dendritic cells derived from CD34+ progenitors to resting human dermal microvascular endothelial cells is down-regulated upon maturation and partially depends on CD11a-CD18, CD11b-CD18 and CD36. Eur J Immunol. 2002;32:3638–50. doi: 10.1002/1521-4141(200212)32:12<3638::AID-IMMU3638>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.Hirakawa S, Hong YK, Harvey N, Schacht V, Matsuda K, Libermann T, Detmar M. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol. 2003;162:575–86. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garlanda C, Dejana E. Heterogeneity of endothelial cells. Specific markers. Arterioscler Thromb Vasc Biol. 1997;17:1193–202. doi: 10.1161/01.atv.17.7.1193. [DOI] [PubMed] [Google Scholar]
- 14.Muller A, Hermanns M, Skryznski C, Nesslinger M, Muller K, Kirkpatrick C. Expression of the endothelial markers PECAM-1, vWF and CD34 in vivo and in vitro. Exp Mol Pathol. 2002;72:221–9. doi: 10.1006/exmp.2002.2424. [DOI] [PubMed] [Google Scholar]
- 15.Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland DR, Baker MA, Greaves MF. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75:2417–26. [PubMed] [Google Scholar]
- 16.Swerlick RA, Garcia-Gonzalez E, Kubota Y, Xu Y, Lawley TJ. Human dermal microvascular endothelial but not human umbilical vein endothelial cells express CD36 in vivo and in vitro. J Immunol. 1992;149:698–705. [PubMed] [Google Scholar]
- 17.Quirici N, Soligo D, Caneva L, Servida F, Bossolasco P, Deliliers G. Differentiation and expansion of endothelial cells from human bone marrow CD133+ cells. Br J Haematol. 2001;115:186–94. doi: 10.1046/j.1365-2141.2001.03077.x. [DOI] [PubMed] [Google Scholar]
- 18.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–12. [PubMed] [Google Scholar]
- 19.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schäfer B, Hossfeld DK, Filder W. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–12. [PubMed] [Google Scholar]
- 20.Eggermann J, Kliche S, Jarmy G, Hoffmann K, Mayr-Beyrle U, Debatin KM, Waltenberger J, Beltinger C. Endothelial progenitor cell culture and differentiation in vitro: a methodological comparison using human umbilical cord blood. Cardiovasc Res. 2003;58:478–86. doi: 10.1016/s0008-6363(03)00252-9. [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Park SY, Kim JM, Kim JW, Kim MY, Yang JH, Kim JO, Choi KH, Kim SB, Ryu HM. Differentiation of endothelial cells from human umbilical cord blodd AC133−CD14+ cells. Ann Hematol. 2005;84:417–22. doi: 10.1007/s00277-004-0988-y. [DOI] [PubMed] [Google Scholar]
- 22.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrokinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–4. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 23.Eichmann A, Corbel C, Natav V, Vaigot P, Breant C, Le Douarin NM. Ligand-dependent development of the endothelial and hemopoietic lineages from embryonic mesodermal cells expressing vascular endothelial growth factor receptor 2. Proc Natl Acad Sci USA. 1997;94:5141–6. doi: 10.1073/pnas.94.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–94. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–13. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bazzoni G, Dejani E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 28.Irjala H, Johansson EL, Reidar G, Alanen K, Salmi M, Jalkanen S. Mannose lymphocyte binding to lymphatic endothelium. J Exp Med. 2001;194:1033–41. doi: 10.1084/jem.194.8.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Rawi MA, Watkins G, Mansel RE, Jiang WG. The effects of interleukin-7 on the lymphangiogenic properties of human endothelial cells. Int J Oncol. 2005;27:721–30. [PubMed] [Google Scholar]
- 30.Neumüller J, Neumüller-Gruber SE, Lipovac M, Mosgoeller W, Vetterlein M, Pavelka M, Huber J. Immunological and ultrstructural characterization of endothelial cell cultures differentiated from human cord blood derived endothelial progenitor cells. Histochem Cell Biol. 2006;126:649–64. doi: 10.1007/s00418-006-0201-6. [DOI] [PubMed] [Google Scholar]


